
Reactive Intermediate Chemistry
.pdf
506 NITRENES
Imidogen can be generated in the gas phase by photolysis or by pyrolysis of hydrazoic acid (HN3) although other precursors find occasional use.17 Exposure of HN3 to either 248 or 266-nm light generates 1NH almost exclusively in its lowest singlet state with a quantum yield near unity.
Singlet NH inserts into the CH bonds of hydrocarbons, much like singlet methylene (see Chapter 7 in this volume). Triplet NH abstracts hydrogen atoms from hydrocarbons to form aminyl (NH2) radicals and alkyl radicals in the same manner as triplet methylene, in spite of the fact that the reactions of 3CH2 are exothermic, whereas some reactions of 3NH are endothermic, depending on the alkane!19 Absolute rate constants for many of these processes have been measured in the gas
phase. However, the gas-phase chemistry of methylene is much more developed than that of imidogen.17–19
Hydrazoic acid is a reagent in the well-known Schmidt reactions in solution, but imidogen is probably not involved in these transformations.20 Systematic mechanistic studies of the photochemistry of HN3 in solution and the chemistry of NH with hydrocarbons, particularly cisand transalkenes as per the detailed studies of CH2,19 have not been performed.
1.1.1. Gas-Phase Spectroscopy. The 336-nm absorption band of triplet imidogen was first observed by Eder21 in 1892 and has been the subject of numerous subsequent studies. The singlet state of NH absorbs at 324 nm. The CASSCF (6,5)/ CASPT2 level of theory predicts transitions at 323 and 293 nm for singlet and triplet imidogen, respectively.22 In each spin state, an electron is promoted from the ‘‘sp’’ hybrid lone pair to a singly occupied 2p orbital as shown below for 1NH.
H |
336 nm |
H |
H |
The singlet and triplet absorptions of imidogen are very similar because the same orbitals are involved in excitation of each spin state. As the transitions are localized on the nitrogen atom, these bands are characteristic of nitrenes in general and will appear in the same spectral region in alkyland arylnitrenes. These bands are diagnostic of triplet alkyland arylnitrenes. Because this wavelength is in a convenient range for laser flash photolysis (LEP) studies, certain alkylnitrenes and many arylnitrenes have now been detected in solution.
Singlet and triplet imidogen have not yet been detected in solution by LFP methods. Future effort should be expended in this direction and it seems safe to predict that such work will be reported in the near future.
Although singlet and triplet imidogen have similar absorption spectra, singlet and triplet methylene do not.15 In fact, most carbenes have rather poor chromophores for UV–vis detection by LFP and must be ‘‘visualized’’ by trapping with pyridine to form ylides.23 The exceptions are arylcarbenes, which have p ! p* transitions localized on the aromatic p system.24


508 NITRENES
The key question, then, is whether singlet methylnitrene is a minimum on the potential energy surface and is a true reactive intermediate with a finite lifetime. Most early calculations found singlet methylnitrene to be protected from rearrangement by sizeable barriers.29 The most recent work, using the CAS(10,8)-MP2 method, predicts that the barrier to rearrangement of singlet methylnitrene is only 1.4 kcal/mol, up from 0.5 kcal/mol at the (12/11) CASSCF/cc-pVTZ level. (CASPT2 predicts a barrier of 2.8–3.8 kcal/mol depending on the basis set.27) So it seems clear that, if singlet methylnitrene does exist as a true reactive intermediate, it will not live long enough to undergo bimolecular reactions and will not be detectable in solution by nanosecond spectroscopy. In fact, singlet methylnitrene has so far eluded detection by femtosecond spectroscopy.30
These conclusions were anticipated by product studies. Alkyl azides are readily available and their thermal and photochemical decomposition reactions have been studied.31,32 In general, light and heat induced decomposition of methyl azide does
not produce a MeN species that can be intercepted in respectable yields with a bimolecular trap.31,32 For example, attempts to trap MeN with cyclohexane as sol-
vent produced only a 0.4% yield of adduct.33 Photolysis of CH3N3 or CD3N3 at cryogenic temperatures fails to produce an IR spectrum attributable to triplet methylnitrene. The IR spectrum of CH2 NH (or CD2 ND) is observed instead.34
The intramolecular chemistry of alkylnitrenes resembles that of alkylcarbenes in general.18,19,35
The photochemistry of tert-butylazide in a nitrogen matrix at 12 K was followed by IR spectroscopy. Only one product, trimethylimine, was observed.36 Thus, the removal of alpha hydrogen from the alkyl azide does not suppress 1,2-migration reactions.
