
Metal-Catalysed Reactions of Hydrocarbons / 04-The Chemisorption of Hydrocarbons
.pdf
THE CHEMISORPTION OF HYDROCARBONS |
183 |
(except perhaps in the trough of the fcc(110) surface or at the foot of a step) by straining usual bond angles (and stretching the imagination). It is unfortunate that more attention is not given to the manner of bonding of highly dehydrogenated species to metal surfaces. Only in the case of Ni(100) was it thought likely that the C––C bond broke initially, forming the singly carbon species methylidyne ( C––H). Ethylidyne or ethylidene may follow the most strongly held species, and then the σ -diadsorbed state (4); finally the π state 2 may appear, but it is by no means certain that the final heats measured (80-140 kJ mol−1) refer just to this state. What is perhaps surprising is that calculated M––C bond strengths lie in so narrow a range, showing only small dependence on surface geometry: for platinum, values lie between about 220 and 270 kJ mol−1 (Table 4.8), while those for nickel are a little lower,23 and for rhodium slightly higher.132 We must therefore conclude that the strength of adsorption depends primarily on the number of C––M bonds formed, although on a given surface the bond energy of each bond increases slightly as the number of bonds formed to the surface decreases.23 Figure 4.2 indicates the energetics of a likely sequence of progressively dehydrogenated species.
C––H). Ethylidyne or ethylidene may follow the most strongly held species, and then the σ -diadsorbed state (4); finally the π state 2 may appear, but it is by no means certain that the final heats measured (80-140 kJ mol−1) refer just to this state. What is perhaps surprising is that calculated M––C bond strengths lie in so narrow a range, showing only small dependence on surface geometry: for platinum, values lie between about 220 and 270 kJ mol−1 (Table 4.8), while those for nickel are a little lower,23 and for rhodium slightly higher.132 We must therefore conclude that the strength of adsorption depends primarily on the number of C––M bonds formed, although on a given surface the bond energy of each bond increases slightly as the number of bonds formed to the surface decreases.23 Figure 4.2 indicates the energetics of a likely sequence of progressively dehydrogenated species.
Fewer results are available for ethyne (see Table 4.9); initial sticking probabilities are consistently high (0.8), and initial heats fall between 190 and 270 kJ mol−1 except for palladium which again stands out with a much lower value (112 kJ mol−1) associated with a di-σ (or di-σ /π ) structure (13 or 12a).23 On Ni(100) the molecule split at low coverage to form methane, which also seemed to occur together with methylene at higher coverages on Ni(110).133 On Rh(100)132 and Pt(211), various rearranged C2 species have been detected. Unfortunately there is little firm information on species existing at high coverage, such as might be intermediates in catalytic processes.
Dumesic and his colleagues have used microcalorimetry25 to determine heats of adsorption of ethene and ethyne on unsupported platinum black133 and silicasupported platinum and platinum-containing bimetallic systems:101,134,135 allocation of observed heats to likely structures was aided by parallel FTIR measurements and bolstered by DFT calculations. Results were obtained at various temperatures between 203 K and ambient, so that, in addition to following the sequence with which adsorbed species were formed as coverage increased, the effects of the temperature dimension were explored, giving results which harmonise with those obtained by LEED and spectroscopic methods. These are summarised in Table 4.10. The values recorded at 203 K represent the molecularly-adsorbed forms 2 and 4,with the latter presumably predominating; they are similar to the terminal values found at 300 K on single crystal surfaces (Table 4.8). Those at 300 K with pure platinum are due to formation of ethylidyne (8) plus a hydrogen atom; progressive addition of tin lowered the number of platinum triplets that could be formed and which are required for ethylidyne, so that the π and di-σ forms could then also appear.101,136 Addition of gold had the same effect,137 but there was calculated to

