
Metal-Catalysed Reactions of Hydrocarbons / 04-The Chemisorption of Hydrocarbons
.pdf
THE CHEMISORPTION OF HYDROCARBONS |
173 |
then be connected to bond lengths using Badger’s rule. Bond lengths so obtained are in fair to very good agreement with those measured directly; they of course correlate with bond orders, but it is better to derive orders via an empirical relation with force constants, namely,
BCC = (kCC /4.2)0.69 |
(4.1) |
Values so obtained are listed in Table 4.6.
Another approach has also been tried. In moving from a π to a di-σ structure, there is also a decrease in the C––C vibration frequency and also in the CH2 scissors (δ) mode.72,96,97 In hydrogen-containing molecules these are strongly coupled and are difficult to disentangle, so it is preferable to use spectral data for deuterated molecules where coupling is weaker. It is then possible to derive a π σ parameter96 defined as the sum of the fractional decreases of the two bands divided by 0.366, a factor which normalises the expression so that for free ethene (C2H4) it is zero and for C2H4Br2 it is unity. Values of this parameter obtained for deuterated molecules (π σ -D) are also given in Table 4.6, and Figure 4.4 shows there to be a reasonable correlation between it and BCC. For many cases there is good agreement between π σ-H and π σ-D96 (e.g. for Zeise’s salt, 0.38 and 0.35 respectively), but in a few cases (e.g. Ni(111) and Fe(110)) there are quite large and unexplained differences. Values of π σ-H shown where necessary in Table 4.6 are in brackets. Ethene co-ordinated to Ag+ is only weakly perturbed, π σ-H being 0.12. Although the ethene C––C bond orders for palladium and platinum are similar on (110) faces, there is a strong propensity for the di-σ structure to be formed on Pt(111) and for the π structure to arise on Pd(111). Examination of 1-butene and 1,3-butadiene by NEXAFS at 95 K on these surfaces has clearly confirmed this.98 Recently published values for Rh(100)74 and Pd(111)82 are in substantial disagreement with those obtained earlier; like the clock that strikes thirteen, they cast an element of doubt on what has gone before.
The validity of this procedure therefore merits some consideration. While it is accepted that a π -bonded species can be comfortably accommodated on a single metal atom, the di-σ species (4) demands two atoms, and it is difficult to envisage structures that are intermediate between them. Papers that deal with π σ parameters carefully avoid drawing structures for the intermediate forms, and discussing how many metal atoms might be needed for their chemisorption. Of course if the di-σ form were actually the metallocyclopropane (3), all would be well, because the analogy with alkene complexes would apply. However, vibrational spectroscopy recognises the di-σ state (4) more certainly than the alternative (3), as the Type A spectrum by which it is defined may be a mixture of π and di-σ forms:17,51 but it may also be a kind of half-way house between them, occurring when there is an increased opportunity for back-donation of d-electrons to enhance the covalent element of the bonding. A further increase of back-donation, and weakening of the

174
TABLE 4.6. Bond Orders (BCC ) and π σ Parameters for C2 D4 (for C2 H4 in brackets) Chemisorbed on Various Single Crystal Surfaces8,66,72,96
Group 8 |
|
|
|
Group 9 |
|
|
|
Group 10 |
|
|
Group 11 |
|
||
Face |
BCC |
π σ |
|
Face |
BCC |
π σ |
|
Face |
BCC |
π σ |
Face |
BCC |
π σ |
|
Fe(110) |
1.20 |
1.00 |
|
|
|
|
|
Ni(111) |
1.33 |
1.04 |
|
Cu (100) |
1.66 |
0.27 |
|
|
(0.55) |
|
|
|
|
|
|
|
(0.80) |
|
|
|
|
Fe(111) |
— |
0.86 |
|
|
|
|
|
Ni(110) |
1.41 |
0.66 |
|
Cu* |
— |
(0.19) |
Ru(001) |
1.35 |
0.78 |
Rh(111) |
1.52 |
0.47 |
|
Pd(100) |
1.38 |
0.70 |
|
Ag* |
1.88 |
(0.09) |
|
|
|
(0.85) |
|
|
|
|
|
Pd(110) |
1.61 |
0.38 |
|
O . . Ag (100) |
— |
(0.14) |
O . . . Ru(001) |
— |
0.42 |
Rh(100) |
1.16 |
0.62 |
|
Pd(111) |
1.61 |
(0.43)(0.87) |
|
|
|
||
|
|
|
|
|
|
(0.39) |
|
O .. Pd(100) |
— |
(0.30) |
|
|
|
|
|
|
|
|
|
|
|
|
Pt(111) |
1.13 |
0.88 |
|
Au* |
— |
(0.25) |
|
|
|
|
|
|
|
|
Pt(110) |
1.51 |
— |
|
|
|
|
|
|
|
|
|
|
|
|
Pt(210) |
1.67 |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)M represents film or foil.