Me |
|
|
Me |
|
Me |
C |
N3 |
h ν |
|
|
|
|
C |
N |
+ N 2 |
||
|
|||||
Me |
|
12 K |
|
|
|
|
|
|
|
||
Me |
|
|
Me |
|
|
In fact, rearrangements of singlet alkylnitrenes generated in frozen matrices cannot even be suppressed by developing strain in imine products such as 1–3, although some triplet nitrene is detected by EPR and UV–vis spectroscopy upon low-temperature photolysis of 1-azidonorbornane.37
h ν |
|
N |
|
+ |
+ |
+ |
|
Ar, 12 K |
N |
|
|
N |
|
||
N3 |
|
3N |
|
1 |
2 |
||
3 |
Photolysis of methyl azide immobilized in a rigid cryogenic matrix does not produce methylnitrene in its triplet ground state, as revealed by EPR spectroscopy.38,39 This observation is in good agreement with Milligan’s study of this system using matrix IR spectroscopy.34 However, triplet sensitized photolysis of methyl azide in

ALKYLNITRENES 509
a frozen matrix does produce the persistent EPR spectrum of triplet methylnitrene.38,39 This experiment again demonstrates the low barrier to isomerization of singlet methylnitrene, which is bypassed upon triplet sensitization.
The solution-phase photochemistry of nine alkylazides was studied by Kyba and Abramovitch.40 Photolysis of tertiary alkyl azides leads cleanly to 1,2 alkyl migration.
R |
|
|
R |
|
R3 |
3 |
|
h ν |
2 |
|
|
C |
N3 |
|
|
|
|
|
C |
N |
+ N 2 |
||
|
|||||
R2 |
|
|
|||
|
|
|
|
|
|
R1 |
|
|
R1 |
|
|
Photolysis of alkyl azides bearing pendant aryl groups does not lead to intramolecular trapping of a nitrene by the aryl group. However, intramolecular capture of the putative nitrene is observed upon pyrolysis of the azide precursor. These observations convinced Kyba and Abramovitch40 that 1,2-migration is concerted with loss of nitrogen from the excited state of the azide, but that a free singlet nitrene is formed upon thermal decomposition. The chemistry of acyl azides will be shown later to exhibit the opposite pattern.
|
|
|
|
R |
|
|
|
|
R |
|
R |
N |
|
|
|
|
|
|
|
|
|
|
|
|
N |
C |
R |
|
|
|
|
|
|
|
N3 |
|
|
|
+ |
|
|
R |
h ν |
|
|
|
|
|
R |
|
|
|
|
|
|
4 |
|
|
R |
H |
|
|
|
|
R |
|
|
||
|
|
|
|
|
|
|
|
∆ |
|
|
N |
|
|
+
h ν
Triplet sensitized LFP studies of simple alkyl azides and systematic product analyses in solution under these conditions have not been reported, although the triplet energies of alkyl azides are 75–80 kcal/mol41 and the quantum yields for azide disappearance with appropriate triplet sensitizers approach unity.
Recently, however, examples of intramolecular triplet sensitization have been described. Alkyl aryl ketones with pendant azido groups in the alkyl moiety were irradiated into the benzoyl chromophore. The excited alkylphenyl ketones undergo intersystem crossing to their triplet states within picoseconds and they can then transfer their triplet energy to the nearby alkyl azido group. This process


CARBONYLNITRENES AND RELATED ACYLNITRENES |
511 |
It is presumed that the barrier-to-1,2-fluorine migration in the singlet nitrene substantially exceeds the analogous barrier-to-hydrogen shift. This difference allows a portion of the nascent singlet nitrene to relax to the lower energy triplet in the matrix.
