
Книги фарма 2 / Bertram G. Katzung-Basic & Clinical Pharmacology(9th Edition)
.pdf
In the brain, processing of the precursor leads primarily to the formation of neurotensin and neuromedin N; these are released together from nerve endings. In the gut, processing leads mainly to the formation of neurotensin and a larger peptide that contains the neuromedin N sequence at the carboxyl terminal. Both peptides are secreted into the circulation after ingestion of food.
Like many other neuropeptides, neurotensin serves a dual function as a neurotransmitter or neuromodulator in the central nervous system and as a local hormone in the periphery. When administered centrally, neurotensin exerts potent effects including hypothermia, antinociception and modulation of dopamine neurotransmission. When administered into the peripheral circulation, it causes vasodilation, hypotension, increased vascular permeability, increased secretion of several anterior pituitary hormones, hyperglycemia, inhibition of gastric acid and pepsin secretion, and inhibition of gastric motility.
Three subtypes of neurotensin receptors designated NT1, NT2, and NT3 have been cloned. NT1 and NT2 receptors belong to the G protein-coupled superfamily; NT3 receptors are structurally different.
Neurotensin agonists that cross the blood-brain barrier have been developed and have potential as potential therapeutic agents for diseases such as schizophrenia and Parkinson's disease (McMahon, 2002).
Neurotensin receptors can be blocked with the nonpeptide antagonists SR142948A and SR48692. SR142948A is a potent antagonist of the hypothermia and analgesia produced by centrally administered neurotensin. It also blocks the cardiovascular effects of systemic neurotensin.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 17. Vasoactive Peptides >
Calcitonin Gene-Related Peptide
Calcitonin gene-related peptide (CGRP) is a member of the calcitonin family of peptides, which also includes calcitonin, adrenomedullin (see below) and amylin. CGRP consists of 37 amino acids and displays approximately 30% structural homology with salmon calcitonin.
Like calcitonin, CGRP is present in large quantities in the C cells of the thyroid gland. It is also distributed widely in the central and peripheral nervous systems, in the cardiovascular system, the gastrointestinal tract, and the urogenital system. CGRP is found with substance P (see above) in some of these regions and with acetylcholine in others.
When CGRP is injected into the central nervous system, it produces a variety of effects, including hypertension and suppression of feeding. When injected into the systemic circulation, the peptide causes hypotension and tachycardia. The hypotensive action of CGRP results from the potent vasodilator action of the peptide; indeed, CGRP is the most potent vasodilator yet discovered.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 17. Vasoactive Peptides >

Adrenomedullin
Adrenomedullin is a 52-amino-acid peptide that was discovered in human adrenal medullary pheochromocytoma tissue. Like CGRP, it is a member of the calcitonin family of peptides. Adrenomedullin is present in several organs including the adrenals, lungs, heart, vascular tissue, and kidneys. It also circulates in the blood.
In animals, adrenomedullin dilates resistance vessels in the kidney, brain, lung, hind limbs, and mesentery, resulting in a marked, long-lasting hypotension. The hypotension in turn causes reflex increases in heart rate and cardiac output. Adrenomedullin also acts on the kidneys to increase sodium excretion, and exerts several endocrine effects including inhibition of aldosterone and insulin secretion. Finally it acts on the central nervous system to increase sympathetic outflow. These diverse actions of adrenomedullin are mediated both by CGRP receptors and unique adrenomedullin receptors, the properties of which are incompletely defined. The major second messenger for both receptors is cAMP.
Circulating adrenomedullin levels increase during intense exercise. Levels are also elevated in patients with hypertension, as well as in patients with renal failure, heart failure, and septic shock.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 17. Vasoactive Peptides >
Neuropeptide Y
Neuropeptide Y is a member of the family that also includes peptide YY and pancreatic polypeptide. Each peptide consists of 36 amino acids. The amino acid sequence of neuropeptide Y is shown below.
Neuropeptide Y is one of the most abundant neuropeptides in both the central and peripheral nervous systems. In the sympathetic nervous system, neuropeptide Y is frequently localized in noradrenergic neurons and apparently functions both as a vasoconstrictor and as a cotransmitter with norepinephrine. Peptide YY and pancreatic polypeptide are both gut endocrine peptides.
