
Reactive Intermediate Chemistry
.pdf992 THE PARTNERSHIP BETWEEN ELECTRONIC STRUCTURE CALCULATIONS
closure to 7a via conrotation, would always lead to disrotatory ring closure. Similarly, in this model, molecules of 7a that opened to 8a via conrotation would always reclose to 7a by this mode of coupled methylene rotations. Thus, if this dynamical model were correct, both conrotatory and disrotatory ring opening of 7b to the (0,0) diradical would contribute only to the fraction of product found to have undergone double methylene rotations in Berson’s experiments.
Consequently, whether the PES calculations by Getty and by Baldwin and coworkers predict that a preference for double methylene rotations should have been observed in the experiments of Berson (and Baldwin), depends on whether the fate of 8b (and the corresponding diradical in Baldwin’s eperiments on 7- 1-13C-1,2,3-d3) is better described by a TST model or by the dynamical model, discussed in the previous paragraph. If the fate of 8b is better described by a TST model, ring opening of 7b to 8b would be expected to make a substantial contribution to the fraction of product found to have undergone net rotation of just one methylene group. On the other hand, if the fate of 8b is dictated by a dynamical effect, which favors ring closure of 8b to 7b by the same mode of coupled methylene rotation by which 8b is formed from 7b, ring opening of 7b to 8b would contribute only to the formation of product observed to have undergone double methylene rotations.
In order to investigate which model for the behavior of 8 is closer to being correct, our group provided Carpenter with an analytical expression, fit to our PES, so that Carpenter could perform semiclassical trajectory calculations on our PES. At the same time, Doubleday and Hase undertook direct dynamics calculations. As discussed in Chapter 21 in this volume, in the latter type of trajectory calculation the forces acting on a molecule at different points on a PES are found by performing electronic structure calculations. For this purpose, Doubleday and Hase used a reparameterized version of AM1 that provided a PES, similar to those calculated by Getty and by Baldwin, Yamaguchi, and Schaefer.
Both types of dynamics calculations gave the same type of results. The calculations found that the ratio of double-to-single methylene rotations is higher than the1 : 1 ratio predicted by application of a TST model to the PES. Ratios of double- to-single rotations between 2.9 and 3.5 were obtained from the direct dynamics calculations of Doubleday et al.,62 and a ratio of 4.7 was computed from the trajectory calculations performed by Carpenter and co-workers.63
Both sets of calculations found that ring closure of 8 preferentially occurs by the same mode of coupled methylene rotations as ring opening of 7. Crudely put, the dynamical behavior of 8 can be predicted by, what would be called in classical mechanics, ‘‘conservation of angular momentum.’’ Chapter 21 in this volume provides examples of other reactions in which dynamical effects cause statistical models, such as TST, to fail to make correct predictions.
4.3.2. Calculations and Experiments on the Stereomutation of 1,1- Difluorocyclopropanes. C F bonds have low-lying unfilled, s* orbitals;64 therefore, C F bonds are strong, hyperconjugative electron acceptors. Consequently, as shown schematically in Figure 22.9, hyperconjugative interaction with

|
APPLICATIONS OF ELECTRONIC STRUCTURE CALCULATIONS 993 |
||||||||
|
F |
|
|
F |
|
F |
|||
R 1 |
R 2 |
R 1 |
|
R2 |
R1 |
|
R 2 |
||
|
|
dis |
|
|
|||||
|
|
|
|
|
|
F |
|
H |
|
|
|
|
|
|
|
|
|
||
H |
R 3 |
|
H |
R3 |
H |
R3 |
|||
|
|
|
|
|
|
HOMO |
|
LUMO |
|
9a, R1 = R2 = R3 = H |
|
10a, R1 = R2 = R3 = H |
|
|
|||||
b, R1 = CH3, R2 = CH3, R3 = H |
|
|
b, R1 = CH3, R2 = CH3, R3 = H |
||||||
c, R1 = CH3, R2 = H, R3 = CH3 |
|
|
c, R1 = CH3, R2 = H, R3 = CH3 |
||||||
Figure 22.9. Disrotatory ring opening of 1,1-difluorocyclopropane (9) to 2,2-difluorocyclo- propane-1,3-diyl (10). The in-phase combination of 2p–p AOs in the highest occupied molecular orbital (HOMO) is stabilized by a bonding interaction with the 2p AO at C2 in the ‘‘p’’ combination of low-lying, C F, antibonding orbitals.
the low-lying, unfilled orbitals of the C F bonds at C2 in 2,2-difluoropropane-1,3- diyl (10a), stabilizes the symmetric combination of 2p–p AOs on the terminal carbons. Therefore, disrotation should be preferred to conrotation in the steromutation of 1,1-difluorocyclopropane (9a).