Triplet methylnitrene has been produced in the gas phase in a corona discharge. This process has allowed its highly resolved absorption spectrum to be recorded and analyzed. 44
Polyfluorination does seem to suppress rearrangements of alkyl azides and to extend the lifetimes of the corresponding singlet nitrenes. Photolysis of 5 in cyclohexane produces insertion adduct 6.45
|
|
|
h ν |
|
|
H |
H2O |
O |
|
|
|
|
|
|
|
||||
CF CHFCF N |
|
CF CHF |
CF |
N |
|
CF3CHF |
N |
||
|
|
||||||||
|
|||||||||
3 |
2 |
3 |
|
3 |
|
2 |
|
|
|
|
5 |
|
|
|
|
|
6 |
H |
|
3. CARBONYLNITRENES AND RELATED ACYLNITRENES
Treatment of amides with bromine in alkaline medium promotes the Hofmann rearrangement, which may or may not involve a free nitrene intermediate.46 The oxidation of primary amides with lead tetraacetate and the resulting Lossen rearran-
gement also produces isocyanates, with the possible intervention of an acylnitrene.3,47
|
O |
|
|
O |
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NaOH |
|
−NaBr |
|
|
|
|
N C O |
||
|
|
|
|
|
|
|
|
|
|
|
|
R |
NH2 |
Br2 |
|
|
|
|
|
|
|
|
|
|
R |
NNaBr |
|
|
R |
N |
|
|
R |
||
|
|
|
|
|
|
|
|
|
|||
Pivaloylazide (7) has received considerable study by Lwowski and Tisue.48 Pyrolysis of this compound in neat cyclohexane, cyclohexene, or 2-methylbutane leads to tert-butylisocyanate in nearly quantitative yields.
O
|
|
∆ |
|
|
|
t-Bu |
N3 |
|
N |
C |
O |
|
|||||
|
t-Bu |
|
|
||
|
7 |
|
|
|
|
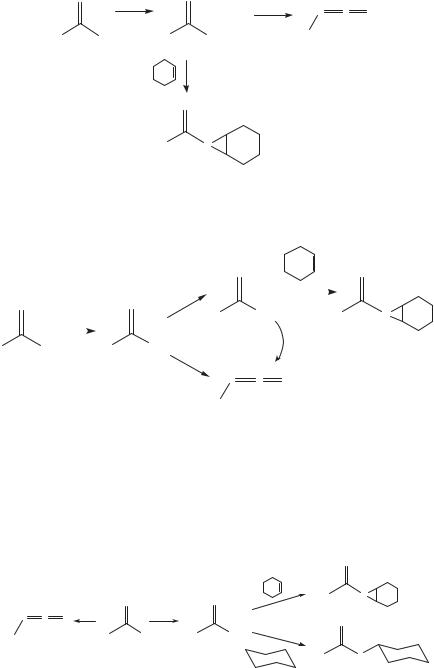
From this result, one can argue that the Curtius rearrangement is a concerted process.
|
O |
|
|
|
N |
|
N |
N C O + N2 |
R |
N |
|
|
|
R |
512 NITRENES
Alternatively, one could conclude the reaction is stepwise, but that the rearrangement of the acylnitrene is much faster than bimolecular chemistry.
|
O |
|
O |
fast |
|
|
|
|
+ N2 |
N C O |
|
|
|
|
|
||
R |
N3 |
R |
N |
|
R |
slow
O
RN
However, Lwowski and co-workers49 demonstrated that photolysis of 7 in cyclohexane, cyclohexeneor 2-methylbutane does lead to the formation of adducts. Therefore, the acylnitrene 8 is a trappable intermediate. The thermal Curtius rearrangement does not involve free nitrenes and it must be a concerted process.
|
|
|
|
|
|
|
O |
|
|
O |
|
O |
|
|
O |
-N2 |
R |
1N |
|
R |
N |
|
|
|
|
|||||||
|
|
|
|
|
||||||
|
|
hν |
|
|
|
|||||
|
|
|
|
|
8 |
× |
|
10 |
||
|
|
|
|
1N |
* |
|
|
|||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
||||
R |
N |
|
R |
|
|
|
|
|||
|
3 |
|
|
3 |
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-N2 |
N |
C |
O |
|
|
R 9
Two other observations are noteworthy. First, the yield of isocyanate (9) produced on photolysis of 7 in methylene chloride (an inert solvent) is 40%. Photolysis of 7 in cyclohexene leads to a 45% yield of aziridine adduct 10 and a 41% yield of isocyanate 9. Trapping the nitrene does not depress the yield of isocyanate! Hence, isocyanate 9 and adduct 10 cannot be derived from the same reactive intermediate. Instead, the isocyanate must be formed from the excited state of the azide, that is, the excited azide (7*) must partition between the formation of isocyanate and nitrene.