(2)There are no data yet for cobalt, osmium or iridium.
(3)The symbol O . . . refers to a surface partly covered by oxygen.
4 CHAPTER
THE CHEMISORPTION OF HYDROCARBONS |
175 |
σ -component, may cause the structure to flip over to that of the two-atom di-σ (4) as being more comfortable in this way, and making perhaps a greater contribution to the relief of surface stress.
The final word on this problem cannot yet be written, but it seems clear that there are species in which the character of the C––C bond is neither substantially double (as in Zeise’s salt) nor single as in ethane. There are indeed considerable variations in both Types A and B vibrational spectra, indicating some flexibility in the kind of structure that can be formed. It seems we must accept that there may be at least two or three different structures co-existing or being capable of being formed at different sites and under different conditions, and that sometimes there may be structures that cannot be reconciled with simple notions of chemical bonding (see Section 4.5.1). This is a situation that does not make the formulation of reaction mechanisms any easier. Two quotations from the literature are apposite to conclude this discussion.
The molecular bonding of ethene is a sensitive probe for the steric and electronic differences between transition metal surfaces.72
As all Transition Metals are characterised by their own peculiarities, extreme care has to be exercised when proposing general principles of chemisorptive bonding.99
Grateful thanks are due to Professor Norman Sheppard for his advice and assistance in drafting this Section.
There is much less information on hydrocarbon chemisorption on alloy surfaces:95 two platinum-tin surfaces analogous to Pt(111), namely, PtSn(2 × 2) containing 25% tin in the surface, and PtSn(√3×√3)R30◦ having 33% tin, have however been examined100 for alkene adsorption. The former has sites of five platinum atoms, and the latter three-atom sites (see Figure 4.5). Tin concentration did not much affect initial sticking probability for either ethene, propene or isobutene, and saturation coverages were similar to those for Pt(111). What was however very marked was the progressive weakening of the adsorption with increasing tin content, due to the loss of trigonal sites, and the extent of dehydrogenation during thermal decomposition was much reduced: in fact all three alkenes desorbed unchanged from the 33% tin surface. There was no sign of
Figure 4.5. Structures of various platinum-tin alloy surfaces having (111) geometry.206 (A) (111) PtSn(2 × 2), with the probable location of an ethene molecule; (B) (111) PtSn(√3 × √3)R30◦; (C) (111) Pt2 Sn(√3 × √3)R30◦.

176 |
CHAPTER 4 |
inductive effects of the methyl substituents on adsorption strength. A structure for ethene on the 25% tin surface was proposed (see Figure 4.5). On silicasupported PtSn bimetallic particles, the π form of ethene was seen at 25% Sn, while at 12.5% tin the diatomic sites needed for the di-σ form occurred more often, and this was then the only structure seen101 (see also Section 4.5 and Table 4.10).