SD(Q)-CI/6-31G*//(2/2)CASSCF/6-31G* calculations confirmed the qualitative expectation that coupled disrotation should be preferred to both conrotation and monorotation in the stereomutation of 9a.65 In addition, unlike the case in 8a, the calculations found that the preference for coupled rotation was enhanced by the addition of methyl groups to the terminal carbons of 10a. Hyperconjugative electron donation from the methyl groups in 10b and 10c enhances electron donation from the symmetric combination of 2p–p AOs on the terminal carbons into the antibonding orbitals of the C F bonds at C2.
It is true that, in general, experiments are more likely than calculations to produce totally unanticipated findings. However, our calculations on 10b and 10c gave a wholly unexpected result—the preference for having both methyl groups on the ‘‘outside’’ in the transoid,transoid conformation (as in 10b), rather than having one on the ‘‘inside’’ in the transoid,cisoid conformation (as in 10c), was computed to be much larger in diradical 10 than in diradical 8. This difference between the two diradicals was shown to have an electronic origin.66
Long-range interaction between the pseudo-p combination of methyl C H bonds on one terminal carbon and the 2p–p AO on the other terminal carbon was found to be bonding in diradicals, such as 8, with hyperconjugatively electondonating bonds at C2. In contrast, the hyperconjugatively electron-accepting C F bonds in 10 were found to make the long-range interaction between the pseudo-p combination of methyl C H bonds on one terminal carbon and the 2p–p AO on the other terminal carbon strongly antibonding in 10c. Since 9b can undergo disrotatory
ring opening to 10b; whereas, 9c must undergo disrotatory ring opening to 10c, disrotatory ring opening was predicted to be much faster for 9b that for 9c.65,66
These computational findings lead to the expectations that, if optically active cisand trans-1,1-difluoro-2-ethyl-3-methylcyclopropanes were both pyrolyzed, they each

994 THE PARTNERSHIP BETWEEN ELECTRONIC STRUCTURE CALCULATIONS
should undergo racemization faster than epimerization; but the cis isomer should racemize much faster than the trans isomer. In order to test these predictions, Scott Lewis, then a graduate student in our group, carried out the syntheses of the two, optically active, 1,1-difluorocyclopropanes, and Feng Tian in Dolbier’s group, improved the syntheses and measured the kinetics of racemization and epimerization.67
For the cis isomer, the ratio of racemization to epimerization was found to be 107 at 274.5 C, and the cis isomer was found to undergo racemization 16.2 times faster than the trans isomer. The ratio of racemization to epimerization for the latter was found to be only 6.6 at 274.5 C. Thus, these experiments confirmed the computational predictions that in 9b and 9c, double methylene rotations are faster than any process that leads to net single methylene rotation, and the preferred mode of double rotation is disrotation, rather than conrotation.65,66
4.3.3. Calculations on the Stereomutation of 1,1-Disilylcyclopropanes. It is to be hoped that future experiments on the stereochemistry of the ring-opening reactions of 1,1-bis(trimethylsilyl)cyclopropanes (11) will test the computational prediction by Anne Skancke (in our group) that the hyperconjugatively electrondonating C Si bonds at C2 in 2,2-bis(trimethylsilyl)propane-1,3-diyl (12a) will result in a substantial preference for ring opening and ring closure by coupled conrotation.68
In agreement with the results of experiments on pyrolysis of 1,1-bis(trimethyl- silyl)cyclopropane,69 additional (2/2)CASPT2/6-31G* calculations predict that a rapid 1,2-silyl shift will occur in 12a, forming 13a.18 However, if cisand trans- 1,1-bis(trimethylsilyl)-2,3-dimethylcyclopropanes (11b and 11c) were pyrolyzed, then, as shown in Figure 22.10, the stereochemistry of ring opening could, presumably, still be inferred from the stereochemistry of the double bonds in the expected rearrangement products (13b and 13c).