|
|
|
|
|
|
O |
|
|
|
|
|
t-Bu |
N |
|
|
O |
|
|
O |
10 |
|
N C O |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
t-Bu |
|
t-Bu |
N * |
t-Bu |
N |
|
|
7* |
3 |
|
8 |
|
|
|
9 |
|
|
|
||
|
|
|
|
t-Bu |
N |
|
|
|
|
|
|
||
|
|
|
|
|
|
H |

CARBONYLNITRENES AND RELATED ACYLNITRENES |
513 |
Similar conclusions concerning the photochemistry of diazo carbonyl compounds and diazirines have been reached using similar experiments and logic.50
The second interesting point to note is that photolysis of 2-naphthoylazide (11, where 2-Np ¼ 2-naphthoyl) in the presence of cis-4-methyl-2-pentene produces azirine 12 stereospecifically.51
|
|
Me |
i-Pr |
|
Me |
|
|
O |
O |
|
O |
|
|
|
|
|
i-Pr |
|||
|
hν |
H |
H |
|
|
|
|
|
|
|
H |
||
2-Np |
N3 -N2 2-Np |
1N |
2-Np |
N |
|
|
|
H |
|||||
|
11 |
× |
|
12 |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
2-Np = |
|
Me |
i-Pr |
|
|
Me |
|
|
|
|
|||
|
|
O |
|
|
O |
|
|
|
|
|
H |
||
|
|
|
|
|
|
|
|
|
H |
H 12 |
+ |
|
H |
|
2-Np |
3N |
|
2-Np |
|
|
|
|
|
N |
i-Pr
This experiment indicates that only the singlet acylnitrene is trapped because it is either the ground state of the nitrene or it relaxes very slowly to a triplet state. However, if the alkene is diluted to a concentration of only 0.01 M, the aziridination reaction remains stereospecific. Extending the nitrene lifetime by diluting the concentration of the trapping agent does not lead to relaxation of a putative excited singlet nitrene to its putative lower energy triplet state.51
O
O
Me |
N3 |
EtO N3
O O
13 |
14 |
|
The observation of a triplet ESR spectrum produced by photolysis of RC(O) N3 in a frozen matrix would prove that RC(O) N has a triplet ground state. Although photolysis of azide esters 1339 and 1452 in matrices does produce triplet nitrene EPR signals, photolysis of RC(O)N3 under the same conditions, does not.51 Thus, one is left again to wonder whether the singlet is the ground state of the nitrene or simply does not find a competitive pathway for intersystem crossing to a lower energy triplet state. These issues were clarified by triplet sensitization experiments by Autrey and Schuster.51

514 |
NITRENES |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
h ν |
|
|
|
|
|
|
|
|
|
|
|
3 TX |
|
TX |
|
|
|
|
Me |
i-Pr |
|
|
||
|
|
|
|
|
|
|
|
|
|
Me |
|||
|
O |
|
|
O |
|
|
O |
|
|
O |
|||
|
|
|
! |
|
|
|
i-Pr |
||||||
|
|
|
|
|
|
|
|
H |
H |
|
|||
|
|
|
|
|
|
|
|
|
|
H |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2-Np |
N3 |
-N2 |
2-Np |
3 N |
|
2-Np |
1 N |
|
|
|
|||
|
|
2-Np |
|
N |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
O |
|
|
|
|
|
|
|
|
|
12 |
i-Pr
TX =
S
A mixture of 2-naphthoylazide and sensitizer 2-isopropylthioxanthone (ET ¼ 65 kcal/mol) was irradiated (l > 385 nm), conditions under which the sensitizer absorbed >95% of the light. Laser flash photolysis experiments demonstrated that the triplet sensitizer is quenched by 2-naphthoylazide at a diffusion controlled rate.