Neuropeptide Y produces a variety of central nervous system effects, including increased feeding (it is one of the most potent orexigenic molecules in the brain), hypotension, hypothermia, and respiratory depression. Other effects include vasoconstriction of cerebral blood vessels, positive chronotropic and inotropic actions on the heart, and hypertension. The peptide is a potent renal vasoconstrictor and suppresses renin secretion, but can cause diuresis and natriuresis. Prejunctional actions include inhibition of transmitter release from sympathetic and parasympathetic nerves; vascular actions include direct vasoconstriction, potentiation of the action of vasoconstrictors, and inhibition of the action of vasodilators.
These diverse effects are mediated by multiple receptors designated Y1 through Y6. All receptors except Y3 have been cloned and shown to be G protein-coupled receptors coupled to mobilization of Ca2+ and inhibition of adenylyl cyclase. Y1 and Y2 receptors are of major importance in the

cardiovascular and other peripheral effects of the peptide. Y4 receptors have a high affinity for pancreatic polypeptide and may be a receptor for this peptide rather than for neuropeptide Y. Y5 receptors are found mainly in the central nervous system and may be involved in the control of food intake. Y6 receptors do not appear to contribute significantly to the physiologic effects of neuropeptide Y in humans.
Selective nonpeptide neuropeptide Y receptor antagonists are now available. The first nonpeptide Y1 receptor antagonist, BIBP3226, is also the most thoroughly studied. In animal models, it blocks the vasoconstrictor and pressor responses to neuropeptide Y. It has a short half-life in vivo.
Structurally related Y1 antagonists include BIB03304 and H 409/22, which has been tested in humans. SR120107A and SR120819A are orally active Y1 antagonists and have a long duration of action. BIIE0246 is the first nonpeptide antagonist selective for the Y2 receptor.
These antagonists have been useful in analyzing the role of neuropeptide Y in cardiovascular regulation. It now appears that the peptide is not important in the regulation of hemodynamics under resting conditions, but may be of increased importance in cardiovascular disorders including hypertension and heart failure. Other studies have implicated neuropeptide Y in feeding disorders, seizures, anxiety, and diabetes, and Y1 and Y5 receptor antagonists have potential as anti-obesity agents (Parker, 2002).
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 17. Vasoactive Peptides >
Urotensin
Urotensin II was originally identified in fish, but isoforms are now known to be present in mammalian species including the mouse, rat, pig, and human. The structure of human urotensin II is shown below.
There are species differences in the structure of urotensin II but the cyclic hexapeptide is conserved in all known isoforms. Major sites of urotensin II expression in humans are the brain, spinal cord, and kidneys. The kidneys may be the major source of circulating urotensin II.
In vitro, urotensin II is a potent constrictor of vascular smooth muscle; its activity depends on the type of blood vessel and the species from which it was obtained. Vasoconstriction occurs primarily in arterial vessels, where urotensin II can be more potent than endothelin 1, making it the most potent known vasoconstrictor. In vivo, urotensin II has complex hemodynamic effects, the most prominent being regional vasoconstriction and cardiac depression. The extent to which the peptide is involved in the regulation of vascular tone and blood pressure in humans is not clear; recent studies have produced conflicting results. The actions of urotensin II are mediated by G proteincoupled receptors that are widely distributed in the brain, spinal cord, heart, vascular smooth muscle, skeletal muscle, and pancreas. Some effects of the peptide including vasoconstriction are mediated by the phospholipase C/IP3/DAG signal transduction pathway.
It has been reported that the expression of urotensin II is upregulated in the heart of humans with end-stage heart failure, but it is not clear if the increase was a cause or consequence of the disease.

Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 17. Vasoactive Peptides >
Preparations Available1
Aprepitant (Emend)
Oral: 80, 125 mg capsules
Bosentan (Tracleer)
Oral: 62.5, 125 mg tablets
1Preparations of angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists are found in Chapter 11: Antihypertensive Agents; preparations of vasopressin are found in Chapter 37: Hypothalamic & Pituitary Hormones.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 17. Vasoactive Peptides >
Chapter 18. The Eicosanoids: Prostaglandins, Thromboxanes,
Leukotrienes, & Related Compounds
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 18. The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds >
The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds:
Introduction
The eicosanoids are oxygenation products of polyunsaturated long chain fatty acids. They are ubiquitous in the animal kingdom and are also found—together with their precursor oils—in a variety of plants. They constitute a very large family of compounds that are not only highly potent but also display an extraordinarily wide spectrum of biologic activity. Because of their biologic activity, the eicosanoids, their specific receptor and enzyme inhibitors, and their plant and fish oil precursors have great therapeutic potential. Their short half-lives—seconds to minutes—make special delivery systems or synthesis of stable analogs mandatory for their clinical use.