Interestingly, the calculations predict that, in the pyrolysis of 11c, formation of cisoid,cisoid diradical 12c should be favored over formation of the transoid,transoid stereoisomer.18 (Since the latter conformer is predicted not to be formed, it is not shown in Fig. 22.10.) The C Si bonds at C2 in 12 are strong hyperconjugative electron donors; so, as discussed in Section 4.3.2, there is a long-range, bonding
|
SiR 3 |
|
|
SiR 3 |
|
|
SiR 3 |
SiR 3 |
|
|
|
|
|
1,2 shift R 3Si |
|||
H |
|
con |
H |
|
|
R2 |
SiR 3 |
|
R 1 |
|
|
R 2 |
+ H |
||||
|
SiR 3 R |
|
|
C SiR 3 |
|
H |
R 1 |
R2 |
H C |
2 |
H |
R1 |
H3C |
H 3C R 1 |
|||
3 |
|
3 |
|
|
|
|||
|
11 |
|
|
12 |
|
|
|
13 |
a, R = R1 = R2 = H; b, R1 = H, R = R2 = CH3; c, R = R1 = CH3, R2 = H
Figure 22.10. Rearrangement products (13), predicted to be formed from 1,1-disilyl- cyclopropanes (11) by conrotatory ring opening, followed by a 1,2-shift of a trimethylsilyl group in the 1,3-diradicals (12) generated. Pyrolysis of 11b should lead to a mixture of (E/Z) isomers (13b), but pyrolysis of 11c is predicted to form only the E stereoisomer (13c).18
996 THE PARTNERSHIP BETWEEN ELECTRONIC STRUCTURE CALCULATIONS
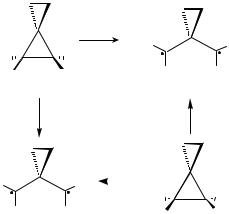
Gajewski’s experiments found that the cis isomer (14a) underwent ring opening by coupled rotation faster than the trans isomer (14b). Gajewski quite reasonably supposed that the transoid,transoid diradical (15a) would be lower in energy than the transoid,cisoid diradical (15b). Since a disrotatory pathway connects 14a with 15a, Gajewski came to the logical, but very surprising, conclusion that the preferred mode of coupled methylene rotations in the stereomutation of 14 must be disrotation, rather than the expected conrotation.
(2,2)CASPT2/6-31G* calculations on the energies of diradicals 15a and 15b, performed by Bill Johnson, then a graduate student in our group, led to a reinterpretation of Gajewski’s experimental results.71 The calculations showed that, although Gajewski’s assumption that 15a is lower in energy than 15b is perfectly reasonable, it turns out to be incorrect.
As discussed in Section 4.3.2, when, in propane-1,3-diyls, the bonds to the substituents at C2 are hyperconjugative electron donors there is a long-range, bonding interaction between the pseudo-p combination of methyl C H bonds on one terminal carbon and the 2p–p AO on the other terminal carbon.66 The cyclopropyl bonds in 15 are such strong electron donors that this effect results in the transoid, cisoid conformer (15b) being computed to be lower in energy than the transoid, transoid conformer (15a).
However, Johnson’s calculations did find that Gajwski was correct in interpreting the faster rate of reaction of 14a, relative to 14b, as meaning that 14a undergoes ring opening to the more stable diradical. However, since the more stable diradical is 15b, rather than 15a, and since 14a is connected to 15b by a conrotatory pathway, Gajewski’s experiments actually confirm the qualitative prediction that 14 should preferentially undergo stereomutation by conrotation.
