
Книги фарма 2 / Bertram G. Katzung-Basic & Clinical Pharmacology(9th Edition)
.pdf
|
|
|
|
|
|
witho |
||
|
|
|
|
|
|
ut a |
||
|
|
|
|
|
|
histor |
||
|
|
|
|
|
|
y of |
||
|
|
|
|
|
|
measl |
||
|
|
|
|
|
|
es or |
||
|
|
|
|
|
|
live |
||
|
|
|
|
|
|
virus |
||
|
|
|
|
|
|
vaccin |
||
|
|
|
|
|
|
ation |
||
|
|
|
|
|
|
on or |
||
|
|
|
|
|
|
after |
||
|
|
|
|
|
|
their |
||
|
|
|
|
|
|
first |
||
|
|
|
|
|
|
birthd |
||
|
|
|
|
|
|
ay |
||
|
|
|
|
|
2. |
|
|
|
|
|
|
|
|
|
Postex |
||
|
|
|
|
|
|
posure |
||
|
|
|
|
|
|
proph |
||
|
|
|
|
|
|
ylaxis |
||
|
|
|
|
|
|
in |
||
|
|
|
|
|
|
unim |
||
|
|
|
|
|
|
muniz |
||
|
|
|
|
|
|
ed |
||
|
|
|
|
|
|
person |
||
|
|
|
|
|
|
s |
||
Measles- |
Live virus |
Subcutaneous |
See Table I–2 |
None |
|
For all |
|
|
mumps-rubella |
|
|
|
|
|
children |
|
|
(MMR) |
|
|
|
|
|
|
|
|
Menningococc |
Bacterial |
Subcutaneous |
One dose |
Every 3 to 5 |
|
1. |
|
|
al vaccine |
polysaccharid |
|
|
years if there |
|
Milita |
|
|
|
es of |
|
|
is continuing |
|
ry |
|
|
|
serotypes |
|
|
high risk of |
|
recruit |
|
|
|
A/C/Y/W-135 |
|
|
exposure |
|
s |
|
|
|
|
|
|
|
|
2. |
|
|
|
|
|
|
|
|
Travel |
|
|
|
|
|
|
|
|
ers to |
|
|
|
|
|
|
|
|
areas |
|
|
|
|
|
|
|
|
with |
|
|
|
|
|
|
|
|
epide |
|
|
|
|
|
|
|
|
mic |
|
|
|
|
|
|
|
|
menin |
|
|
|
|
|
|
|
|
gococ |
|
|
|
|
|
|
|
|
cal |
|
|
|
|
|
|
|
|
diseas |
|
|
|
|
|
|
|
|
e |
|
|
|
|
|
|
|
|
3. |
|
|
|
|
|
|
|
|
Indivi |
|
|
|
|
|
|
|
|
duals |
|
|

|
|
|
|
|
|
with |
||
|
|
|
|
|
|
asplen |
||
|
|
|
|
|
|
ia, |
||
|
|
|
|
|
|
compl |
||
|
|
|
|
|
|
ement |
||
|
|
|
|
|
|
deficie |
||
|
|
|
|
|
|
ncy, |
||
|
|
|
|
|
|
or |
||
|
|
|
|
|
|
proper |
||
|
|
|
|
|
|
din |
||
|
|
|
|
|
|
deficie |
||
|
|
|
|
|
|
ncy |
||
|
|
|
|
|
4. |
|
|
|
|
|
|
|
|
|
Contr |
||
|
|
|
|
|
|
ol of |
||
|
|
|
|
|
|
outbre |
||
|
|
|
|
|
|
aks in |
||
|
|
|
|
|
|
closed |
||
|
|
|
|
|
|
or |
||
|
|
|
|
|
|
semi- |
||
|
|
|
|
|
|
closed |
||
|
|
|
|
|
|
popula |
||
|
|
|
|
|
|
tions |
||
|
|
|
|
|
5. |
|
|
|
|
|
|
|
|
|
Colleg |
||
|
|
|
|
|
|
e |
||
|
|
|
|
|
|
fresh |
||
|
|
|
|
|
|
men |
||
|
|
|
|
|
|
who |
||
|
|
|
|
|
|
live in |
||
|
|
|
|
|
|
dormit |
||
|
|
|
|
|
|
ories |
||
Mumps |
Live virus |
Subcutaneous |
One dose |
None |
|
Adults born |
|
|
|
|
|
|
|
|
after 1956 |
|
|
|
|
|
|
|
|
without a |
|
|
|
|
|
|
|
|
history of |
|
|
|
|
|
|
|
|
mumps or |
|
|
|
|
|
|
|
|
live virus |
|
|
|
|
|
|
|
|
vaccination |
|
|
|
|
|
|
|
|
on or after |
|
|
|
|
|
