
Книги фарма 2 / Bertram G. Katzung-Basic & Clinical Pharmacology(9th Edition)
.pdf
Patency of the fetal ductus arteriosus is now generally believed to depend on local PGE2 and PGI2 synthesis. In certain types of congenital heart disease (eg, transposition of the great arteries, pulmonary atresia, pulmonary artery stenosis), it is important to maintain the patency of the neonate's ductus arteriosus before surgery. This is done with alprostadil, PGE1. Like PGE2, PGE1 is a vasodilator and an inhibitor of platelet aggregation, and it contracts uterine and intestinal smooth muscle. Adverse effects include apnea, bradycardia, hypotension, and hyperpyrexia. Because of rapid pulmonary clearance, the drug must be continuously infused at an initial rate of 0.05–0.1 g/kg/min, which may be increased to 0.4  g/kg/min. Prolonged treatment has been associated with ductal fragility and rupture.
g/kg/min. Prolonged treatment has been associated with ductal fragility and rupture.
In delayed closure of the ductus arteriosus, COX inhibitors are often used to inhibit synthesis of PGE2 and PGI2 and so close the ductus. Premature infants in whom respiratory distress develops due to failure of ductus closure can be treated with a high degree of success with indomethacin. This treatment often precludes the need for surgical closure of the ductus.
Blood
As noted above, eicosanoids are involved in thrombosis because TXA2 promotes platelet aggregation and PGI2 inhibits it. Aspirin inhibits platelet COX to produce a mild clotting defect. The mildness of this defect is supported by the fact that very modest hemostatic defects are noted in patients with diseases involving deficiencies of platelet COX and thromboxane synthase—eg, these patients have no history of increased or decreased bleeding. Blockade of either of these two enzymes inhibits secondary aggregation of platelets induced by ADP, by low concentrations of thrombin and collagen, or by epinephrine. Thus, these platelet enzymes are not necessary for platelet function but may amplify an aggregating stimulus.
Epidemiologic studies in the USA and United Kingdom indicate that low doses of aspirin reduce the risk of death due to infarction but may increase overall mortality rates due to hemorrhagic stroke (see Chapter 34: Drugs Used in Disorders of Coagulation). It is now difficult to find patients at risk for thromboembolism—as in orthopedic surgery or angioplasty for coronary artery stenosis—who do not take aspirin. The beneficial effects of aspirin are discussed in greater detail in Chapter 34: Drugs Used in Disorders of Coagulation.
Respiratory System
PGE2 is a powerful bronchodilator when given in aerosol form. Unfortunately, it also promotes coughing, and an analog that possesses only the bronchodilator properties has been difficult to obtain.
PGF2 and TXA2 are both strong bronchoconstrictors and were once thought to be primary mediators in asthma. However, the identification of the peptidoleukotrienes—LTC4, LTD4, and LTE4—expanded the role of eicosanoids as important mediators in asthma and other immune responses. As described in Chapter 20: Drugs Used in Asthma, leukotriene receptor inhibitors (eg, zafirlukast, montelukast) are effective in asthma. A lipoxygenase inhibitor (zileuton) has also been used in asthma but is not as popular as the receptor inhibitors. It remains unclear whether leukotrienes are partially responsible for the acute respiratory distress syndrome.
Corticosteroids and cromolyn are also useful in asthma. Corticosteroids inhibit eicosanoid synthesis and thus limit the amounts of eicosanoid mediator available for release. Cromolyn appears to inhibit the release of eicosanoids and other mediators such as histamine and platelet-activating factor from mast cells.

Gastrointestinal System
The word "cytoprotection" was coined to signify the remarkable protective effect of the E prostaglandins against peptic ulcers in animals at doses that do not reduce acid secretion. These prostaglandins were independently discovered also to inhibit gastric acid secretion (at higher doses). Since then, numerous experimental and clinical investigations have shown that the PGE compounds and their analogs protect against peptic ulcers produced by either steroids or NSAIDs. Misoprostol is an orally active synthetic analog of PGE1 available in Europe and the USA for ulcer treatment. The FDA-approved indication is for prevention of NSAID-induced peptic ulcers. The drug is administered at a dosage of 200 g four times daily. This and other PGE analogs (eg, enprostil) are cytoprotective at low doses and inhibit gastric acid secretion at higher doses. The adverse effects are abdominal discomfort and occasional diarrhea; both effects are dose-related. More recently, dosedependent bone pain and hyperostosis have been described in patients with liver disease who were given long-term PGE treatment. This adverse effect can be explained by a PGE-induced, EP4- mediated acceleration of osteoclast and osteoblast activity. Recurrent calcium oxalate kidney stones were described in the same group of patients. This may be related to PGE-induced hypercalciuria.