The adsorption of ethene and ethene-d4 on Pt3Cu(111) at 95 K gave both the π and di-σ forms, bonding as for Pt(111): they continued to exist in equilibrium and to desorb unchanged.102 The surface composition was actually Pt85Cu15, and there was no evidence for any ligand effect. It may however be that the π form resides on the copper and the di-σ on the platinum, as was suggested by similar work on the p(1 × 1)CuRh(111) surface, and on partial overlayers of gold or copper on ruthenium. Peculiar behaviour was shown by the platinum-rich Pt50Ni50(111) surface, which seemingly failed to adsorb ethene under conditions where Pt(111) does so readily. The behaviour of the PdCu(111) surface has also been compared with Pd(111) using EELS.103
To conclude this section, we should note what has not been discussed – and why. The emphasis has been on trying to understand how the nature of the surface metal atoms influences the type of hydrocarbon structure formed, and although there is a great deal of information available on the structures formed by higher and cyclic alkenes, alkadienes, alkynes and benzene, some of which will be touched on below, it is only with ethene that a sufficiently wide range of surfaces has been investigated to make comparisons between them possible. A further omission, to be remedied in Section 4.7, is any attempt to provide a theoretical basis for the observations any more profound than that outlined above. The reason for this delay is that further useful information comes from studying the detailed structures of certain adsorbed molecules (Section 4.5) and the manner of their thermal decomposition (Section 4.6).
4.5.STRUCTURES AND PROPERTIES OF CHEMISORBED HYDROCARBONS104−110
In this Section we shall consider (i) detailed structure determinations of ethene, ethyne and benzene, (ii) measurements of the heats of chemisorption of hydrocarbons on single crystals and small particles, and (iii) their spectroscopic characterisation.
4.5.1. Detailed Structures of Chemisorbed Alkenes
The structures displayed in Tables 4.2 and 4.3, and discussed above, are usually qualitatively identified by vibrational spectroscopy using pattern recognition
THE CHEMISORPTION OF HYDROCARBONS |
177 |
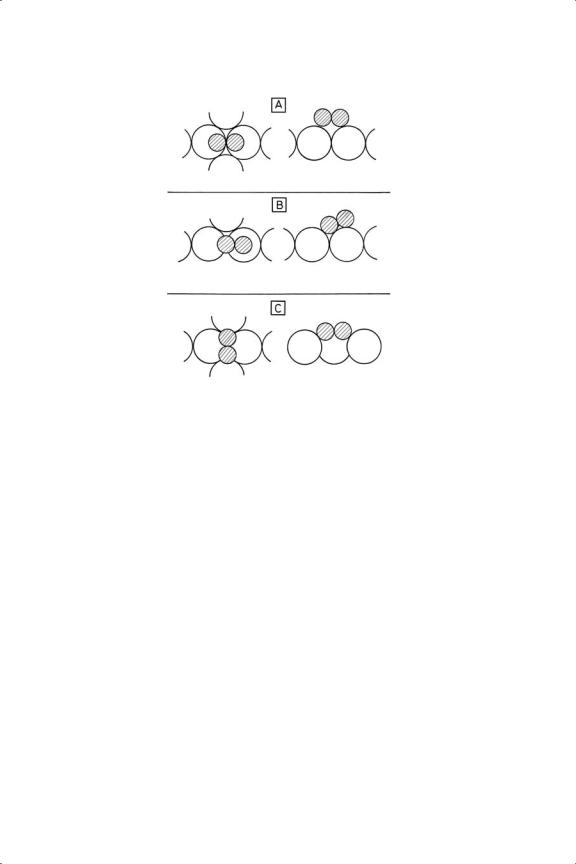
Figure 4.6. Experimentally determined structures of ethene and ethyne chemisorbed on various sin-
gle crystal surfaces. (A) Ethene on Ni(111) by PED;20,116 (b) ethene on Ni(110);21 (C) ethyne on Ni(111).21,50,147
involving frequencies and intensities; there is no attempt to define the exact location of the carbon atoms with respect to the lattice of metal atoms with which they are in contact. It is possible to do this by photoelectron diffraction (PED; see Section 4.3) and by LEED intensity analysis.111 Results are available for ethene on Ni(111)20 and (110),100 and Pt(111),112,113 and for ethyne on Ni(111), Pd(111) and Cu(111), and the important conclusions are shown diagrammatically in Figure 4.6. While these techniques reveal the structures with considerable certainty, the precise C––C bond length is sometimes difficult to pin down, it sometimes appearing to be even longer than the bond in ethane. In the case