Arachidonic Acid & Other Polyunsaturated Precursors
Arachidonic acid is the most abundant and the most important of the precursors of the eicosanoids. Arachidonic acid is a 20-carbon (C20) fatty acid that contains four double bonds beginning at the omega-6 position to yield a 5,8,11,14-eicosatetraenoic acid (designated C20:4–6). For eicosanoid synthesis to occur, arachidonate must first be released or mobilized from membrane phospholipids by one or more lipases of the phospholipase A2 (PLA2) type (Figure 18–1). At least three phospholipases mediate arachidonate release from membrane lipids: cardiac PLA2 (cPLA2), cytosolic PLA2, and secretory PLA2. In addition, arachidonate is also released by a combination of phospholipase C and diglyceride lipase. These lipase pathways are interdicted by corticosteroids. The precise mechanisms are still conjectural.

Figure 18–1.
Pathways of arachidonic acid release and metabolism.
Following mobilization, arachidonic acid is oxygenated by four separate routes: the cyclooxygenase (COX), lipoxygenase, P450 epoxygenase, and isoprostane pathways (Figure 18–1). A number of factors determine the type of eicosanoid synthesized: (1) the species, (2) the type of cell, and (3) the cell's particular phenotype. The pattern of eicosanoids synthesized also frequently reflects (4) the manner in which the cell is stimulated. Finally, an important factor governing the pattern of eicosanoid release is (5) the nature of the precursor polyunsaturated fatty acid that has been esterified in specific membrane phospholipids. For example, homo- -linoleic acid (C20:3–6), which is trienoic, yields products that differ from those derived from arachidonate (C20:4–6), which has four double bonds. Similarly, the products derived from eicosapentaenoic acid (C20:5–3), which has five double bonds, are also quantitatively different. This is the basis for using as nutritional supplements in humans the structurally different fatty acids obtained from cold water fish or from plants. An example of the significance of the polyunsaturated fatty acid precursors is evident when one considers thromboxane A (TXA) derived from the COX pathway. TXA2 is synthesized from arachidonate, a tetraenoic acid and is a powerful vasoconstrictor and aggregator of platelets. However 5,8,11,14,17-eicosapentaenoic acid yields TXA3, which is relatively inactive. In theory, dietary eicosapentaenoate substitution for arachidonate should minimize thrombotic events due to the displacement of tetraenoic arachidonate in the membrane by a pentaenoic acid.
-linoleic acid (C20:3–6), which is trienoic, yields products that differ from those derived from arachidonate (C20:4–6), which has four double bonds. Similarly, the products derived from eicosapentaenoic acid (C20:5–3), which has five double bonds, are also quantitatively different. This is the basis for using as nutritional supplements in humans the structurally different fatty acids obtained from cold water fish or from plants. An example of the significance of the polyunsaturated fatty acid precursors is evident when one considers thromboxane A (TXA) derived from the COX pathway. TXA2 is synthesized from arachidonate, a tetraenoic acid and is a powerful vasoconstrictor and aggregator of platelets. However 5,8,11,14,17-eicosapentaenoic acid yields TXA3, which is relatively inactive. In theory, dietary eicosapentaenoate substitution for arachidonate should minimize thrombotic events due to the displacement of tetraenoic arachidonate in the membrane by a pentaenoic acid.

Synthesis of Eicosanoids
Products of Prostaglandin Endoperoxide Synthases (Cyclooxygenases)
Two unique COX isozymes convert arachidonic acid into prostaglandin endoperoxide. PGH synthase-1 (COX-1) is constitutively expressed, ie, it is always present. In contrast, PGH synthase-2 (COX-2) is inducible, ie, its expression varies markedly depending on the stimulus. The two isozymes also differ in function in that COX-1 is widely distributed and has "housekeeping" functions, eg, gastric cytoprotection. Two-fold to four-fold increases occur following humoral stimulation. In contrast, COX-2 is an immediate early response gene product in inflammatory and immune cells and expression is stimulated ten-fold to eighteen-fold by growth factors, tumor promoters, and cytokines. Lipopolysaccharide (endotoxin) is particularly potent in this respect. However, COX-2 is also involved in normal renal development and vascular prostacyclin production. Several additional COX isoforms have been described recently (Chandrasekharan, 2002).
The synthases are important because it is at this step that the nonsteroidal anti-inflammatory drugs (NSAIDs) exert their therapeutic effects (see Chapter 36: Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout).