Gastrointestinal side effects seen in many patients using NSAIDs may be reduced by the recent introduction of selective inhibitors of COX-2 that spare gastric COX-1 so that the natural cytoprotection by locally synthesized PGE2 is undisturbed (see Chapter 36: Nonsteroidal AntiInflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout).
Immune System
Monocyte-macrophages are the only principal cells of the immune system that can synthesize all the eicosanoids. T and B lymphocytes are interesting exceptions to the general rule that all nucleated cells produce eicosanoids. However, in a B lymphoma cell line, there is non-receptor-mediated uptake of LTB4 and 5-HETE. Interaction between lymphocytes and monocyte-macrophages may cause the lymphocytes to release arachidonic acid from their cell membranes. The arachidonic acid is then used by the monocyte-macrophages for eicosanoid synthesis. In addition to these cells, there is evidence for eicosanoid-mediated cell-cell interaction by platelets, erythrocytes, PMNs, and endothelial cells.
The eicosanoids modulate the effects of the immune system, as illustrated by the cell-mediated immune response. As shown in Figure 18–5, PGE2 and PGI2 affect T cell proliferation in vitro as corticosteroids do. T cell clonal expansion is attenuated through inhibition of interleukin-1 and interleukin-2 and class II antigen expression by macrophages or other antigen-presenting cells. The leukotrienes, TXA2, and platelet-activating factor stimulate T cell clonal expansion. These compounds stimulate the formation of interleukin-1 and interleukin-2 as well as the expression of interleukin-2 receptors. The leukotrienes also promote interferonrelease and can replace interleukin-2 as a stimulator of interferon- . These in vitro effects of the eicosanoids agree with in vivo findings in animals with acute organ transplant rejection, as described below.
. These in vitro effects of the eicosanoids agree with in vivo findings in animals with acute organ transplant rejection, as described below.
Figure 18–5.

Modulation of macrophage and lymphocyte interactions by eicosanoids, platelet-activating factor, and corticosteroids. Corticosteroids, PGE2, and possibly PGI2 inhibit the expression of interleukin- 1 (IL-1) and its effect on T lymphocytes. Platelet-activating factor (PAF), LTB4, and LTD4 increase IL-1 expression. Similar inhibitory and stimulant effects are exerted on the action of interferon- on the macrophage and on the action of interleukin-2 (IL-2). Agents marked with an asterisk are suspected, but not yet proved, to have the effects indicated. (DR, class II MHC [major histocompatibility complex] receptor; T, T lymphocytes.) (Modified and reproduced, with permission, from Foegh ML, Ramwell PW: PAF and transplant immunology. In: Braquet P [editor]: The Role of Platelet Activating Factor in Immune Disorders. Karger, 1988.)
on the macrophage and on the action of interleukin-2 (IL-2). Agents marked with an asterisk are suspected, but not yet proved, to have the effects indicated. (DR, class II MHC [major histocompatibility complex] receptor; T, T lymphocytes.) (Modified and reproduced, with permission, from Foegh ML, Ramwell PW: PAF and transplant immunology. In: Braquet P [editor]: The Role of Platelet Activating Factor in Immune Disorders. Karger, 1988.)
Cell-Mediated Organ Transplant Rejection
Acute organ transplant rejection is a cell-mediated immune response. Administration of PGI2 to renal transplant patients has reversed the rejection process in some cases. Experimental in vitro and in vivo data show that PGE2 and PGI2 can attenuate T cell proliferation and rejection, which can also be seen with drugs that inhibit TXA2 and leukotriene formation. In organ transplant patients, urinary excretion of TXB2, a metabolite of TXA2, increases during acute rejection. Corticosteroids, the primary drugs used for treatment of acute rejection due to their effects on lymphocytes, inhibit both phospholipase and COX-2 activity.
Inflammation
Aspirin has been used to treat arthritis for nearly a century, but its mechanism of action—inhibition of COX activity—was not discovered until 1971. Aspirin and other anti-inflammatory agents that inhibit COX are discussed in Chapter 36: Nonsteroidal Anti-Inflammatory Drugs, DiseaseModifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout. COX-2 appears to be the form of the enzyme associated with cells involved in the inflammatory process. The prostaglandins are not chemoattractants, but the leukotrienes and some of the HETEs (eg, 12HETE) are strong chemoattractants. PGE2 inhibits both antigen-driven and mitogen-induced B

lymphocyte proliferation and differentiation to plasma cells, resulting in inhibition of IgM synthesis. The concomitant elevation of serum IgE and monocyte PGE2 synthesis, seen in patients with severe trauma and patients with Hodgkin's disease, is explained by PGE2 ability to enhance immunoglobulin class switching to IgE.