184
TABLE 4.9. Initial Sticking Probabilities (σ 0 ) and Heats of Adsorption (− H ) of Ethene on Single Crystal Suraces at 300 K: Species Identified and C––M Bond Strengths (DCM ) (kJ mol−1 )
Surface |
σ 0 |
− H0 |
− Hfin |
|
Species proposed |
|
Code |
DCM |
Species Proposed |
Code |
References |
||||||||||||||||||||||||||
Ni (100) |
0.81 |
264 |
? |
|
|
|
|
|
|
|
|
|
CH |
|
— |
204 |
|
|
HC |
|
|
|
C |
21 |
131 |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
Ni (110) |
0.80 |
190 |
50 |
|
|
HC |
|
|
|
|
|
|
C–– |
21 |
— |
|
|
CH2 + |
|
|
|
CH |
— |
133 |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
Rh (100) |
0.83 |
210 |
80 |
|
CH––CH |
|
|
|
|
|
or ––CH |
|
C |
|
16 or 22 |
273 |
? |
|
|
|
|
|
— |
132 |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
Pd (100) |
0.83 |
112 |
? |
|
––HC |
|
|
|
|
|
CH–– |
|
12/A |
177 |
? |
|
|
|
|
|
— |
131 |
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
Pt (211) |
0.84 |
270 |
80 |
|
|
|
|
C |
|
|
CH2 |
|
17 or 18 |
220, 255 |
|
|
|
|
C |
|
|
|
|
|
— |
312 |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||
− H0 is the initial and − |
Hfin the final heat (measured at high coverage). The first-named species are those proposed at low coverage, and the second-named at high coverage. |
||||||||||||||||||||||||||||||||||||
4 CHAPTER

THE CHEMISORPTION OF HYDROCARBONS |
185 |
TABLE 4.10. Initial Heats of Adsorption of Ethene and of isoButene
|
|
|
Heat of adsorption/kJ mol−1 |
|
|
Alkene |
Catalyst |
300 K |
203 K |
Reference |
|
|
|
|
|
|
|
C2 H4 |
Pt film |
148 |
— |
141 |
|
|
Pt black |
160 |
120b |
133 |
|
|
Pt/SiO2 |
157 |
125 |
101 |
|
|
Pt/SiO2 |
145 |
— |
135 |
|
|
Pd/SiO2 |
170 |
— |
135 |
|
|
Pt-10Au/SiO2a |
140 |
100 |
137 |
|
|
Pt7 Sn/SiO2a |
135 |
100 |
101 |
|
|
Pt3 Sn/SiO2a |
129 |
98 |
101 |
|
|
PtSn/SiO c |
115 |
— |
135 |
|
|
2 |
|
|
|
|
iso-C4 H8 |
Pt/SiO2 |
160 |
— |
135 |
|
|
Pd/SiO2 |
190 |
— |
135 |
|
|
PtSn/SiO c |
125 |
— |
135 |
|
|
2 |
|
|
|
|
a The numbers are the relative molar amounts. b 173 K.
c Pt/Sn ratios of 2/1 and 2/3 gave very similar results. Values for 300 K are for alkylidynes.
be a consequential move of charge from the 6sp to the 5d levels of the platinum with a net increase of charge at the expense of the gold, corresponding to the latter’s greater electronegativity (see Chapter 1): this caused stronger adsorption of ethylidyne, counteracting the geometric effect. The effect of tin was to increase the population of both the 5d and 6sp levels. Early work gave no evidence for the chemisorption of hydrocarbons on Au(111) or stepped gold surfaces,138 but more recently weak adsorption of ethene on Au(100) and (111) has been detected,139 the desorption activation energy being about 40 kJ mol−1. There is also spectroscopic evidence for weak adsorption of ethene on Au/SiO2.137 Heats of adsorption of ethyne on Pd/SiO2 were higher than for Pt/SiO2, as were the greater values for isobutene (Table 4.10). Values for propyne on Pt/Al2O3 decreased slightly with increasing dispersion, but a more marked difference in the same sense was found with Pd/Al2O3, especially at coverages between 0.1 and 0.5, where values were greater than for Pt/Al2O3.140
On platinum black at 173 K, ethyne formed a π σ structure (either 13 or 15), and at 300 K some ethylylidyne (16A) was also observed,133 the heats liberated being respectively 210 and 220 kJ mol−1.
Initial heats of adsorption of ethene reported many years ago13,14 for condensed metal films were remarkably similar to those given in Tables 4.8 and 4.10 (Fe, 272; Ni, 232; Rh, 210 kJ mol−1). Plus c¸a change, plus c’est la memeˆ chose.