Indomethacin and sulindac are slightly selective for COX-1. Meclofenamate and ibuprofen are approximately equipotent on COX-1 and COX-2, while celecoxib and rofecoxib preferentially inhibit COX-2. The steroidal anti-inflammatory drugs such as dexamethasone can inhibit COX-2 gene expression. Aspirin acetylates and inhibits both enzymes to different extents.
Both COXs promote the uptake of two molecules of oxygen by cyclization of arachidonic acid to yield a C9–C11 endoperoxide C15 hydroperoxide (Figure 18–2). This product is PGG2, which is then rapidly modified by the peroxidase moiety of the COX enzyme to add a 15-hydroxyl group that is essential for biologic activity. This product is PGH2. Both endoperoxides are highly unstable. Analogous families—PGH1 and PGH3 and all their subsequent products—are derived from homo- - linolenic acid and eicosapentaenoic acid, respectively.
- linolenic acid and eicosapentaenoic acid, respectively.
Figure 18–2.
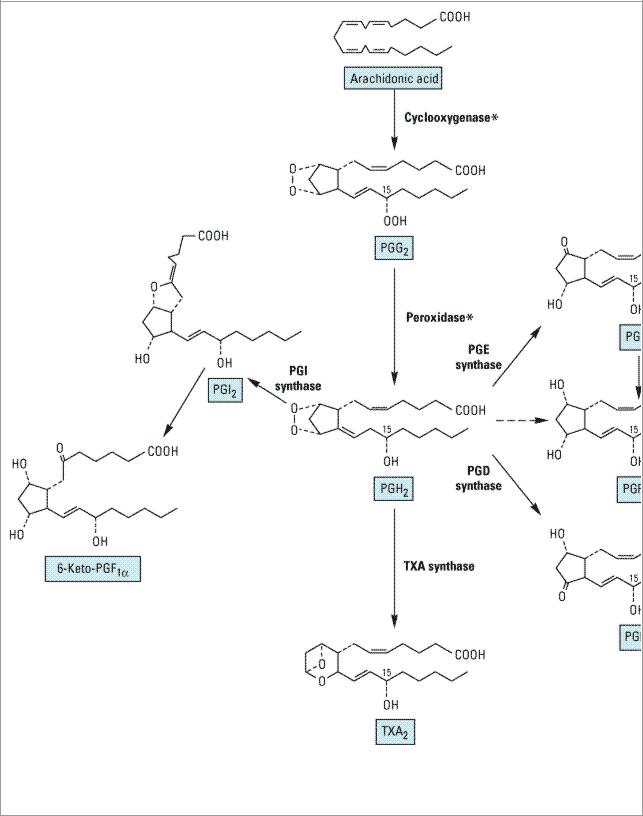
Prostaglandin and thromboxane biosynthesis. Compound names are enclosed in boxes. The asterisks indicate that both cyclooxygenase and peroxidase steps are catalyzed by the single enzyme prostaglandin endoperoxide (PGH) synthase.
PGH2 then yields the prostaglandins, thromboxane, and prostacyclin by separate pathways. The prostaglandins differ from each other in two ways: (1) in the substituents of the pentane ring (indicated by the last letter, eg, E and F in PGE and PGF) and (2) in the number of double bonds in the side chains (indicated by the subscript, eg, PGE1, PGE2). Several products of the arachidonate series are of current clinical importance. Alprostadil (PGE1) is used for its smooth muscle relaxing

ulcer and in combination with mifepristone for terminating early pregnancies. PGE2 and PGF2 are used in obstetrics. Latanoprost and several similar compounds are topically active PGF2 derivatives used in ophthalmology. Prostacyclin (PGI2, epoprostenol) is synthesized mainly by the vascular endothelium and is a powerful vasodilator and inhibitor of platelet aggregation. In contrast, thromboxane (TXA2) has undesirable properties (aggregation of platelets, vasoconstriction). Therefore TXA2 receptor antagonists and synthesis inhibitors are developed for cardiovascular indications.
All the naturally occurring COX products rapidly undergo metabolism by 
 -oxidation, -oxidation, and oxidation of the key 15-hydroxyl group to the corresponding ketone. These inactive metabolites can be determined in blood and urine by immunoassay or mass spectrometry as a measure of the in vivo synthesis of their parent compounds.
-oxidation, -oxidation, and oxidation of the key 15-hydroxyl group to the corresponding ketone. These inactive metabolites can be determined in blood and urine by immunoassay or mass spectrometry as a measure of the in vivo synthesis of their parent compounds.