4.3.5. Calculations and Experiments on the Ground States of Cyclo- pentane-1,3-diyls. Our calculations on 2,2-difluoropropane-1,3-diyl (10)65,66
led to another prediction72 that was subsequently tested by us experimentally and
confirmed.73 Both (2,2)CASSCF and SD-CI calculations predict the triplet to be the ground state of propane-1,3-diyl (8)60,66 and cyclopentane-1,3-diyl (16a),74 a cyclic
derivative of 8. Prior experiments by Closs had shown that the ground state of 16a is, in fact, a triplet;75 and Dougherty, Adam, Wirz, and their co-workers76 found the same to be true for the 1,3-diphenyl derivative (16b) (Fig. 22.12).76
In contrast, our (2,2)CASSCF and (2,2)CASPT2/6-31G* calculations predicted that hyperconjugation with the fluorines is strong enough in both diradical 1065,66 and in the cyclic derivative 16c72 to make a singlet the ground state of each diradical. In the lowest singlet state both nonbonding electrons can occupy the MO, formed from the in-phase combination of 2p–p AOs on the terminal atoms and stabilized by mixing with the C F antibonding orbitals. However, in the triplet only one nonbonding electron can occupy this MO.
In collaboration with Adam and Wirz, our group confirmed this prediction experimentally, by synthesizing a derivative of 16d and showing that it does have a singlet ground state.73 Subsequently, a derivative of 16e, in which the geminal fluorines at C2 in 16d were replaced by ethoxy groups, was also found to have

CONCLUSION AND OUTLOOK |
997 |
XX
R R
16a, X = R = H; b, X = H, R = Ph;
c, X = F, R = H; d, X = F, R = Ph;
e, X = OCH2CH3, R = Ph;
f , X = OCH3, R = Ph;
g, X = SiH3, R = H
Figure 22.12. Cyclopentane-1,3-diyl (16a) has been both calculated74 and found75 to have a triplet ground state, and the 1,3-diphenyl derivative (16b) has also been found to have a triplet ground state.76 In contrast, calculations on 16c72 and 16g79 have predicted both diradicals should have singlet ground states, and experiments on derivatives of 16d,73 16e,77and 16f78 found these three diradicals do, in fact, have singlet ground states.
a singlet ground state.77 Most recently, Abe et al.78 measured the lifetimes of derivatives of 16f, with different substituents in the para positions of the phenyl rings. The longest lived singlet diradical was, as might have been expected, found to be the one with the substituents in the aromatic ring that are the best electron donors. Donation of electrons to the 2p–p AOs on C1 and C3 increases the amount of electron donation from the in-phase combination of these AOs into the low-lying antibonding orbitals of the C O bonds at C2.
We also predicted that hyperconjugatively electron-donating, as well as electronaccepting bonds at C2, can confer a singlet ground state on derivatives of 16. Our (2,2)CASSCF and CASPT2/6-31G* calculations found that the silyl substituents at C2 in 16g should make this diradical a ground-state singlet too.79
However, in contrast to the case for singlet diradical 16c, singlet 16g was calculated to have a high barrier-to-ring closure. Unlike the fluorines in 16c, the silyl groups in 16g cause conrotatory closure to be strongly preferred; but disrotation is required for closure of diradical 16. Unfortunately, the predictions of a singlet ground state and a high barrier-to-ring closure in diradical 16g are unlikely to be testable experimentally, because, as in the case of singlet diradical 12, a 1,2-silyl shift is calculated to occur rapidly in singlet 16g.18
5. CONCLUSION AND OUTLOOK
Section 2 described some of the properties of RIs that can be computed, using the methodology discussed in Section 3. Section 4 provided several examples of how electronic structure calculations can be used to obtain information about RIs that is crucial to interpreting the results of experiments, but that would be either difficult or impossible to obtain experimentally, Examples of such information, discussed in
998 THE PARTNERSHIP BETWEEN ELECTRONIC STRUCTURE CALCULATIONS
Section 4, are the nature of the lowest singlet state of phenylnitrene (1b),30 the charge distribution in cubyl cation 4,45 and the existence of a long-range, bonding interaction that counterintuitively makes diradical 15b lower in energy than diradical 15a.71 Electronic structure calculations have obviously become very useful tools for studying, understanding, and predicting the behavior of RIs.
Future advances in computer hardware and software will surely make it possible to perform electronic structure calculations at higher and higher levels of theory on larger and larger molecules. Suppose that, at some time in the not too distant future, the properties of RIs can be computed at a level of theory so high that, as shown by repeated comparisons with experiments, the results of calculations at this level can be guaranteed to be as accurate as experimental measurements. If the cost of calculating the geometries, energies, and spectra of RIs at this level of theory were significantly less than the cost of measuring these properties, would there be any point in doing experiments to measure them?