More recently values have been obtained141 for a number of hydrocarbons on platinum films (see Table 4.10 for ethene), but adsorbed states could not be identified, and in many cases (e.g. methane, ethane) substantial decomposition must have occurred.

186 |
CHAPTER 4 |
4.5.5. Characterisation by Other Spectroscopic Methods
The UPS technique was briefly introduced in Section 4.3. It was extensively applied to ethene, ethyne and benzene chemisorbed at low temperatures (100–200 K) on single crystal surfaces of nickel, copper, palladium, iridium and platinum (Table 4.1), and, notwithstanding its constraint in observing all adsorbed species, it led to the conclusion that under these conditions the molecules were adsorbed without dissociation or rearrangement, i.e. in π or π σ states. This agrees with the conclusions reached by the other techniques discussed above.
Ethylidyne (8) has been recognised on Pt/SiO2 at 300 K using the SEDOR NMR technique applied to heavily 13C-labelled ethene;27,28 the C––C bond length was 149 pm. This seemed to occur on large platinum particles, where areas of (111) face are most likely;136 it was also seen by SIMS on platinum black,142 but on small particles vinylidene (17) predominated. Similar SEDOR experiments with ethyne showed 75% vinylidene and 25% ethyne as 12A or 15.143 Adsorbed benzene was shown to rotate freely at 300 K, and cyclopropane was adsorbed, but not strongly, i.e. without loss of hydrogen.27
Many other methods have been used to look at chemisorbed states of hydrocarbons, but none of them give direct structural information. The decrease in magnetic susceptibility of paramagnetic metals accompanying adsorption led to estimates of the number of bonds formed between each molecule and the surface,144,145 and work function changes (Table 4.1) indicated the direction of charge movement at the surface. Techniques such as LEED, SEXAFS, HREELS, PED and FTIR, utilising the interaction of electrons or electromagnetic radiation with the adsorbed layer, remain of paramount importance for structure determination.
4.5.6. C6 Molecules
The reader may be surprised to see so little about higher alkenes, and especially those having six carbon atoms, on which considerable work has been done. Because of the greater number of options for decomposition by C––H bond breaking, their pristine states are harder to access: a few leading references are given at the end of the chapter, and further attention to these molecules will appear in Chapters 12 to 14.
4.6.THERMAL DECOMPOSITION OF CHEMISORBED HYDROCARBONS
The modes of decomposition of chemisorbed hydrocarbon as the temperature is raised from an initial low value to ambient or even higher have been investigated extensively and in very great detail, using all the techniques of structure assessment

THE CHEMISORPTION OF HYDROCARBONS |
187 |
referred to above. It should by now be quite clear that chemisorbed molecules, especially alkenes, exist at low temperatures in states that will change to more strongly bound configurations given the necessary (often minimal) thermal input (see Figure 4.2), in accordance with the principle of maximum bond formation stated in Section 4.2. Recognition of such events may therefore be necessary in order to find the primeval adsorbed state, but with those techniques currently limited to ambient temperature (e.g. calorimetry on single crystals) understanding the ways of decomposition is unavoidable. More generally the multiplicity of states and pathways has proved an irresistible challenge both to experimentalists and theoreticians, and the subject has acquired a momentum that perhaps outweighs its significance to catalysis. Nevertheless a rich area of surface chemistry has been disclosed, and the chemical explanation of what goes on is by no means accomplished.
And what goes on depends on (i) the molecule, (ii) the metal, and (iii) the surface geometry in the case of single crystals8,146 (to which our attention will be largely confined). A molecule such as ethene, which is the most widely studied, if chemisorbed at low temperature can on heating undergo either (a) desorption unchanged or (b) dissociation by loss of one or more hydrogen atoms, which may then attack other species with the formation of ethane. These events have been recognised from the time of the work on metal films,13,14 and the dehydrogenated species, sometimes called ‘acetylenic residues’, have since time immemorial been the curse of anyone trying to study catalysed reactions of alkenes. Knowing the cause of the problem does not however necessarily make for happiness. It is also evident that dehydrogenation of an alkene may lead to species also formed by the corresponding alkyne, although adsorption via the stronger triple bond makes for greater stability. The alkane can also by loss of hydrogen form equivalent species, although usually only at higher temperatures. The formalism of these various species was depicted for C3 hydrocarbons in Table 4.3.