Rheumatoid Arthritis
In rheumatoid arthritis, immune complexes are deposited in the affected joints, causing an inflammatory response that is amplified by eicosanoids. Lymphocytes and macrophages accumulate in the synovium, while PMNs localize mainly in the synovial fluid. The major eicosanoids produced by PMNs are leukotrienes, which facilitate T cell proliferation and act as chemoattractants. Human macrophages synthesize the COX products PGE2 and TXA2 and large amounts of leukotrienes.
Infection
The relationship of eicosanoids to infection is not well defined. The association between the use of the anti-inflammatory steroids and increased risk of infection is well established. However, the NSAIDs do not seem to alter patient responses to infection.
Glaucoma
Latanoprost, a stable long-acting PGF2 derivative, was the first prostanoid used for glaucoma. The success of latanoprost has stimulated development of similar prostanoids with ocular hypotensive effects, and bimatoprost, travaprost, and unoprostone are now available. These drugs act at the FP receptor and are administered as drops into the conjunctival sac once or twice daily. Adverse effects include irreversible brown pigmentation of the iris and eyelashes, drying of the eyes, and conjunctivitis.
Dietary Manipulation of Arachidonic Acid Metabolism
Because arachidonic acid is derived from dietary linoleic and 
 -linolenic acids, which are essential fatty acids, the effects of dietary manipulation on arachidonic acid metabolism have been extensively studied. Two approaches have been used. The first adds corn, safflower, and sunflower oils, which contain linoleic acid (C18:2), to the diet. The second approach adds oils containing eicosapentaenoic (C20:5) and docosahexaenoic acids (C22:6), so-called omega-3 fatty acids, from cold water fish. Both types of diet change the phospholipid composition of cell membranes by replacing arachidonic acid with the dietary fatty acids. It has been claimed that the synthesis of both TXA2 and PGI2 is reduced and that changes in platelet aggregation, vasomotor spasm, and cholesterol metabolism follow.
-linolenic acids, which are essential fatty acids, the effects of dietary manipulation on arachidonic acid metabolism have been extensively studied. Two approaches have been used. The first adds corn, safflower, and sunflower oils, which contain linoleic acid (C18:2), to the diet. The second approach adds oils containing eicosapentaenoic (C20:5) and docosahexaenoic acids (C22:6), so-called omega-3 fatty acids, from cold water fish. Both types of diet change the phospholipid composition of cell membranes by replacing arachidonic acid with the dietary fatty acids. It has been claimed that the synthesis of both TXA2 and PGI2 is reduced and that changes in platelet aggregation, vasomotor spasm, and cholesterol metabolism follow.
As indicated above, there are many possible oxidation products of the different polyenoic acids. It is probably naive to ascribe the effects of dietary intervention reported thus far to such metabolites. Carefully controlled clinical studies will be needed before these questions can be satisfactorily answered. However, subjects on diets containing highly saturated fatty acids clearly show increased platelet aggregation when compared with other study groups. Such diets (eg, in Finland and the USA) are associated with higher rates of myocardial infarction than are more polyunsaturated diets (eg, in Italy).

Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 18. The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds >
Preparations Available
Nonsteroidal Anti-Inflammatory Drugs Are Listed in Chapter 36: Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout.