It might be argued that theoretical predictions, no matter how good the record of success of the method used to make them, must always be verified experimentally. However, putting aside this philosophical issue, there would still be a very good reason to do experiments. Nature has demonstrated an amazing ability to surprise experimentalists, by providing not only unexpected solutions to puzzles that experimentalists have sought to solve, but also by furnishing new and previously unimagined puzzles. Without experiments, these puzzles would not be discovered; and, without these puzzles, electronic structure calculations would never be performed, in order to solve them.
Of course, calculations done ‘‘in silico,’’ like experiments done in silica, have the potential for yielding unexpected results. Several examples of theoretical results, which were very surprising when first obtained, were provided in Section 4. However, experimental research has an even greater propensity than computational studies for making unexpected discoveries, far removed from the questions that initially motivated the research.
For example, if, in studying the reaction of methylcubane (6) with tert-butoxyl radicals, Della et al.52 were only trying to see whether this reaction could be used to generate cubylcarbinyl radical, their finding that tert-butoxyl radical preferentially abstracts a hydrogen from the cube, rather than from the methyl group, was only indirectly related to the question they were actually trying to answer. However, without the stimulus provided by Della’s experimental results, we would never have been motivated to try to solve the puzzle of why the stronger C H bond is preferentially broken in this reaction.
In fact, all of the computational projects described in Section 4, were motivated by the results of experiments. Without the puzzles provided by the experimental results, the calculations would never have been performed. Therefore, if calculations ever did replace experiments, computational chemists would very likely become the victims of the success of the theoretical methods they helped to develop.
The focus in this chapter, on how electronic structure calculations can be used by experimentalists who are studying RIs, is due to the expectation that most of the
SUGGESTED READING |
999 |
readers will be experimentalists. However, computational chemists might interpret this chapter (especially Section 4) rather differently. They might read this chapter as a treatise on how experiments on RIs can serve computational chemists, by providing them with problems that can be addressed by performing calculations.
Neither perspective is entirely correct. Calculations can be very useful in designing, executing, and interpreting experiments; and experimental results motivate many, if not most, electronic structure calculations. As indicated by the title of this chapter, electronic structure calculations and experiments really have become partners in the study of RIs.
ACKNOWLEDGMENT
My research was generously supported by the National Science Foundation. I am grateful to Professor Thomas Bally, Professor Barry Carpenter, Professor Chris Cramer, Professor Ernest Davidson, Professor Matt Platz, and Professor Daisy Zhang for reading and commenting on various drafts of this chapter. I am also very grateful to Dr. David Hrovat for proofreading the penultimate draft and for being the major contributor to much of the research from my group during the past 18 years.
SUGGESTED READING
C.J. Cramer, Essentials of Computational Chemistry, John Wiley & Sons, Inc., New York, 2002. This monograph provides an authoritative, detailed, and very readable treatise on the methods that are currently used for performing electronic structure calculations, which are described briefly in Section 3.
W. J. Hehre, L. Radom, P. von R. Schleyer, and J. A. Pople, Ab Initio Molecular Orbital Theory, Wiley-Interscience, New York, 1986. For an excellent discussion of basis sets and how they are constructed see, pages 65–88.
The following is a small collection of papers that use the results of electronic structure calculations to explain and/or predict the results of experiments on different types of RIs:
M.Mauksch, V. Gogonea, H. Jiao, and Paul. von R. Schleyer, ‘‘The Remarkable Nature of the
Monocyclic (CH)þ9 Cation. A Heilbronner Mo¨bius Aromatic System Revealed,’’ Angew. Chem. Int. Ed. Engl. 1998, 37, 2395. B3LYP calculations provide an interpretation of
puzzling experimental results, obtained nearly three decades earlier.