of ethene on Ni(111) there was a 7% expansion of the Ni––Ni distance between the first and second layers. With ethene on Ni(110)21 the C––C axis seemed to be not exactly aligned with the rows of nickel atoms, nor were the carbon atoms located directly above the nickel nuclei. There was an interesting long-range order, dictated by the need to minimise overlap of the van der Waals radii. While some structures conform to traditional models, that is, they may safely be described as di-σ states, that found on Ni(110) does not fit easily into the canon of chemisorbed states as shown in Table 4.6, nor would an MO description of its bonding be an easy matter. On Pt(111) there were two non-equivalent sites, depending on whether or not there was a platinum atom below the trigonal hole over which one of the carbon atoms

178 |
CHAPTER 4 |
sat, but the two structures are very similar. It appears that atoms in the second layer do not contribute significantly to the chemisorption bonds. Ethene π -adsorbed on Pt(111) saturated with the di-σ form had its plane tilted with respect to the surface, but the C C bond was parallel to it.90
C bond was parallel to it.90
4.5.2. Structures of Chemisorbed Ethyne
The chemisorption of ethyne has commanded somewhat less attention than that of ethene, although there have been numerous studies of it using vibrational spectroscopy, LEED, UPS and other techniques.40,50,114−118 Table 4.2 shows a number of the structures that have been proposed, together with some organometallic analogues. Much experimental evidence suggests that on Pt(111) the lowtemperature form is likely to have the di-σ /π structure 13; the C––C bond has stretched from 120 pm to about 135 pm, i.e. intermediate between the lengths of single and double bonds, and the linearity of the molecule has been lost, giving a C––C––H angle of approximately 125◦.119 There is also some evidence for the occurrence of the purely π forms 11 and 14.50 Further structural input comes from PED;122 structures have been devised for ethyne on Ni(111),120 Pd(111)121 and Cu(111)122 (see Figure 4.6). Calorimetric work, to be considered in the next section, latter also contributes information on thermal stability, being performed at 300 K. Little ethyne desorbed unchanged from Pt(111) on heating, it mainly decomposing to carbon and gaseous hydrogen, but introduction of tin into this surface diminished the disruption of the molecule, partially in the case of Pt3Sn p(2 × 2) and almost totally with Pt2Sn(√3 × √3)R30◦. Quite unexpectedly the major product on this latter surface was then benzene, with some butadiene as intermediate; this may be due to there being a hexagon of platinum atoms surrounding each tin atom123 (see Figure 4.5). The conversion of ethyne to benzene on palladium surfaces will be considered in Chapter 9. No attempt seems yet to have been made to apply the π σ formalism in a quantitative way to the forms of chemisorbed ethyne.
RAIRS studies of propyne on Ni(111) and on Cu(110) showed that addition of the methyl group made little difference to the interaction of the triple bond to the surface, the adsorbed states being well described by structure 15 in Table 4.2.