Alprostadil
Penile injection (Caverject, Edex): 5, 10, 20, 40  g sterile powder for reconstitution
g sterile powder for reconstitution
Penile pellet (Muse): 125, 250, 500, 1000 
 g
g
Parenteral (Prostin VR Pediatric): 500 g/mL ampules
Bimatoprost (Lumigan)
Ophthalmic drops: 0.03% solution
Carboprost tromethamine (Hemabate)
Parenteral: 250 

 g carboprost and 83 g tromethamine per mL ampules
g carboprost and 83 g tromethamine per mL ampules
Dinoprostone [prostaglandin E2] (Prostin E2, Prepidil, Cervidil)
Vaginal: 20 mg suppositories, 0.5 mg gel, 10 mg controlled release system
Epoprostenol [prostacyclin] (Flolan)
Intravenous: powder to make 3, 5, 10, 15 g/mL
Latanoprost (Xalatan)
Topical: 50  g/mL ophthalmic solution
g/mL ophthalmic solution
Misoprostol (Cytotec)
Oral: 100 and 200 g tablets
Monteleukast (Singulair)
Oral: 5 mg chewable, 10 mg tablets
Travaprost (Travatan)
Ophthalmic solution: 0.0004%
Treprostinil (Remodulin)

Parenteral: 1, 2.5, 5, 10 mg/mL for continuous subcutaneous infusion
Unoprostone (Rescula)
Ophthalmic solution 0.15%
Zafirleukast (Accolate)
Oral: 10, 20 mg tablets
Zileuton (Zyflo))
Oral: 600 mg tablets
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 18. The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds >
Chapter 19. Nitric Oxide, Donors, & Inhibitors
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 19. Nitric Oxide, Donors, & Inhibitors >
Nitric Oxide, Donors, & Inhibitors: Introduction
Nitric oxide (NO) is a gaseous signaling molecule that readily diffuses across cell membranes and regulates a wide range of physiologic and pathophysiologic processes including cardiovascular, inflammation, immune and neuronal functions.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 19. Nitric Oxide, Donors, & Inhibitors >
Discovery of Endogenous Nitric Oxide
Early observations of the biologic role of endogenously generated NO were made in rodent macrophages and neutrophils: In vitro exposure of these cells to endotoxin lipopolysaccharide released significant amounts of nitrite and nitrate into the cell culture medium. Furthermore, injection of endotoxin in vivo elevated urinary nitrite and nitrate, the two oxidation products of nitric oxide. This nitric oxide was found to originate from oxidation of the guanidino group of L- arginine.
The second observation was made by Furchgott and Zawadzki in 1980 using isolated vascular smooth muscle preparations. They discovered that following stimulation with acetylcholine or carbachol, the endothelium released a short-lived vasodilator, which—unlike endothelium-derived prostacyclin—was not blocked by cyclooxygenase inhibitors. They named this vasodilator endothelium-derived relaxing factor (EDRF) since it promoted relaxation of precontracted smooth muscle preparations. Other workers confirmed and extended these findings. In 1987, by comparing the pharmacologic and biochemical properties of the suspect molecule, three independent groups reported that EDRF and nitric oxide are the same molecule. It was later reported that other vasodilator molecules may be a part of EDRF, but it appears clear that nitric

oxide provides the major part of its activity. Subsequent studies revealed that nitric oxide was generated by many cells and was, like the eicosanoids (see Chapter 18: The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds), found in almost all tissues. The major exogenous donors of nitric oxide (nitrates, nitrites, nitroprusside) have been discussed (see Chapter 11: Antihypertensive Agents and Chapter 12: Vasodilators & the Treatment of Angina Pectoris).
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 19. Nitric Oxide, Donors, & Inhibitors >
Biologic Synthesis & Inactivation of Nitric Oxide
Synthesis
Nitric oxide, written as NO. or simply NO, is a highly diffusible stable gas composed of one atom each of nitrogen and oxygen. It is synthesized by a family of enzymes that are collectively called nitric oxide synthase, NOS (EC 1.14.13.49). Three isoforms of NOS have been identified (Table 19–1). These isoforms are heme-containing flavoproteins employing L-arginine as a substrate and requiring NADPH, flavin adenine dinucleotide, and tetrahydrobiopterin as cofactors.
Phosphorylation also has differential regulatory effects on the activity of NOS. For example, phosphorylation significantly reduces the activity of NOS-1, whereas phosphorylation of NOS-3 by a serine-threonine protein kinase activates the enzyme. Furthermore, NOS-2 expression is tightly controlled by several transcription factors.
Table 19–1. Properties of the Three Isoforms of Nitric Oxide Synthase (NOS).