|
|
|
their first |
|
|
|
|
|
|
|
|
birthday |
|
|
Pneumococcal |
Bacterial |
Intramuscular |
One dose |
Repeat after |
|
1. |
|
|
vaccine |
polysaccharid |
or |
|
5 years in |
|
Adults |
|
|
|
es of 23 |
subcutaneous |
|
patients at |
|
65 |
|
|
|
serotypes |
|
|
high risk |
|
years |
|
|
|
|
|
|
|
|
of age |
|
|
|
|
|
|
|
|
2. |
|
|
|
|
|
|
|
|
Person |
|
|
|
|
|
|
|
|
s at |
|
|

|
|
|
|
|
|
increa |
||
|
|
|
|
|
|
sed |
||
|
|
|
|
|
|
risk |
||
|
|
|
|
|
|
for |
||
|
|
|
|
|
|
pneum |
||
|
|
|
|
|
|
ococc |
||
|
|
|
|
|
|
al |
||
|
|
|
|
|
|
diseas |
||
|
|
|
|
|
|
e or its |
||
|
|
|
|
|
|
compl |
||
|
|
|
|
|
|
ication |
||
|
|
|
|
|
|
s |
||
Poliovirus |
Inactivated |
Subcutaneous |
See Table I–2 for |
One-time |
|
1. For |
|
|
vaccine, |
viruses of all |
|
childhood schedule. |
booster dose |
|
all |
|
|
inactivated |
three |
|
Adults: Two doses 4 |
for adults at |
|
childr |
|
|
(IPV) |
serotypes |
|
to 8 weeks apart, |
increased |
|
en |
|
|
|
|
|
and a third dose 6 to |
risk of |
|
2. |
|
|
|
|
|
12 months after the |
exposure |
|
Previo |
|
|
|
|
|
second |
|
|
usly |
|
|
|
|
|
|
|
|
unvac |
|
|
|
|
|
|
|
|
cinate |
|
|
|
|
|
|
|
|
d |
|
|
|
|
|
|
|
|
adults |
|
|
|
|
|
|
|
|
at |
|
|
|
|
|
|
|
|
increa |
|
|
|
|
|
|
|
|
sed |
|
|
|
|
|
|
|
|
risk |
|
|
|
|
|
|
|
|
for |
|
|
|
|
|
|
|
|
occup |
|
|
|
|
|
|
|
|
ational |
|
|
|
|
|
|
|
|
or |
|
|
|
|
|
|
|
|
travel |
|
|
|
|
|
|
|
|
expos |
|
|
|
|
|
|
|
|
ure to |
|
|
|
|
|
|
|
|
poliov |
|
|
|
|
|
|
|
|
iruses |
|
|
Rabies |
Inactivated |
Intramuscular |
Preexposur |
Serologic |
|
1. |
|
|
|
virus |
(IM) or |
e: Three |
testing every |
|
Preex |
|
|
|
|
intradermal |
doses (IM or |
6 months to |
|
posur |
|
|
|
|
(ID) |
ID) at days |
2 years in |
|
e |
|
|
|
|
|
0, 7, and 21 |
persons at |
|
proph |
|
|
|
|
|
or 28 |
high risk |
|
ylaxis |
|
|
|
|
|
Postexposur |
|
|
in |
|
|
|
|
|
e: Five- |
|
|
person |
|
|
|
|
|
doses (IM |
|
|
s at |
|
|
|
|
|
only) at days |
|
|
risk |
|
|
|
|
|
0, 3, 7, 14, |
|
|
for |
|
|
|
|
|
and 28 |
|
|
contac |
|
|
|
|
|
|
|
|
t with |
|
|
|
|
|
|
|
|
rabies |
|
|

|
|
|
|
|
|
|
|
|
|
virus |
||
|
|
|
|
|
|
|
|
|
2. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Poste |
||
|
|
|
|
|
|
|
|
|
|
xposu |
||
|
|
|
|
|
|
|
|
|
|
re |
||
|
|
|
|
|
|
|
|
|
|
proph |
||
|
|
|
|
|
|
|
|
|
|
ylaxis |
||
|
|
|
|
|
|
|
|
|
|
(admi |
||
|
|
|
|
|
|
|
|
|
|
nister |
||
|
|
|
|
|
|
|
|
|
|
with |
||
|
|
|
|
|
|
|
|
|
|
rabies |
||
|
|
|
|
|
|
|
|