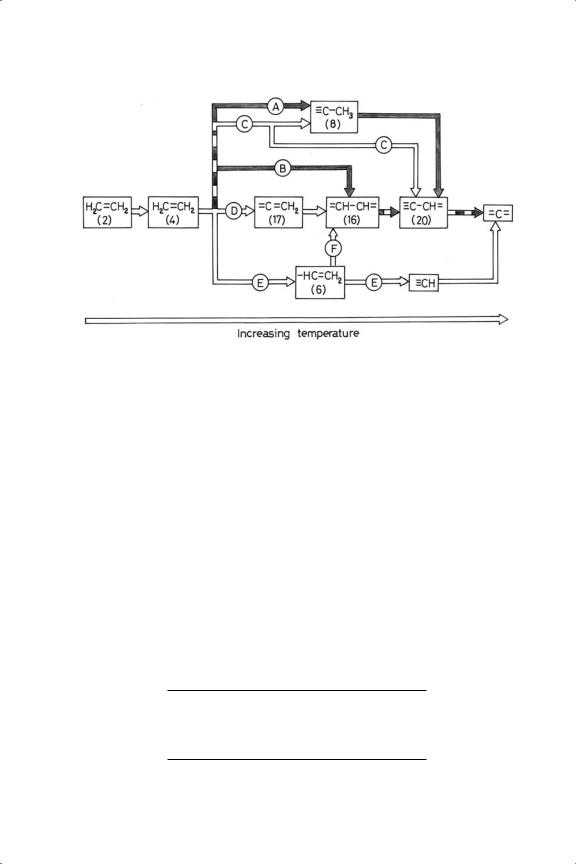
The following discussion is confined mainly to ethene. A survey of the very extensive literature (see Further Reading section) strongly suggests that the pathway of decomposition depends greatly on the arrangement of atoms in the crystal face used. On faces of trigonal symmetry (fcc(111), cph(0001), fcc(100)−(5 × 20)), and on stepped surfaces exhibiting terraces of this type, ethylidyne (8) has usually been observed at room temperature, but on Ni(111) (and the corresponding faces of iron and tungsten) di-σ ethene dehydrogenated to ethyne in the form shown in Figure 4.6: this process has been closely observed by PED147 (route B in Figure 4.9). On the square (100) plane however the bonding carbon atoms more often form only one or two σ bonds to the surface. The various processes are summarised in Figure 4.9; it is not certain that the π form always precedes the di-σ , although it may be a normal transient state as indicated in Figure 4.2. Recognised routes followed by the π form are shown in Table 4.11, together with some of the surfaces on which they occur: the egregious behaviour of palladium, already noted
188 |
CHAPTER 4 |
Figure 4.9. Reactions of chemisorbed ethene on faces of three-fold symmetry (dark lines) and on faces of four-fold symmetry (open lines): (A) Re, Ru, Rh, Pd, Ir, Pt; (B) W, Fe, Ni; (C) Rh; (D) Pt; (E) Ni, Pd; (F) Ni.
several times, is once again very evident. Rough surfaces such as the fcc(110), either in its normal (1 × 1) or reconstructed (1 × 2) form, and higher index stepped surfaces, have shown additional features (Figure 4.10). Ethylidyne could not form on Pt(110) because of overlap of the van der Waals radii of the methyl group and the metal atoms;148 this reinforces a point often ignored in the drawing of adsorbed species. Instead a further loss of hydrogen occurred via vinylidene (17) to the strange-looking isomeric form ethylylidyne (16A), which could be accommodated in (110) troughs or at the foot of steps of the right kind. The tendency to find single carbon fragments is a logical consequence of its formation. The importance of step density in the reactions of adsorbed ethene has however been questioned.149,150
Much attention has been given to the way in which di-σ adsorbed ethene (4) is transformed into ethylidyne, especially on Pt(111), and every conceivable technique, theoretical as well as experimental, has been applied to this problem (see Further Reading section). The activation energy for the conversion as measured
TABLE 4.11. Reactions of π -Adsorbed Ethene
|
C2 H4 (g) |
|
|||
−−−−→ di-σ C |
H |
4 |
|||
|
−−−−→ |
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
π -C2 H4 |
+2H |
H |
6 |
|
|
|
−−−−−H→ ? 2 |
|
|
||
|
C |
|
|
|
|
|
−−−−→ |
|
|
|
|
|
|
|
|
|
|
(i). . . Pd (100, 110, 111)
(ii). . . Pt (100)
(iii). . . Pt (110)
(iv). . . Pt (210)

THE CHEMISORPTION OF HYDROCARBONS |
189 |
Figure 4.10. Additional reactions of chemisorbed ethene on stepped surfaces: (A) Pt(110); (B) Ni(110);
(C) Pd(110), Pt(210). Note, reactions depicted in the previous figure may also occur on terraces.
