
Reactive Intermediate Chemistry
.pdf
476 ATOMIC CARBON
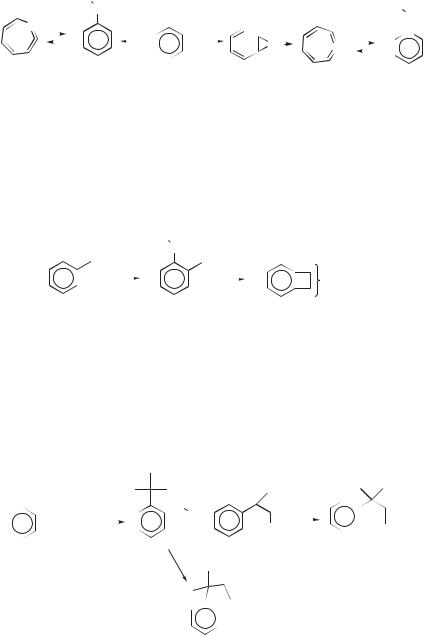
ethylene, vinylacetylene (29), and benzene as the only isolable products.64 Attempts to trap 28 were unsuccessful. The ethylene and 29 were postulated to arise from the known retro Diels–Alder reaction of an intermediate 28.65 However, the use of 13C atoms revealed that the label in 29 was distributed on both alkyne carbons and the vinyl CH. Hence, an additional pathway to 28 involving an initial C H insertion to give vinylcarbene (30), which then ring expands, was postulated. Since the label is unequally distributed between C1 and C3 in 29, it seem likely that a direct cleavage of 30 to ethylene and 29 competes with ring expansion (Eq. 22). In this case, the ratio of DBA to C H insertion was 0.87:1.64
|
|
|
|
|
|
|
|
|
|
C: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
+ *C |
|
|
+ |
* |
C: |
|
|
|
|
29 |
|
|
|
C |
|
H |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
30 H |
|
|
|
+ |
|
|
|
|
|
|||||||||||
*C = 13C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
* |
|
|
|
|
|
|
|
|
|
* |
|
|
|
|
|
|
|
|
ð22Þ |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
C |
|
|
|
|
|
|
|
|
C* |
C |
|
|
* |
|
|
|
|
* |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
29 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
C |
|
H |
||||||||
|
+ |
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
29 |
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
28 |
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
28 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
The competing ring expansion and cleavage in carbene 30 was confirmed by generating the deuterium labeled carbene 30a by the C atom deoxygenation of aldehyde 31 (Eq. 23).64 While the origin of the benzene in these reactions has not been established, several pathways to energetic cyclohexadiene followed by loss of hydrogen may be envisioned.
O |
C |
|
|
|
|
|
|
H |
|
|
C |
|
|
D |
|||
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
||||||||||
C |
|
C: |
|
|
|
|
+ |
|
|
|
+ |
||||||
|
CO + |
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
||||||||||
D |
|
|
|
D |
|
|
D |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
30a |
|
|
|
|
|
C |
|
H |
|||||||
31 |
|
|
|
|
|
|
|
D |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
C |
|
D |
ð23Þ |
|
|
|
|
||
|
|
|
|
||
|
|
|
Carbon atoms react with norbornadiene 32 in a manner analogous to their reaction with cyclopentene.66 Thus, DBA and vinyl C H insertion both generate bi- cyclo[3.2.1]octa-2,3,5-triene (33), which undergoes a known67 [3.3] sigmatropic rearrangement to endo-6-ethynylbicyclo[3.1.0]hex-3-ene (34). When 13C atoms are employed, the label distribution indicates that the three pathways in Eq. 24 are involved. The DBA gives tricyclopropylidene (35), which opens to 33 (path a). The C H insertion gives vinylcarbene (36), which either cleaves directly to 34 (path b) or ring expands to 33 (path c). In this case, the ratio of DBA to C H insertion by C atoms is 1.08:1. In neither 28 nor 33 is cumulene formation from the


478 ATOMIC CARBON
Reaction of CF with benzene generates the 7-fluoronorcaradien-7-ly radical (39), which abstracts hydrogen (from added isobutane) and opens to 7-fluorocyclohepta- triene (40). Cycloheptatriene (10) is trapped as tropylium fluoroborate (41) by the addition of BF3 (Eq. 27).73 An additional product of CF þ benzene is fluorobenzene (42), in which labeling studies demonstrate that the attacking carbon contains
the fluorine in 42. The interesting transfer of CH in Eq. 28 is proposed to account for the formation of 42.73,74
|
|
F |
H F |
|
H F |
|
BF4- |
|
. |