4.5.3. Structures of Chemisorbed Benzene
Although in the earlier sections we have omitted mention of the chemisorption of benzene and other aromatic molecules so as not to muddy the waters unnecessarily, there have been a number of structure determinations of chemisorbed benzene that are conveniently described at this point. On surfaces of trigonal symmetry (i.e. fcc (111) and cph (001)) the molecule sometimes forms disordered overlayers, which can be forced into long-range order by co-adsorbing carbon
THE CHEMISORPTION OF HYDROCARBONS |
179 |
monoxide112 or nitric oxide.124 The general conclusion is that it remains at most only slightly distorted, that is, essentially flat and parallel to the surface,40 but several studies appear to show an alternation in the C––C bond lengths, i.e. a change towards a cyclohexatriene structure, with a simultaneous buckling of the plane of the ring125 (but not on Pd(111)112). On Pt(100)(2 × 1) this form was seen125 at low coverage, and reacted with hydrogen; a less distorted planar form succeeded it at high coverage, but was not reactive. Similar results were obtained with Pt(111).126 The existence of different forms at different coverages (as with ethene) may help to explain some of the discrepancies that are found in the literature. Once again however the C––C distances can rarely be found exactly, and the differences in bond lengths are often within experimental error. An exception is the case of Ni(110) where bond lengths are measurable by PED with unusual precision; they were all the same (145 pm) and only a little longer than in the
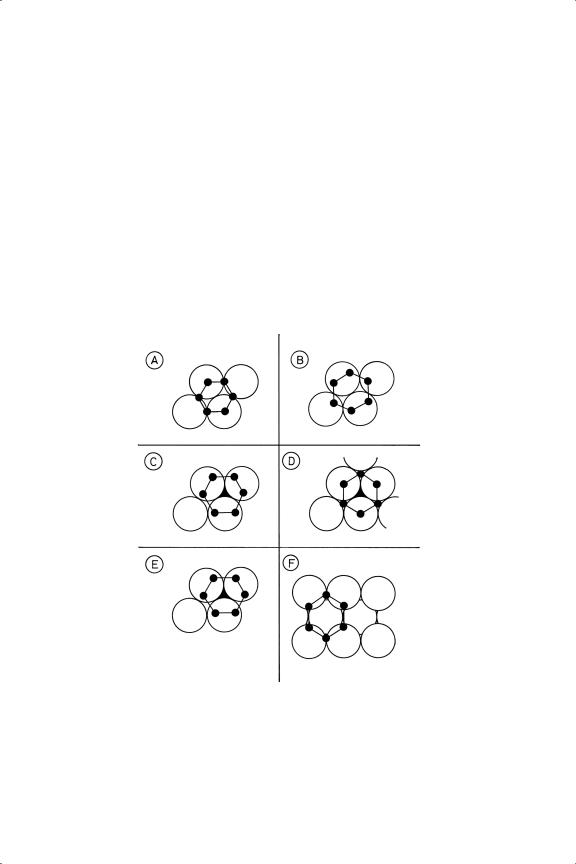
Figure 4.7. Experimentally determined structures of benzene chemisorbed on various single crystal surfaces. (A) On Pt(111) (disordered);111 (B) on Pt(111) (ordered with co-adsorbed CO);111 (C) on
Rh(111) (ordered with co-adsorbed CO);112 (D) on Ru(001);112 (E) on Ni(111) (ordered with coadsorbed NO: the same structure is found without NO);112,124,127,128 (F) on Ni(110).22 In (C), (D) and
(E) the molecules are adsorbed over a cph site.

180 |
CHAPTER 4 |
free molecule (139 pm). The structures shown in Figure 4.7 reveal that on the close-packed surfaces of nickel, ruthenium, rhodium and palladium the centre of the benzene molecule lies directly over an hcp hole whereas on platinum it is over a bridge site. There have been three determinations of the structure of benzene on Ni(111), two by LEED112,127 and one by PED;128 they agree on the orientation of the molecule (Figure 4.7), but not entirely on the extent of bond length alternation or on the amount of distortion of the nickel surface layer, which is more at low coverage.128 The very long C––C distances found by LEED are not supported by vibrational spectroscopy measurements. The orientation was different on Ru(001), where the larger atomic radius allowed a mode of packing that is forbidden with
¯
the smaller nickel atom.128 An unusual structure was observed on Co(1010). Benzene molecules have also been individually resolved by STM: on Rh(111) when co-adsorbed with carbon monoxide at 4 K, they formed a (3 × 3) structure in which two different sites could be distinguished. They have also been observed on Pt(111), Cu(111) and Pd(110).129,130
For further references to work on chemisorbed benzene, see the Further Reading section at the end of the chapter.