by the accompanying hydrogen desorption is about 43 kJ mol−1. The arguments based on the observations verge on the theological, but it seems likely151 that there is first isomerisation to ethylidene (5) and then dehydrogenation to ethylidyne. STM revealed that its formation proceeds by island growth on a surface saturated with ethene.130 LEED structure determinations of ethylidyne have been performed on the (111) faces of rhodium111,112,152 and platinum.153 On the former it sat on an hcp trigonal hole, and the atoms to which it was attached moved away from the bulk, as did the underlying atom: the surface was therefore corrugated. On the latter it resided on an fcc hole, a difference for which there is no logical explanation. All stages of the decomposition of adsorbed ethene on Pt(111) from 160 to 800 K have been followed by STM. At 230 K the molecule coexists with ethylidyne, the ethene being ordered and the ethylidyne randomly arranged: its formation during heat proceeds by island growth.129,130
Self-hydrogenation of adsorbed ethene to ethane using hydrogen atoms released when some of the molecules decompose is apparently confined to platinum, several of whose surfaces show this reaction: on other metals, the hydrogen atoms desorb as molecules, leaving dehydrogenated species behind; these end up as carbon.146
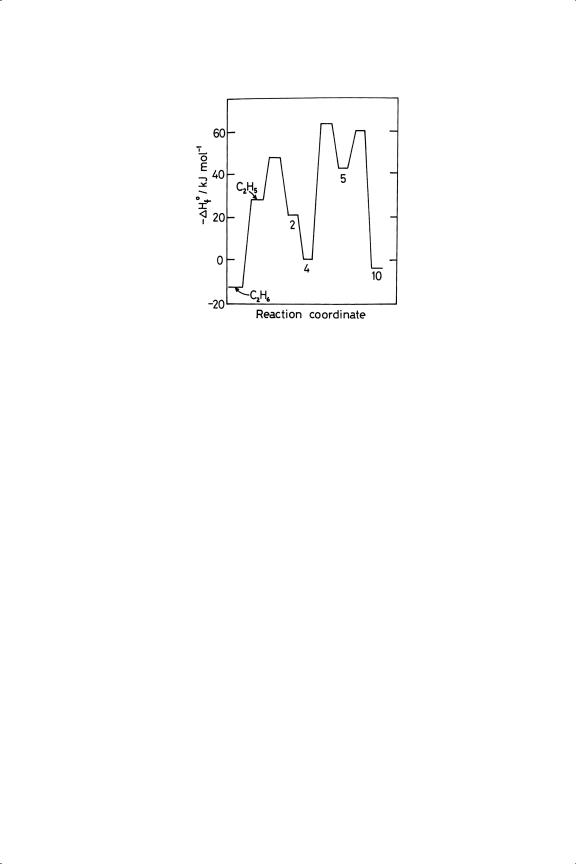
The entire energy profile for conversion of ethane through ethene to ethylidyne on Pt(111) is shown in Figure 4.11.
Formation of ethylidyne is not confined to single crystal surfaces; it has been detected on a number of supported metals, including Pt/SiO2,28,154 Pd/SiO2155, ,156 Rh/Al2O3157and even Ni/SiO2,158 and it is presumably mainly responsible for the all-too-frequently observed deactivation during the catalysis of hydrocarbon reactions. Nor does it arise only from ethene: alkylidynes have also been formed on Ni/Al2O3 from higher 1-alkenes,159 but they were unstable, and reverted to ethylidyne at higher temperatures. It is also formed from ethyne, both on supported
190 |
CHAPTER 4 |
Figure 4.11. Energy profile for the transition of C2 H6 via C2 H5 to π and di-σ C2 H4 , then to  CH––CH3 , and finally to
CH––CH3 , and finally to  C––CH3 , on Pt(111).207 Compare with Figure 4.2 which starts with gaseous C2 H4 , but does not include
C––CH3 , on Pt(111).207 Compare with Figure 4.2 which starts with gaseous C2 H4 , but does not include  CH––CH3 as in intermediate.