|
|
|
Isoform Names |
|
|
|
|
|
|
Property |
|
NOS-1 |
NOS-2 |
|
NOS-3 |
|
|
|
|
|
|
|
|
|
|
|
|
Other names |
|
nNOS (neuronal |
iNOS (inducible NOS) |
|
eNOS (endothelial |
|
|
|
|
|
NOS) |
|
|
NOS) |
|
|
|
Tissue |
|
Neuronal, epithelial |
Macrophages, smooth muscle |
|
Endothelial cells |
|
|
|
|
|
cells |
cells |
|
|
|
|
|
Expression |
|
Constitutive |
Transcriptional induction |
|
Constitutive |
|
|
|
|
|
|
|
|
|
|
|
|
Calcium |
|
Yes |
No |
|
Yes |
|
|
|
requirement |
|
|
|
|
|
|
|
|
Chromosome |
|
12 |
17 |
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
Approximate mass |
|
150–160 kDa |
125–135 kDa |
|
133 kDa |
|
|
|
|
|
|
|
|
|
|
|
Formation of nitric oxide from L-arginine and several nitric oxide donors is shown in Figure 19–1. Activation of NOS by the influx of extracellular calcium and binding of calmodulin, as in the case of the constitutive enzyme, or following the activation of the inducible NOS (NOS-2) by cytokines, results in the metabolism of L-arginine to L-citrulline and nitric oxide. The conversion of L-arginine to nitric oxide and L-citrulline is inhibited by several arginine competitors such as NG-monomethyl- L-arginine (below). Some nitric oxide donors, eg, oxygenated nitroprusside, spontaneously generate
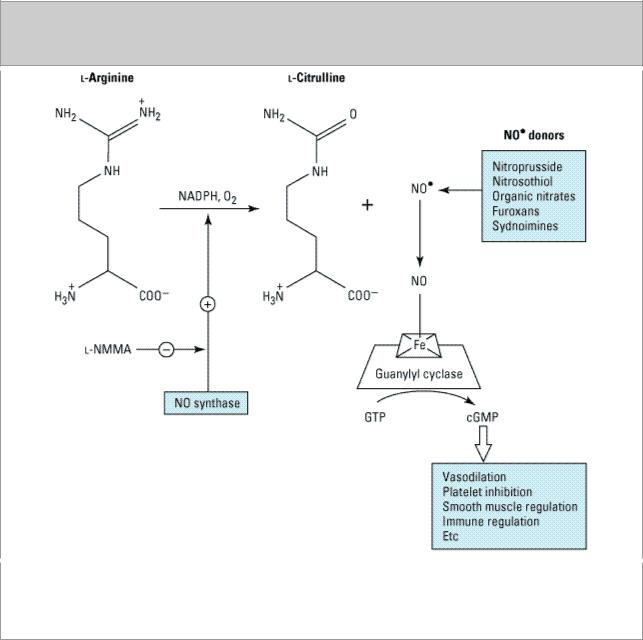
such as nitroglycerin, require the presence of a thiol compound such as cysteine. Once generated, nitric oxide interacts with the heme moiety of soluble guanylyl cyclase in the cytoplasm of cells (Figure 19–1, right). This results in allosteric transformation and activation of the enzyme and leads to the formation of 3',5'-cyclic-guanosine monophosphate (cGMP) from GTP. Activation of the soluble guanylyl cyclase by nitric oxide can be inhibited by methylene blue. The affinity of nitric oxide for iron is also responsible for its inhibitory effect on several enzymes by interacting with the iron-sulfur centers of these enzymes. Inhibition of enzymes such as cytochrome P450 by nitric oxide is a major problem in inflammatory liver disease and can be reversed by NO synthase inhibitors. Carbon monoxide, another gaseous compound produced endogenously from the catabolism of heme, shares many of the properties of nitric oxide such as activation of soluble guanylyl cyclase. However, unlike nitric oxide, which has an extra electron, carbon monoxide is a stable molecule in the presence of oxygen. The affinity of nitric oxide for hemoglobin is several orders of magnitude greater than that of carbon monoxide. Nitric oxide undergoes both oxidative and reductive reactions, resulting in the formation of a variety of oxides of nitrogen (Table 19–2).
Figure 19–1.
Nitric oxide generation from L-arginine and nitric oxide donors and the formation of cGMP. L- NMMA inhibits nitric oxide synthase. Some of the nitric oxide donors such as furoxans and organic nitrates and nitrites require a thiol cofactor such as cysteine or glutathione to form nitric oxide.

Table 19–2. Oxides of Nitrogen.