|
|
|
BF3 |
||
. |
|
R-H |
|
|
|
||
. F-C: + |
|
|
|
|
|
|
+ |
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
39 |
|
40 |
41 |
|||
ð27Þ
F |
|
|
F |
|
|
H |
H |
|
|
|
|
H |
|
|
|
|
|
|
|
|
|||||||
|
|
. |
|
|
|
|
. |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
* C . |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
* C |
|
|
|
Ph-H |
|
|
|
|
. |
|
|
|
|
H C . |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
*C |
|
* C |
|
|
* C |
+ |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
F |
|
F |
|
|
|
|
|
|
|
|
|
|
||||||
39 |
|
|
|
|
|
|
|
|
|
|
|
|
F |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
F |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
42 |
|||||||
ð28Þ
Like carbenes, C atoms insert into the weaker C Cl and C Br bonds. For example, reaction of C with CCl4 may be rationalized by assuming an initial C Cl insertion to give the chlorocarbene 43 (Eq. 29).16 However, C also abstracts Cl from CCl4 to give CCl, which has been trapped by cyclohexene to give 7-chloronor- caran-7-yl radicals (44). These radicals abstract both H and Cl from substrate (Eq. 30).75 Control experiments demonstrate that CCl2 is not involved in this reaction. Reaction of C with chlorofluorocarbons results in abstraction of both F and Cl with the latter predominating as would be expected from a consideration of the relative bond strengths. An example is shown in Eq. 31.75 Abstraction of halogen by C can also be used to generate CBr and CI. Thus, reaction of C with CBrF3 and CIF3 in the presence of cyclohexene results in trapping products of the bromoand iodonorcaranyl radicals, respectively.76
|
: |
|
|
|
|
|
|
|
|
C + Cl-CCl3 |
|
C |
|
CCl3 |
|
|
Cl2C |
|
CCl2 |
|
|
|
|
|
|||||
|
|
|
|||||||
|
|
Cl |
|
|
|
||||
|
43 |
|
|
ð29Þ |
|||||
|
|
|
|
CCl4 |
|||||
|
|
|
|
|
Cl3C |
CCl3 |
|||
|
|
|
|
|
|
|
C |
||
|
|
|
|
|
|
Cl |
Cl |
||


REACTIONS OF ATOMIC CARBON |
481 |
CH groups in the aromatic ring of 56 are unlabeled indicates that neither the m- nor p-tert-butylphenylcarbene 59 and 60, if formed, rearranges to 58. Addition of HBF4 to the cold reactor bottom following the 13C þ tert-butylbenzene reaction results in the formation of tert-butyltropylium fluoroborate (61) labeled in the 3- and 4- positions in a 1:2 ratio, and no excess 13C in the 2-position (Eq. 36). These results are consistent with formation of carbenes 58–60 by C H insertion followed by intramolecular trapping of 58, and rearrangement of 59 and 60 to tert-butyl- cycloheptatetraenes (62), which are trapped by HBF4. Calculations indicate that the first step of the phenylcarbene rearrangement, ring expansion of 51, must surmount a barrier of 13 kcal/mol to give 53.80 However, rearrangement back to 51 (in this case interconverting 58–60) must cross a barrier of 30 kcal/mol, and it may be that initial ring expansion of 59 and 60 leads to cycloheptatetraenes 62 which, lacking the energy to rearrange further, are trapped by HBF4. A similar result is observed when 58 and 60 are generated by deoxygenation of the corresponding aldehydes. Thus, 58 is trapped intramolecularly while 60 ring expands to a tertbutylcycloheptatetraene that is trapped as the tropylium salt.
|
|
|
|
|
|
|
|
|
BF4- |
|
|
|
* |
|
|
|
|
|
|
HBF4 |
* |
+ |
|
||
+ C |
|
|
+ |
|
|
|
|
+ |
|
|||
|
|
|
* C: |
60 |
* |
|
|
C |
C |
* |
||
*C = 13C |
|
62 |
|
|
|
|||||||
59 H * C: |
1 |
: |
2 |
|||||||||
|
||||||||||||
|
|
|
H |
|
|
|
61 |
|
61 |
|
||
ð36Þ
An intermediate cycloheptatetraene can also be trapped in the addition of carbon to benzene itself. When 13C atoms react with benzene-d6 and HBF4 is added, tropylium fluoroborate (41), containing deuterium on the labeled carbon, is observed.