4.5.4. Heats of Adsorption
The measurement of heats of adsorption has a long and honourable history. The first calorimetric results for hydrocarbons were obtained by Otto Beeck using condensed metal films,13,14 but the worth of such results is limited if the structure of the adsorbed species is not known and the exact process to which the heat release relates cannot be defined. The recent development of a calorimetric technique applicable to very thin single crystals (Section 4.3) is significant because simultaneous measurement of sticking probability is possible, and plots of differential heat versus coverage often show more or less well-defined plateaux (see Figure 4.8 for an example), which by comparison with other methods (LEED, HREELS etc.) permit identification of the adsorbed structures formed at various coverages with high confidence. This allows estimation of the bond strengths of covalent C––M bonds in each type of species, with the aid of assumed values for C––C, C––H and M––H bond strengths (see Table 4.7) and certain plausible assumptions, at least in those cases where there is no π component.
It was hinted above that one of the factors determining what structure is formed is in fact surface coverage, and the importance of the calorimetric method is that it informs about the sequence in which adsorbed states are formed as coverage increases at constant temperature. The most reactive sites are first used to take the most strongly held species, utilising the greatest number of C––M bonds, in line with the principle proposed in Section 4.2; progressively more weakly-held species form as coverage rises (Figure 4.2).90 The information obtained is thus complementary to that obtained in the study of thermal decomposition (Section 4.6),
THE CHEMISORPTION OF HYDROCARBONS |
181 |
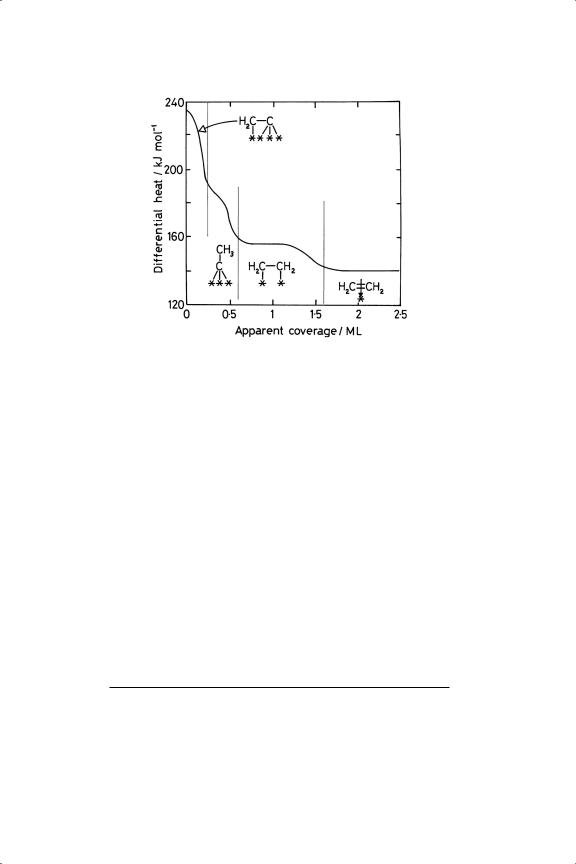
Figure 4.8. Dependence of heat of adsorption of ethene upon coverage for the Pt(110)(1×2) surface; the structures thought to be formed at each stage are shown.23
but the order of events is reversed; things seen predominantly at low temperature are those found at high coverage at room temperature, where only sites of low reactivity remain. Since catalytic reactions usually involved fully occupied surfaces, the relevance of UHV studies at low temperature is reinforced.