Y.Osamura, H. F. Schaefer, III, S. K. Gray, and W. H. Miller, ‘‘Vinylidene: A Very Shallow
Minimum on the C2H2 Potential Energy Surface. Static and Dynamic Considerations.’’ J. Am. Chem. Soc. 1981, 103, 1904. At the time these calculations were published, there was no convincing experimental evidence that H2C C: is an energy minimum, rather than a TS. The prediction that it is an energy minimum was subsequently confirmed experi-
mentally by S. M. Burnett, A. E. Stevens, C. S. Feigerle, and W. C. Lineberger,
‘‘Observation of Vinylidene by Photoelectron Spectroscopy of the Vinylidene (C2H2 ) Ion,’’ Chem. Phys. Lett. 1983, 100, 124.
1000 THE PARTNERSHIP BETWEEN ELECTRONIC STRUCTURE CALCULATIONS
K.N. Houk, N. G. Rondan, and J. Mareda, ‘‘Theoretical Studies of Halocarbene Cycloaddition Selectivities. A New Interpretation of Negative Activation Energies and Entropy Control of Selectivities,’’ Tetrahedron 1985, 41, 1555. Calculations on carbene addition reactions led to a general explanation of why it is possible for very exothermic, bimolecular reactions to have negative activation enthalpies.
D. M. Smith, S. D. Wetmore, and L. Radom, ‘‘Theoretical Studies of Coenzyme-B12- Dependent Carbon-Skeleton Rearrangements,’’ in Theoretical Biochemistry—Processes and Properties of Biological Systems, L. A. Ericksson, Ed., Elsevier, Amsterdam, The Netherlands, 2001, pp. 183–214. Electronic structure calculations are applied to the understanding and prediction of how enzymes can lower the barriers to the 1,2-shifts in radicals that occur in reactions catalyzed by B12.
F.Ogliaro, N. Harris, S. Cohen, M. Filatov, S. P. de Visser, and S. Shaik, ‘‘A Model ‘Rebound’ Mechanism of Hydroxylation by Cytochrome P450. Stepwise and Effectively Concerted Pathways, and Their Reactivity Patterns,’’ J. Am. Chem. Soc. 2000, 122, 8977. Calculations explain puzzling aspects of cytochrome P450 hydroxylation reactions in terms of two, different, reactive spin states of the enzyme.
T.Bally, S. Bernhard, S. Matzinger, J.-L. Roulin, G. N. Sastry, L. Truttmann, Z. Zhu, A. Marcinek, J. Adamus, R. Kaminski, J. Gebicki, F. Williams, G.-F. Chen, and M. P. Fu¨lscher, ‘‘The Radical Cation of syn-Tricyclooctadiene and Its Rearrangement Products,’’ Chem. Eur. J. 2000, 6, 858. A combination of CASPT2 calculations of UV spectra and B3LYP and CCSD(T) calculations of PESs is used to identify the intermediates formed sequentially in the rearrangement of the radical cation of the syn dimer of cyclobutadiene and to understand why they differ from those formed from the anti dimer.
D.A. Hrovat, J. H. Hammons, C. D. Stevenson, and W. T. Borden, ‘‘Calculations of the
Equilibrium Isotope Effects on the Reductions of Benzene-d6 and Cyclooctatetraene-d8,’’ J. Am. Chem. Soc. 1997, 119, 9523. B3LYP/6-31þG* calculations on the title compounds
and on the radical anions formed from them show that the very large difference between the equilibrium isotope effects, found by Stevenson, is due to an inverse isotope effect on the planarization of the COT ring. This explanation was subsequently confirmed by KIE measurements, carried out by C. D. Stevenson, E. C. Brown, D. A. Hrovat, and W. T. Borden, ‘‘Isotope Effects on the Ring Inversion of Cyclooctatetraene,’’ J. Am. Chem. Soc. 1998, 120, 8864.
REFERENCES
1.Review: I. Shavitt, Tetrahedron 1985, 41, 1531.
2.See, inter alia: (a) B. R. Beno, K. N. Houk, and D. A. Singleton, J. Am. Chem. Soc. 1996, 118, 9984; (b) A. J. DelMonte, J. Haller, K. N. Houk, K. B. Sharpless, D. A. Singleton, T. Strassner, and A. A. Thomas, J. Am. Chem. Soc. 1997, 119, 9907; (c) A. E. Keating, S. R. Merrigan, D. A. Singleton, and K. N. Houk, J. Am. Chem. Soc. 1999, 121, 3933.