Name |
Symbol |
Known Function |
|
|
|
Nitric oxide |
NO |
Vasodilator, platelet inhibitor, immune regulator, neurotransmitter |
|
|
|
|
|
|
Nitroxyl anion |
NO- |
Smooth muscle relaxant |
|
|
|
|
|
|
Nitrogen dioxide |
NO2 |
Free radical, nitrosating agent, lung irritant |
|
|
|
|
|
|
Nitrous oxide |
N2O |
Anesthetic |
|
|
|
|
|
|
Dinitrogen trioxide |
N2O3 |
Nitrosating agent |
|
|
|
|
|
|
Dinitrogen tetraoxide |
N2O4 |
Nitrosating agent |
|
|
|
|
|
|
Nitrite |
NO2- |
Produce NO at acidic pH |
|
|
|
|
|
|
Nitrate |
NO3- |
Stable oxidation product of NO |
|
|
|
|
|
|
Inactivation
Nitric oxide is inactivated by heme and by the free radical, superoxide. Thus, scavengers of superoxide anion such as superoxide dismutase may protect nitric oxide, enhancing its potency and prolonging its duration of action. Conversely, interaction of nitric oxide with superoxide may generate the potent tissue-damaging moiety, peroxynitrite (ONOO-), which has a high affinity for sulfhydryl groups and thus inactivates several key sulfhydryl-bearing enzymes. This effect of peroxynitrite is regulated by the cellular content of glutathione. Since glutathione is the major intracellular soluble sulfhydryl-containing compound, factors that regulate the biosynthesis and decomposition of glutathione may have important consequences.
Glutathione also interacts with nitric oxide under physiologic conditions to generate S- nitrosoglutathione, a more stable form of nitric oxide. Nitrosoglutathione may serve as an endogenous long-lived adduct or carrier of nitric oxide. Vascular glutathione is decreased in diabetes mellitus and atherosclerosis, and this may account for the increased incidence of cardiovascular complications in these conditions. Ischemia followed by reperfusion is another situation in which endothelial function is compromised owing to increased production of free radicals, resulting in reduced nitric oxide formation.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 19. Nitric Oxide, Donors, & Inhibitors >
Inhibitors of Nitric Oxide
In theory, several methods are available for reducing nitric oxide levels in tissues and thus

inhibiting its actions. Drugs may inhibit the uptake of L-arginine into cells, thus depriving the NOS isoforms of substrate. Other methods include deprivation of the cofactors and calmodulin antagonists; inhibitors of NOS synthesis; inhibitors of binding of arginine to NOS, and scavengers of nitric oxide. The most important thus far have been inhibitors of NOS. Unfortunately, the selectivity of these inhibitors for the individual isoforms is incomplete. Most of these inhibitors are substrate analogs (Table 19–3).
Table 19–3. Some Inhibitors of Nitric Oxide Synthesis or Action.
Inhibitor |
Mechanism |
Comment |
|
|
|
Ng–Monomethyl–L–arginine (L–NMMA) |
NOS inhibition |
May act as substrate in some |
|
|
tissues |
|
|
|
Ng–Nitro–L–arginine methyl ester (L– |
NOS inhibition |
Less selective NOS inhibitor |
NAME) |
|
|
|
|
|
|
|
|
7–Nitroindazole |
NOS inhibition |
Markedly selective for NOS–1 in |
|
|
vivo |
|
|
|
S–Methylthiocitrulline |
NOS inhibition |
Partially selective for NOS–1 |
|
|
|
Heme |
Nitric oxide |
|
|
scavenger |
|
|
|
|
Protein inhibitor of NOS |
Unknown |
Endogenous inhibitor found in |
|
mechanism |
brain |
|
|
|
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 19. Nitric Oxide, Donors, & Inhibitors >
Effects of Nitric Oxide
Nitric oxide has major effects that are mediated by activation of cytoplasmic soluble guanylyl cyclase and stimulated production of cGMP, an important second messenger. In addition, nitric oxide can produce several reactive nitrogen derivatives by interaction with molecular oxygen and superoxide radicals (Table 19–2). These highly unstable molecules react with a variety of proteins, lipids, nucleic acids, and metals (especially iron) in cells (Davis, 2001). The remainder of this chapter discusses some of the second messenger-mediated effects of nitric oxide and the effects of inhibition of its production.
Vascular Effects
Nitric oxide has a significant effect on vascular smooth muscle tone and blood pressure. As stated previously, it is released by acetylcholine and other endothelium-dependent vasodilators. It may play a role in the normal regulation of vascular tone as suggested by the fact that a reduction of nitric oxide synthesis (caused by knockout mutations, infusion of NOS inhibitors such as L-NMMA, or by injury to the vascular endothelium) increases vascular tone and elevates mean arterial pressure. The effects of vasopressor drugs are increased by inhibition of NOS. As described in Chapter 12: Vasodilators & the Treatment of Angina Pectoris and shown in Figure 12–2, increased cGMP synthesis by guanylyl cyclase results in smooth muscle relaxation.