|
|
immu |
||
|
|
|
|
|
|
|
|
|
|
ne |
||
|
|
|
|
|
|
|
|
|
|
globul |
||
|
|
|
|
|
|
|
|
|
|
in) |
||
Rubella |
|
Live virus |
|
Subcutaneous |
|
One or two doses (at |
|
None |
|
Adults born |
|
|
|
|
|
|
|
|
least 28 days apart) |
|
|
|
after 1956 |
|
|
|
|
|
|
|
|
|
|
|
|
without a |
|
|
|
|
|
|
|
|
|
|
|
|
history of |
|
|
|
|
|
|
|
|
|
|
|
|
rubella or live |
|
|
|
|
|
|
|
|
|
|
|
|
virus |
|
|
|
|
|
|
|
|
|
|
|
|
vaccination |
|
|
|
|
|
|
|
|
|
|
|
|
on or after |
|
|
|
|
|
|
|
|
|
|
|
|
their first |
|
|
|
|
|
|
|
|
|
|
|
|
birthday |
|
|
Tetanus- |
|
Toxoids |
|
Intramuscular |
|
Two doses 4–8 |
|
Every 10 |
|
1. All |
|
|
diphtheria (Td |
|
|
|
|
|
weeks apart, and a |
|
years |
|
adults |
|
|
or DT)3 |
|
|
|
|
|
third dose 6–12 |
|
|
|
who |
|
|
|
|
|
|
|
|
months after the |
|
|
|
have |
|
|
|
|
|
|
|
|
second |
|
|
|
not |
|
|
|
|
|
|
|
|
|
|
|
|
been |
|
|
|
|
|
|
|
|
|
|
|
|
immu |
|
|
|
|
|
|
|
|
|
|
|
|
nized |
|
|
|
|
|
|
|
|
|
|
|
|
as |
|
|
|
|
|
|
|
|
|
|
|
|
childr |
|
|
|
|
|
|
|
|
|
|
|
|
en |
|
|
|
|
|
|
|
|
|
|
|
|
2. |
|
|
|
|
|
|
|
|
|
|
|
|
Postex |
|
|
|
|
|
|
|
|
|
|
|
|
posure |
|
|
|
|
|
|
|
|
|
|
|
|
proph |
|
|
|
|
|
|
|
|
|
|
|
|
ylaxis |
|
|
|
|
|
|
|
|
|
|
|
|
if > 5 |
|
|
|
|
|
|
|
|
|
|
|
|
years |
|
|
|
|
|
|
|
|
|
|
|
|
has |
|
|
|
|
|
|
|
|
|
|
|
|
passed |
|
|
|
|
|
|
|
|
|
|
|
|
since |
|
|
|
|
|
|
|
|
|
|
|
|
last |
|
|
|
|
|
|
|
|
|
|
|
|
dose |
|
|
Typhoid, |
|
Live bacteria |
|
Oral |
|
Four doses |
|
Four doses |
|
Risk of |
|
|
Ty21a oral |
|
|
|
|
|
administered every |
|
every 5 |
|
exposure to |
|
|

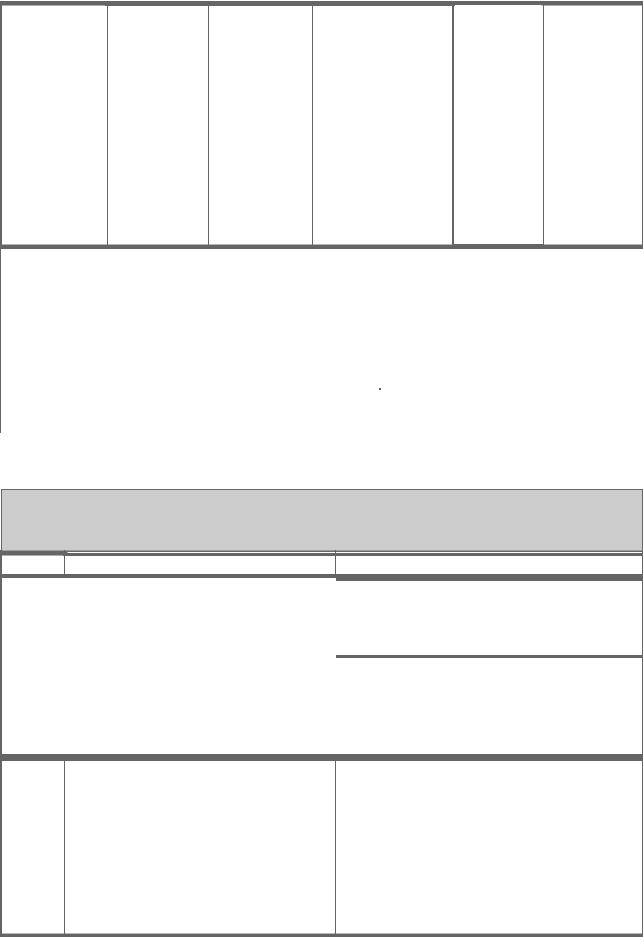
expos ed to yellow fever virus 2. Travel ers to areas where yellow fever occurs
1 Dosages for the specific product, including variations for age, are best obtained from the manufacturer's package insert. Does not include all combination products.
2 One dose unless otherwise indicated.
3 Td = Tetanus and diphtheria toxoids for use in persons  7 years of age (contains less diphtheria toxoid than DPT and DT). DT = Tetanus and diphtheria toxoids for use in persons < 7 years of age (contains the same amount of diphtheria toxoid as DPT).
7 years of age (contains less diphtheria toxoid than DPT and DT). DT = Tetanus and diphtheria toxoids for use in persons < 7 years of age (contains the same amount of diphtheria toxoid as DPT).
Current recommendations for routine active immunization of children are given in Table I–2.