3.(a) C. J. Cramer, Essentials of Computational Chemistry, John Wiley & Sons, Inc., New York, 2002. (b) The quotation is from page 212.
4.(a) P. C. Hariharan and J. A. Pople, Theor. Chim. Acta 1973, 28, 213; (b) R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople, J. Chem. Phys. 1980, 72, 650; (c) M. J. Frisch, J. A. Pople, and J. S. Binkley, J. Chem. Phys. 1984, 80, 3265. (d) For an excellent discussion
REFERENCES 1001
of basis sets and how they are constructed see, W. J. Hehre, L. Radom, P. von R. Schleyer, and J. A. Pople, Ab Initio Molecular Orbital Theory, Wiley-Interscience, New York, 1986, pp. 65–88.
5.T. H. Dunning, Jr., J. Chem. Phys. 1989, 90, 1007
6.(a) C. Mo¨ller and M. S. Plesset, Phys. Rev. 1934, 46, 618; (b) J. A. Pople, J. S. Binkley, and R. Seeger, Int. J. Quantum Chem. 1976, S10, 1.
7.(a) R. J. Bartlett and G. D. Purvis, Int. J. Quant. Chem. 1978, 14, 516; (b) R. J. Bartlett and J. F. Stanton, in Reviews in Computational Chemisty, Vol. 5, K. B. Lipkowitz and D. B. Boyd, Eds., VCH Publishers, New York, 1994, pp. 65–169.
8.J. A. Pople, M. Head-Gordon, and K. Raghavachari, J. Chem. Phys. 1987, 87, 3700.
9.(a) L. A. Curtiss, K. Raghavachari, G. W. Trucks, and J. A. Pople, J. Chem. Phys. 1991, 94, 7221; (b) L. A. Curtiss, P. C. Redfern, Raghavachari, V. Rassolov, and J. A. Pople,
J. Chem. Phys. 1998, 109, 7764.
10.M. R. Nyden and G. A. Petersson, J. Chem. Phys. 1981, 75, 1843 (1981).
11.GAUSSIAN98, Revision A.7, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1998.
12.B. O. Roos, Adv. Chem. Phys. 1987, 69, 339.
13. T. Bally and W. T. Borden, in Reviews in Computational Chemisty, Vol. 13, K. B. Lipkowitz and D. B. Boyd, Eds., Wiley-VCH Publishers, New York, 1999,
pp. 1–97.
14.M. DuPuis, C. Murray, and E. R. Davidson, J. Am. Chem. Soc. 1991, 113, 9756.
15.W. T. Borden and E. R. Davidson, Acc. Chem. Res. 1996, 29, 67.
˚
16. (a) K. Andersson, P. A. Malmqvist, B. O. Roos, A. J. Sadlej, and K. Wolinski, J. Phys.
1990 ˚
Chem. , 94, 5483. (b) K. Andersson, P.-A. Malmqvist, and B. O. Roos, J. Chem. Phys. 1992, 96, 1218.
17. K. Andersson, M. R. A. Blomberg, M. P. Fu¨lscher, G. Karlstro¨m, V. Kello¨, R. Lindh,
˚
P.-A. Malmqvist, J. Noga, J. Olsen, B. O. Roos, A. J. Sadlej, P. E. M. Siegbahn, M. Urban, and P.-O. Widmark, MOLCAS-4, University of Lund, Sweden.
18.D. A. Hrovat and W. T. Borden, unpublished results.
19.D. A. Hrovat, K. Morokuma, and W. T. Borden, J. Am. Chem. Soc. 1994, 116, 1072.
20.D. A. Hrovat and W. T. Borden, J. Am. Chem. Soc. 1994, 116, 6327.
21.T. Bally, S. Bernhard, S. Matzinger, J.-L. Roulin, G. N. Sastry, L. Truttmann, Z. Zhu, A. Marcinek, J. Adamus, R. Kaminski, J. Gebicki, F. Williams, G.-F. Chen, and M. P. Fu¨lscher, Chem. Eur. J. 2000, 6, 858.
22.A. D. Becke, J. Chem. Phys. 1993, 98, 5648.
23.C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 1988, 37, 785.