CH––CH3 as in intermediate.
metals28,156 and on trigonal-symmetry single-crystal surfaces,160−164 necessarily with the concomitant formation of other species in which the H/C ratio is less than two.
4.7. THEORETICAL APPROACHES16,24,165−167
There is already a very considerable literature on the application of theoretical procedures to our understanding of the chemisorbed states of hydrocarbons, and no doubt its growth is set to continue. At the outset we need to ask ourselves what expectations we have of such work, and what questions and problems we would like to see addressed. There would seem to be two different classes of question demanding attention. The first is a detailed and profound insight into the structure and bonding of individual molecules and their fragments on specific crystal planes or adsorption sites. The second is a more qualitative and broad-brush look over the whole field, to discern what factors may explain satisfactorily the trends we have observed, with particular reference to apparently anomalous behaviour. It may well be that in the course of time the questions of the second type will be answered more precisely by the quantitative methods now being applied to questions of the first type, and indeed one can see already a start in this direction.
Early work on the quantum mechanical analysis of chemisorption and chemisorbed states is admirably covered in Clark’s monograph.165 Application of the extended Huckel¨ molecular orbital method in the hands of A. B.

THE CHEMISORPTION OF HYDROCARBONS |
191 |
Anderson116,117,165,166 gave results which have broadly stood the test of time, and the procedure has been developed by R. Hoffmann to give a detailed account of the bonding of ethene to surfaces of the Group 10 metals;68 this again points to palladium being unlike the others, the ethene molecule preferring an atop site (presumably in the π -form) rather than a two-fold site. What has revolutionised theoretical chemistry is the use of density functional theory (DFT).24,168 It is unnecessary to attempt to explain it here, as the exposition would only interest its practitioners, and is in any case fully described elsewhere; the experimental chemist, lacking the skills and dare one say the interest in the ritual, has perforce to take its results on trust, while noting its limitations and uncertainties.
It is indeed a very powerful and convincing tool, and is capable of devising the most stable location of an adsorbed molecule or fragment, the type of best bonding, bond lengths and angles, and heats of adsorption119 to an accuracy of about ± 20 kJ mol−1. The computational procedure contains a number of disposable parameters, which have to be evaluated and optimised (‘calibrated’) with reference to experimental facts. Thus for example employing a ‘basis set’ that treats the valence and outermost core electrons of platinum (i.e. 5s2 5p6 5d9 6s1) explicitly, and lumps the inner electrons together as effective core potentials accounting for mass-velocity and relativistic effects, gives very satisfactory results, but including the 5s and 5 p electrons in the core potential gives worse results, because their orbitals are similar in size to those of the valence electrons. Once the calibration is done, the machinery can be applied predictively to systems for which facts are lacking. The power of the method may be shown by calculations made on a ten-atom tetrahedral cluster of platinum atoms, exposing triangular faces;119 energy minimisation however led to some distortion of the fcc structure, and the normal bond length of 277 pm was reduced to an average of 272 pm. This agrees with experimental findings on small metal particles (Section 2.4). DFT has been further applied to ethene chemisorbed on Pt(111) and Cu(111).169