Table I–2. Recommended Routine Childhood Immunization Schedule.1
Age |
Immunization |
Comments |
|
|
|
|
|
Infants born to seronegative mothers: |
|
Birth to |
|
Hepatitis B vaccine (HBV) |
|
|
|
|
2 |
|
|
|
|
Administration should begin at birth, with the |
|
months |
|
|
|
|
second dose administered at least 4 weeks |
|
|
|
|
|
|
after the first dose. |
|
|
|
|
|
|
|
|
Infants born to seropositive mothers:
Should receive the first dose within 12 hours after birth (with hepatitis B immune globulin), the second dose at 1–2 months of age, and the third dose at 6 months of age.
|
2 |
Diphtheria and tetanus toxoids and |
|
|
|
months |
acellular pertussis vaccine (DTaP), |
|
|
|
|
inactivated poliovirus vaccine (IPV), |
|
|
|
|
Haemophilus influenzae type b |
|
|
|
|
conjugate vaccine (Hib)2 pneumococcal |
|
|
|
|
conjugate vaccine (PCV) |
|
|
|
|
|
|
|
|
|
|
|
|
|
1–4 |
HBV |
The second dose should be given at least 4 |
|
|
months |
|
weeks after the first dose. |
|
|
|
|
|
|

4 |
DTaP, Hib2, IPV, PCV |
|
months |
|
|
|
|
|
6 |
DTaP, Hib2, PCV |
|
months |
|
|
|
|
|
6–18 |
HBV, IPV |
The third dose of HBV should be given at |
months |
|
least 16 weeks after the first dose and at least |
|
|
8 weeks after the second dose, but not before |
|
|
age 6 months. |
|
|
|
6–23 |
Influenza, split virus vaccine |
Two doses 1 month apart are recommended |
months |
|
for children 9 years who are receiving |
|
|
influenza vaccine for the first time. |
|
|
|
12–15 |
Measles-mumps-rubella vaccine |
|
months |
(MMR), Hib2, PCV |
|
|
|
|
|
|
|
12–18 |
DTaP at 15–18 months, varicella |
DTaP may be given as early as age 12 months. |
months |
vaccine |
Varicella vaccine is recommended at any visit |
|
|
after the first birthday for susceptible children. |
|
|
Susceptible children 13 years of age should |
|
|
receive two doses given at least 4 weeks apart. |
|
|
|
4–6 |
DTaP IPV, MMR |
The second dose of MMR should be routinely |
years |
|
administered at 4–6 years of age but may be |
|
|
given during any visit if at least 4 weeks have |
|
|
elapsed since administration of the first dose. |
|
|
The second dose should be given no later than |
|
|
age 11–12 years. |
|
|
|
11–12 |
Diphtheria and tetanus toxoids (Td) |
Vaccination is recommended if at least 5 years |
years |
|
has elapsed since administration of the last |
|
|
dose of DTaP. Routine booster doses of Td |
|
|
should be given every 10 years thereafter. |
|
|
|
1Adapted from MMWR Morb Mortal Wkly Rep 2002;31:31.