This result is consistent with an initial C D insertion by carbon to give 51-d6, which ring expands to 53-d6, and is trapped by HBF4 (Eq. 37).77i
+ *C |
|
|
|
|
|
|
HBF4 |
+ |
H |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||||
|
* |
D |
* |
|
|
* |
BF4- |
|||
d6 |
C |
D |
|
d5 |
D |
|
||||
d5 |
|
|
d5 |
|
|
|
||||
*C = 13C |
51-d6 |
|
|
|
53-d6 |
|
|
41-d6 |
|
|
ð37Þ
Several other substituted benzenes have been reacted with carbon and the substituted phenylcarbene or cycloheptatetraene have been trapped in an intramolecular reaction. Thus, phenol reacts with C atoms to give tropone (63) and traces of the o-, m-, and p-cresols.81 In this case, an initial o-, m-, or p-hydroxyphenylcarbene (64) is postulated to ring expand to a hydroxycycloheptatetraene that traps itself by intramolecular proton transfer (Eq. 38). A competing reaction of 64 is


REACTIONS OF ATOMIC CARBON |
483 |
Cocondensation of arc generated carbon with naphthalene (67) at 77 K generates cyclobuta[de]naphthalene (68) and the 1- and 2-methylnaphthalenes (69a,b).84 The intermediacy of the 1- and 2-naphthylcarbenes (70a,b) is postulated. Intersystem crossing produces triplet 70a,b that abstract hydrogen, while intramolecular trapping of singlet 70a gives 68 in a known reaction (Eq. 40).85 Generation of 70a and 70b independently by the deoxygenation of the corresponding naphthaldehydes results in the formation of 68 and 69a,b. The fact that 68 is formed when 2-naphthaldehyde is deoxygenated indicates that 170b rearranges to 170a under the energetic reaction conditions. This rearrangement has been shown to occur at high temperatures85 and has been studied computationally.86
CH3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
||
|
|
+ C |
|
|
|
|
|
+ |
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
67 |
|
|
77 K |
|
|
|
|
|
|
|
|
|
|||||
|
68 |
|
69a |
R-H |
69b |
||||||||||||
via |
H |
|
|
|
|
|
R-H |
||||||||||
|
|
|
|
|
|
||||||||||||
|
|
|
|
H |
|||||||||||||
|
|
170a |
+ |
|
|
|
H |
isc |
|
+ |
|
|
|
H |
|||
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
170b |
|
|
|
370a |
|
370b |
||||||||
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
ð40Þ |
||||
Cocondensation of C with a 1:1 mixture of 67-d0 and 67-d8 generates 68-d0 and 68-d8 in a 0.12 ratio as a result of a large kH/kD for isc in 70a,b. This unusually large kH/kD is postulated to result from the generation of the singlet state of 70a,b with excess energy and it is the isotope effect upon the degradation of the energetic carbene to the triplet state that is observed. The large isotope effect on isc leads to more triplet products from 70a,b-d0 than from 70a,b-d8. Thus, when 70a,b are trapped with the 2-butenes, 4–18% of the carbene adducts are formed nonstereospecifically from C þ 67-d0, while addition of the carbenes from C þ 67-d8 to the 2-butenes produces only stereospecific adducts (Eq. 41). A similar result is observed when triplet 70a,b are trapped with oxygen (Eq. 42).84
|
|
|
|
|
|
|
|
|
|
1-N |
|
2-N |
|
67-d0 |
+ C |
|
|
|
|
|
1-N |
+ |
2-N |
+ |
|
||
|
|
|
|
|
+ |
|
|||||||
|
|
|
|
|
|
|
|
|
+ |
|
|
|
ð41Þ |
|
|
|
|
1.00 |
0.19 |
0.05 |
0.70 |
0.04 |
|||||
67-d8 |
+ C |
|
|
|
|
|
|
0.21 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
1.00 |
0.29 |
0 |
|
|||||||||
|
|
|
|
|
|||||||||
N= Naphthyl
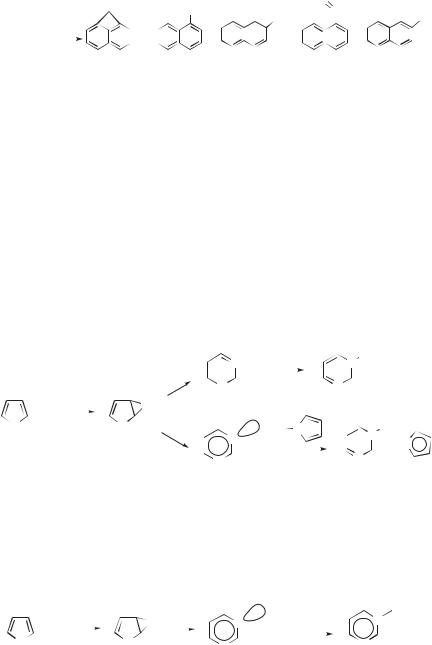



 *
* OCH
OCH S
S
 CH
CH