Some values of the calculated parameters are given in Table 4.12; bond lengths and angles agree very well with those found, and many of the heats of adsorption are quite accurate. (The experimental heats in this Table are either taken Tables 4.8 or 4.9 or are those from which the bond energies in Table 4.8 were obtained). The di-σ form of ethene was located at a two-fold bridging site, as observed by PED on Ni(111) (Figure 4.6A); the π -form adopted an atop position on an edge atom, with the C––C axis either parallel or at right angles to the edge. This conclusion was supported by other calculations showing that this form is the more stable when an atom of co-ordination number less than seven is available,119 and supports the experimental finding of its stability on stepped surfaces (e.g. Pt(210)). In practice this species is likely to rotate freely except at very low temperatures. Ethyne was predicted to sit over a trigonal hole, making σ bonds to two atoms on one side and a π bond to an atom on the other; this is similar but not identical to the structure predicted by vibrational spectroscopy and found by PED on Ni(111). Structures

192 CHAPTER 4
TABLE 4.12. Estimated Bond Lengths (C––C) and Calculated Heats of Adsorption
(− |
Hads )24,119,187,305,343,354 and Comparison with Experimental Values23,101,133 |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
− Hads /kJ mol−1 |
|
Surface |
Structure |
Code |
C-C/pm |
Calc. |
23 |
101,133 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pt(111) |
di-σ -C2 H4 |
4 |
150,148 |
171,122 |
136 |
120 |
||||||||||||
Pt(111) |
π-C2 H4 (i) |
2 |
141 |
103 |
120 |
— |
||||||||||||
Pt(111) |
π-C2 H4 (ii) |
2 |
141 |
137 |
|
|
||||||||||||
Pt(111) |
CH3 ––C |
|
|
|
|
|
|
|
8 |
152 |
127 |
125 |
115 |
|||||
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
||||||||||||
Pt(111) |
Di-σ ,π-C2 H2 |
13 |
141 |
209 |
— |
210 |
||||||||||||
Pt(111) |
Di-σ ,π-C2 H2 |
18 |
144 |
278 |
290 |
— |
||||||||||||
Pt(111) |
H2 C |
|
|
|
C |
|
|
|
|
|
17 |
134 |
262 |
— |
— |
|||
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|||||||||||||
Pt(111) |
π- C2 H2 |
11 |
125 |
88 |
— |
— |
||||||||||||
Pt(111) |
H2 C |
|
|
CH–– |
6 |
134 |
49 |
— |
— |
|||||||||
|
||||||||||||||||||
|
|
|
||||||||||||||||
Pt(111) |
−CH2 ––CH |
|
|
6A |
150 |
78 |
— |
— |
||||||||||
|
|
|||||||||||||||||
|
|
|
|
|||||||||||||||
Pt(111) |
CH3 ––CH |
|
|
5 |
151 |
87 |
— |
— |
||||||||||
|
||||||||||||||||||
|
|
|
|
|
||||||||||||||
Pd(111) |
Di-σ -C2 H4 |
4 |
150 |
48,60 |
— |
— |
||||||||||||
Pd(111) |
π- C2 H4 |
2 |
142 |
34,30 |
— |
— |
||||||||||||
The two forms of π -C2 H4 vary in the orientation of the C––C axis (see text). Experimental heats are corrected for the adsorption of hydrogen atoms where dissociation occurs, assuming − Hads (H2 ) = 90 kJ mol−1 .
for vinylidene (di-σ π (18) and di-σ (17)), for ethylidyne (8) and for C1 species have been advanced, with characteristics close to those observed (see Table 4.12). Such is the confidence now attending these calculations that the probable accuracy of a LEED structure for ethene on Pt(111) can be queried.24
While a Pt10 cluster of this type is a not unreasonable model for a small metal particle, since particles of this size or even smaller do indeed show catalytic behaviour, it may not be quite large enough to simulate a single crystal surface. The procedure only examines one species at a time, and so the manner of their packing, and possible overlap of van der Waals radii as a factor in determining structure,124 are not considered. The latter was shown to be important in the behaviour of ethene on Pt(110).8 It also identifies the most stable site, although not ignoring others that may be relevant in catalysis. A triangular six-atom face is also not large enough to accommodate species such as 16 (di-σ, di-π ethyne). A further problem for the chemist is the translation of the results into a molecular orbital description, as the orbital structure of the cluster lies buried deep within the computational procedure and is reluctant to emerge (see however references 68, 99). These comments do not demean the value of DFT in these systems, and present limitations may well be overcome in future developments.
Recent work on DFT has concentrated on the metals of Group 10 (Ni, Pd, Pt) and on C1 and C2 species (see Further Reading section). Di-σ ethene on Pd(111) is reported as showing a low binding energy, that for the π -form being even lower (see Table 4.12), but the distinction appears to be less marked at low coverage. Other metals have been less studied (for CH3 . . . Rh6, see reference 170) and faces