2Three Hib conjugate vaccines are available for use: (a) oligosaccharide conjugate Hib vaccine (HbOC), (b) polyribosylribitol phosphate-tetanus toxoid conjugate (PRP-T), and (c) Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) (PRP-OMP). Children immunized with PRP-OMP at 2 and 4 months of age do not require a dose at 6 months of age.
Katzung PHARMACOLOGY, 9e > Section X. Special Topics > Appendix I: Vaccines, Immune Globulins, & Other Complex Biologic Products >
Passive Immunization
Passive immunization consists of transfer of immunity to a host using preformed immunologic products. From a practical standpoint, only immunoglobulins have been utilized for passive immunization, since passive administration of cellular components of the immune system has been technically difficult and associated with graft-versus-host reactions. Products of the cellular immune system (eg, interferons) have also been used in the therapy of a wide variety of hematologic and

infectious diseases (Chapter 56: Immunopharmacology).
Passive immunization with antibodies may be accomplished with either animal or human immunoglobulins in varying degrees of purity. These may contain relatively high titers of antibodies directed against a specific antigen or, as is true for pooled immune globulin, may simply contain antibodies found in most of the population. Passive immunization is useful for (1) individuals unable to form antibodies (eg, congenital agammaglobulinemia); (2) prevention of disease when time does not permit active immunization (eg, postexposure); (3) for treatment of certain diseases normally prevented by immunization (eg, tetanus); and (4) for treatment of conditions for which active immunization is unavailable or impractical (eg, snakebite).
Complications from administration of human immunoglobulins are rare. The injections may be moderately painful and rarely a sterile abscess may occur at the injection site. Transient hypotension and pruritus occasionally occur with the administration of IGIV products, but generally are mild. Individuals with certain immunoglobulin deficiency states (IgA deficiency, etc) may occasionally develop hypersensitivity reactions to immune globulin that may limit therapy. Conventional immune globulin contains aggregates of IgG; it will cause severe reactions if given intravenously. However, if the passively administered antibodies are derived from animal sera, hypersensitivity reactions ranging from anaphylaxis to serum sickness may occur. Highly purified immunoglobulins, especially from rodents or lagomorphs are the least likely to cause reactions. To avoid anaphylactic reactions, tests for hypersensitivity to the animal serum must be performed. If an alternative preparation is not available and administration of the specific antibody is deemed essential, desensitization can be carried out.
Antibodies derived from human serum not only avoid the risk of hypersensitivity reactions but also have a much longer half-life in humans (about 23 days for IgG antibodies) than those from animal sources (5–7 days or less). Consequently, much smaller doses of human antibody can be administered to provide therapeutic concentrations for several weeks. These advantages point to the desirability of using human antibodies for passive protection whenever possible. Materials available for passive immunization are summarized in Table I–3.