Products of Lipoxygenase
The metabolism of arachidonic acid by the 5-, 12-, and 15-lipoxygenases results in the production of hydroperoxyeicosatetraenoic acids (HPETEs), which rapidly convert to hydroxy derivatives (HETEs) and leukotrienes (Figure 18–3). The most actively investigated leukotrienes are those produced by the 5-lipoxygenase present in inflammatory cells (PMNs, basophils, mast cells, eosinophils, macrophages). This pathway is of great interest since it is associated with asthma and anaphylactic shock. Stimulation of these cells elevates intracellular Ca2+, and releases arachidonate; incorporation of molecular oxygen by 5-lipoxygenase then yields the unstable epoxide leukotriene A4 (LTA4). This intermediate either converts to the dihydroxy leukotriene B4 (LTB4) or conjugates with glutathione to yield leukotriene C4 (LTC4), which undergoes sequential degradation of the glutathione moiety by peptidases to yield LTD4 and LTE4. These three products are called cysteinyl leukotrienes or peptidoleukotrienes.
Figure 18–3.

Leukotriene biosynthesis. The asterisks indicate that both the lipoxygenase and dehydrase reactions are driven by the single enzyme 5-lipoxygenase. (GGTP,  -glutamyltranspeptidase.)
-glutamyltranspeptidase.)
LTC4 and LTD4 are potent bronchoconstrictors and are recognized as the primary components of the slow-reacting substance of anaphylaxis (SRS-A) that is secreted in asthma and anaphylaxis. There are four current approaches to anti-leukotriene drug development: 5-lipoxygenase enzyme inhibitors, leukotriene receptor antagonists, inhibitors of an important membrane-bound 5- lipoxygenase activating protein (FLAP), and phospholipase A2 inhibitors. Two selective leukotriene receptor antagonists are currently used for treatment of asthma.

Another group of 5-lipoxygenase products are the lipoxins LXA and LXB. Their biologic roles are still to be defined.
Epoxygenase Products
Specific isozymes of microsomal P450 monooxygenases convert arachidonic acid to four epoxyeicosatrienoic acids (EETs) (Figure 18–1). These are the 5,6-, 6,9-, 11,12-, and 14,15-oxido products. Each EET has two stereoisomers. These epoxides are unstable and rapidly form the corresponding dihydroxyeicosatrienoic (DHET) acid, eg, 5,6-DHET. Unlike the prostaglan-dins, both the EETs and the DHETs can be incorporated into phospholipids that then act as storage sites. The epoxygenase products are active on smooth muscle cells and in cell signaling pathways and are thought to have important roles in renal function.
Isoprostanes
The generation of isoprostanes from arachidonic acid is another potentially important pathway. The isoprostanes are prostaglandin stereoisomers. Because prostaglandins have many asymmetric centers, they have a large number of potential stereoisomers. Prostaglandin synthase (COX) is not needed for the formation of the isoprostanes, and aspirin and other nonsteroidal inhibitors of COX should not affect the isoprostane pathway. The primary epimerization mechanism is peroxidation of arachidonate by free radicals. Peroxidation occurs while arachidonic acid is still esterified to the membrane phospholipids. Thus, unlike prostaglandins, these stereoisomers are "stored" as part of the membrane. The importance of the isoprostane pathway lies in the relatively large amounts of these products (ten-fold greater in blood and urine than the COX-derived prostaglandins) and their potent vasoconstrictor effects in the renal and other vascular beds. It has been proposed that isoprostanes play an important role in the hepatorenal syndrome.
Katzung PHARMACOLOGY, 9e > Section IV. Drugs with Important Actions on Smooth Muscle > Chapter 18. The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds >
Basic Pharmacology of Eicosanoids
Mechanisms & Effects of Eicosanoids
Receptor Mechanisms
The eicosanoids act in an autocrine and paracrine fashion. These ligands bind to receptors on the cell surface, and pharmacologic specificity is determined by receptor density and type on different cells. A number of the membrane receptors and their subtypes have been cloned. All of these receptors appear to be G protein-linked; properties of the best-studied receptors are listed in Table 18–1.
Table 18–1. Some Properties of Prostanoid Membrane Receptors.
Receptor |
Endogenous |
G protein, Second |
Smooth |
Result of Receptor Knockout |
Type |
Agonist |
Messenger |
Muscle |
|
|
|
|
Tone |
|
|
|
|
|
|
DP |
PGD2 |
Gs, inc cAMP |
+/– |
Dec allergic bronchial responses |
|
|
|
|
|
|
|
|
|
|