Direct photolysis of 2-naphthoylazide (11) in cyclohexane produces the corresponding isocyanate (15) in 50% yield along with C H insertion adduct 16.
|
O |
h ν |
|
|
O |
|
|
|
|
|
|
|
|
|
N C |
O + |
|
2-Np |
N3 |
2-Np |
15 |
2-Np |
N |
|
11 |
|
|
H |
|
|
|
|
|
|
16
In the presence of 0.2 M cis-4-methyl-2-pentene, the yield of isocyanate 15 is unchanged and a 46% yield of aziridine 12 is observed. Upon sensitized photolysis, the yield of isocyanate drops to 5%, demonstrating that triplet sensitization has been achieved and that the isocyanate is a product of reaction of the singlet excited state of the azide precursor. However, triplet sensitized photolysis of 11 in the presence of cis-4-methyl-2-pentene gives aziridine 12 with complete retention of stereospecificity! When the triplet acyl nitrene is generated intentionally the products of singlet nitrene reactions are obtained! Thus, singlet 2-naphthoylnitrene must be lower in energy than the corresponding triplet nitrene and it must rearrange rather slowly to isocyanate!52 Direct photolysis of 11 in the presence of an alkene (e.g., cyclohexene) can be summarized as shown.
|
|
|
|
|
|
O |
|
|
O |
|
O |
|
|
-N2 |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
O |
1N |
|
R |
N |
||
|
|
h |
ν |
R |
|
||||
|
|
|
|
R |
1N * |
|
× |
|
|
R |
N |
|
|
|
|||||
|
3 |
|
|
|
3 |
|
|
|
|
|
|
|
|
|
N |
C |
O |
|
|
|
|
|
|
|
-N2 |
|
|
|
|
|
|
|
|
|
R |
|
|
|
|

CARBONYLNITRENES AND RELATED ACYLNITRENES |
515 |
In subsequent work, Sigman et al.,52 studied aroyl azides 14 and 17–18.
O |
N3 |
O |
N |
|
|
3 |
|
Me |
O |
Me |
O |
|
17 |
|
18 |
Each of these azides contains an internal sensitizing group. Upon LFP the triplet states of all three azides are observed. However, in the case of 17 and 18, only singlet nitrene derived products are formed. Photolysis of 17 and 18 at 8 K in fluorolube fails to produce persistent triplet EPR spectra. It seems clear that these aroyl nitrenes also have singlet ground states. As mentioned previously, photolysis of 14 does produce a triplet nitrene EPR spectrum.
Recent high-level calculations support the conclusion that benzoyl nitrene has a singlet ground state.53,54 According to CCSD(T)/cc-pVTZ calculations on HCNO the distance between the oxygen and nitrogen atoms and the C N bond length are unusually small, whereas the C O bond length is much longer than in a carbonyl group in the singlet state (in contrast to the triplet state where all these values are normal). The minimum energy geometry of 1HCON is intermediate between that of an acylnitrene and an oxazirine. Presumably, it is this bonding interaction that lowers the energy of the closed-shell singlet nitrene–oxazirine and this hybrid species becomes more stable than the corresponding triplet nitrene. Singlet benzoylnitrene– oxazirine has recently been observed as a persistent species in argon by IR spectroscopy. Its vibrational spectrum is in good agreement with theory.54
1.222 Å |
O |
1.307 Å |
O |
O |
|
O |
|
|
|||||
|
|
|
|
|
|
|
H C |
|
H C |
1.816 Å H |
C |
|
H C |
|
|
|||||
1.378 Å |
N |
1.260 Å |
N |
N |
|
N |
|
|
|
|
|
|
|
3HCON |
|
1 HCON |
|
|
|
|
3.1. Nitrene Esters
Carboethoxynitrene (19) can be generated in solution by base-catalyzed a-elimina- tion of 20 or upon photolysis of azide 13. The nitrene undergoes addition to double bonds and formal CH insertion reactions.55
The reaction of carboethoxynitrene with cisand trans-4-methyl-2-pentene was studied as a function of alkene concentration. At large alkene concentrations, aziridination is stereospecific. Upon dilution of the alkene, the stereospecificity is lost.56 These results are completely analogous to studies of carbenes in which a

 F
F