Table I–3. Materials Available for Passive Immunization.1
Indication |
Product |
Dosage |
Comments |
|
|
|
|
Black widow |
Antivenin |
One vial (6000 units) IV or IM. |
For persons with |
spider bite |
(Latrodectus |
|
hypertensive |
|
mactans), equine |
|
cardiovascular disease or |
|
|
|
age < 16 or > 60 years. |
|
|
|
|
Bone marrow |
Immune globulin |
500 mg/kg IV on days 7 and 2 |
Prophylaxis to decrease |
transplantation |
(intravenous)2 |
prior to transplantation and |
the risk of infection, |
|
|
then once weekly through day |
interstitial pneumonia, and |
|
|
90 after transplantation. |
acute graft-versus-host |
|
|
|
disease in adults |
|
|
|
undergoing bone marrow |
|
|
|
transplantation. |
|
|
|
|
Botulism |
Botulism |
Consult the CDC.3 |
Treatment and prophylaxis |
|
antitoxin |
|
of botulism. Available |
|
(trivalent, types |
|
from the CDC.3 Ten to 20 |
|
|
|
|

|
A, B, and E), |
|
percent incidence of serum |
|
equine |
|
reactions. |
|
|
|
|
|
|
|
|
Chronic |
Immune globulin |
Initial dose of 400 mg/kg IV |
CLL patients with |
lymphocytic |
(intravenous)3 |
every 3–4 weeks. Dosage |
hypogammaglobulinemia |
leukemia (CLL) |
|
should be adjusted upward if |
and a history of at least |
|
|
bacterial infections occur. |
one serious bacterial |
|
|
|
infection. |
|
|
|
|
Cytomegalovirus |
Cytomegalovirus |
Consult the manufacturer's |
Prophylaxis of CMV |
(CMV) |
immune globulin |
dosing recommendations. |
infection in bone marrow, |
|
(intravenous) |
|
kidney, liver, lung, |
|
|
|
pancreas, heart transplant |
|
|
|
recipients. |
|
|
|
|
Diphtheria |
Diphtheria |
20,000–120,000 units IV or IM |
Early treatment of |
|
antitoxin, equine |
depending on the severity and |
respiratory diphtheria. |
|
|
duration of illness. |
Available from the CDC.3 |
|
|
|
Anaphylactic reactions in |
|
|
|
7% of adults and serum |
|
|
|
reactions in 5–10% of |
|
|
|
adults. |
|
|
|
|
|
|
|
|
Hepatitis A |
Immune globulin |
Preexposure |
Preexposure and |
|
(intramuscular) |
prophylaxis: 0.02 |
postexposure hepatitis A |
|
|
mL/kg IM for |
prophylaxis. The |
|
|
anticipated risk of 3 |
availability of hepatitis A |
|
|
months, 0.06 mL/kg for |
vaccine has greatly |
|
|
anticipated risk of > 3 |
reduced the need for |
|
|
months, repeated every |
preexposure prophylaxis. |
|
|
4–6 months for |
|
|
|
continued exposure. |
|
|
|
Postexposure: 0.02 |
|
|
|
mL/kg IM as soon as |
|
|
|
possible after exposure |
|
|
|
up to 2 weeks. |
|
|
|
|
|
Hepatitis B |
Hepatitis B |
0.06 mL/kg IM as soon as |
Postexposure prophylaxis |
|
immune globulin |
possible after exposure up to 1 |
in nonimmune persons |
|
(HBIG) |
week for percutaneous |
following percutaneous, |
|
|
exposure or 2 weeks for sexual |
mucosal, sexual, or |
|
|
exposure. 0.5 mL IM within 12 |
perinatal exposure. |
|
|
hours after birth for perinatal |
Hepatitis B vaccine should |
|
|
exposure. |
also be administered. |
|
|
|
|
HIV-infected |
Immune globulin |
400 mg/kg IV every 28 days. |
HIV-infected children with |
children |
(intravenous)2 |
|
recurrent serious bacterial |
|
|
|
infections or |
|
|
|
hypogammaglobulinemia. |
|
|
|
|
Kawasaki disease |
Immune globulin |
400 mg/kg IV daily for 4 |
Effective in the prevention |
|
(intravenous)2 |
consecutive days within 4 days |
of coronary aneurysms. |
|
|
after the onset of illness. A |
For use in patients who |
|
|
|
|

|
|
|
|
|
|
single dose of 2 g/kg IV over |
|
meet strict criteria for |
|||
|
|
|
|
|
|
10 hours is also effective. |
|
Kawasaki disease. |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Measles |
|
Immune globulin |
|
|
Normal hosts: 0.25 |
|
|
Postexposure prophylaxis |
|
|
|
|
|
(intramuscular) |
|
|
mL/kg IM. |
|
|
(within 6 days after |
|
|
|
|
|
|
|
|
Immunocompromised |
|
|
exposure) in nonimmune |
|
|
|
|
|
|
|
|
hosts: 0.5 mL/kg IM |
|
|
contacts of acute cases. |
|
|
|
|
|
|
|
|
(maximum 15 mL for |
|
|
|
|
|
|
|
|
|
|
|
all patients). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Idiopathic |
|
Immune globulin |
|
|
Consult the manufacturer's |
|
|
Response in children with |
|
|
|
thrombocytopenic |
|
(intravenous)2 |
|
|
dosing recommendations for |
|
|
ITP is greater than in |
|
|
|
purpura (ITP) |
|
|
|
|
the specific product being used. |
|
|
adults. Corticosteroids are |
|
|
|
|
|
|
|
|
|
|
|
the treatment of choice in |
|
|
|
|
|
|
|
|
|
|
|
adults, except for severe |
|
|
|
|
|
|
|
|
|
|
|
pregnancy-associated ITP. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Primary |
|
Immune globulin |
|
|
Consult the manufacturer's |
|
|
Primary |
|
|
|
immunodeficiency |
|
(intravenous)2 |
|
|
dosing recommendations for |
|
|
immunodeficiency |
|
|
|
disorders |
|
|
|
|
the specific product being used. |
|
|
disorders include specific |
|
|
|
|
|
|
|
|
|
|
|
antibody deficiencies (eg, |
|
|
|
|
|
|
|
|
|
|
|
X-linked |
|
|
|
|
|
|
|
|
|
|
|
agammaglobulinemia) and |
|
|
|
|
|
|
|
|
|
|
|
combined deficiencies (eg, |
|
|
|
|
|
|
|
|
|
|
|
severe combined |
|
|
|
|
|
|
|
|
|
|
|
immunodeficiencies). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rabies |
|
Rabies immune |
|
|
20 IU/kg.The full dose should |
|
|
Postexposure rabies |
|
|
|
|
|
globulin |
|
|
be infiltrated around the wound |
|
|
prophylaxis in persons not |
|
|
|
|
|
|
|
|
and any remaining volume |
|
|
previously immunized |
|
|
|
|
|
|
|
|
should be given IM at an |
|
|
with rabies vaccine. Must |
|
|
|
|
|
|
|
|
anatomic site distant from |
|
|
be combined with rabies |
|
|
|
|
|
|
|
|
vaccine administration. |
|
|
vaccine. |
|
|
|
Respiratory |
|
Palivizumab |
|
|
15 mg/kg IM once prior to the |
|
|
For use in infants and |
|
|
|
syncytial virus |
|
|
|
|
beginning of the RSV season |
|
|
children younger than 24 |
|
|
|
(RSV) |
|
|
|
|
and once monthly until the end |
|
|
months with chronic lung |
|
|
|
|
|
|
|
|
of the season. |
|
|
disease or a history of |
|
|
|
|
|
|
|
|
|
|
|
premature birth ( 35 |
|
|
|
|
|
|
|
|
|
|
|
weeks' gestation). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSV immune |
|
|
750 mg/kg IV once prior to the |
|
|
As for palivizumab. |
|
|
|
|
|
globulin |
|
|
beginning of the RSV season |
|
|
Palivizumab is preferred |
|
|
|
|
|
|
|
|
and once monthly until the end |
|
|
for selected high-risk |
|
|
|
|
|
|
|
|
of the season. |
|
|
children, but RSV-IGIV |
|
|
|
|
|
|
|
|
|
|
|
may be preferred for |
|
|
|
|
|
|
|
|
|
|
|
selected high-risk children. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rubella |
|
Immune globulin |
|
|
0.55 mL/kg IM. |
|
|
Nonimmune pregnant |
|
|
|
|
|
(intramuscular) |
|
|
|
|
|
women exposed to rubella |
|
|
|
|
|
|
|
|
|
|
|
who will not consider |
|
|
|
|
|
|
|
|
|
|
|
therapeutic abortion. |
|
|
|
|
|
|
|
|
|
|
|
Administration does not |
|
|
|
|
|
|
|
|
|
|
|
prevent rubella in the fetus |
|
|
|
|
|
|
|
|
|
|
|
of an exposed mother. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