Results obtained for the chemisorption of ethene at 300 K on a number of single surfaces are given in Table 4.8. Initial sticking coefficients are generally high (0.6–0.8), while initial heats are variable between 120 and 305 kJ mol−1; the Pd(100) surface has however been distinguished from the rest by only being able to adsorb ethene reversibly at 300 K, so that no adsorbed structure could be determined and no decomposition took place.131 In many cases, dehydrogenated species such as ethylidyne (8) or ethylylidyne (16A) or tetra-σ -ethyne (16) are first formed: the ethenylidyne structure (22) was also proposed, although the disposition of the bonds about the carbon atoms can only be accommodated by the surface
TABLE 4.7. Average Bond Strengths (kJ mol−1 ) Used for
Calculating the C––M Bond Strengths Shown in Table 4.8
C |
|
|
C |
962 |
Ni––H |
266 |
|
||||||
|
|
|||||
|
|
|||||
C |
|
|
C |
733 |
Rh––H |
255 |
|
|
|||||
|
||||||
C––C |
376 |
Pd––H |
270 |
|||
C––H |
412 |
Pt––H |
250 |
|||
|
|
|
|
|
|
|
The C––H bond strength depends on its environment.

182
|
TABLE 4.8. |
Initial Sticking Probabilities (σ0 ) and Heats of Adsorption (− H ) of Ethene at 300 K: |
|
|
||||||||||||||||||||||||||
|
|
Species Identified at Various Stages of Coverage and Corresponding C––M Bond Strengths |
|
|
|
|||||||||||||||||||||||||
Surface |
σ0 |
− H0 |
− Hfin |
Species proposed |
Code |
DCM Species proposed |
Code |
DCM |
Code |
DCM |
Code |
Reference |
||||||||||||||||||
Ni (100) |
0.82 |
203 |
100 |
|
|
|
|
|
|
CH |
— |
204 |
−−−−→ CCH |
21,22 |
— |
— |
— |
— |
131 |
|||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||
Ni (110) |
0.78 |
120 |
80 |
|
|
|
|
|
CCH |
21,22 |
204 |
−−−−→ |
? |
— |
— |
— |
— |
— |
133 |
|||||||||||
Rh (100) |
0.88 |
175 |
100 |
|
––HC |
|
C |
|
|
|
|
22 |
268 |
−−−−→ |
? |
— |
— |
— |
— |
— |
132 |
|||||||||
|
|
|
||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||
Pd (100) |
0.75 |
73 |
— |
|
|
|
|
|
— |
— |
— |
|
|
|
|
— |
— |
— |
— |
— |
— |
131 |
||||||||
Pt (100) |
0.71 |
305 |
135 |
|
|
|
CH––CH |
|
|
16 |
246 −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−→ 4 |
|
253 |
2 |
310 |
|||||||||||||||
|
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
||||||||||||||||||||||||||
Pt (111) |
0.67 |
195 |
80 |
|
|
|
|
|
C––CH3 |
8 |
238 −→ |
|
|
CH––CH3 |
5 |
250 −−−−−−−−−−−−−−→ 2 |
23 |
|||||||||||||
|
|
|
|
|
|
|
||||||||||||||||||||||||
Pt (100)hex |
0.75 |
213 |
130 |
|
|
|
CH––CH |
|
|
16 |
223 −→ |
|
|
C––CH3 |
8 |
230−−→4 |
−−−−−−−−→ 2 |
310 |
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
Pt (110)(1× 2) |
0.85 |
235 |
140 |
|
|
|
|
|
C––CH2 |
16A |
229 −→ |
|
|
C––CH3 |
8 |
239−−→4 |
−−−−−−−−→ 2 |
23 |
||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
||||||||||||||||||||||||
Pt (211) |
0.84 |
180 |
110 |
|
|
|
CH––CH |
|
|
16 |
262 −→ |
|
|
C––CH3 |
8 |
274−−→4 |
−−−−−−−−→ 2 |
311 |
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
Pt (311)(1× 2) |
0.84 |
220 |
80 |
|
|
|
|
|
C––CH2 |
16A |
273−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−→ 2 |
311 |
||||||||||||||||||
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
||||||||||||||||||||||||||
− H0 is the initial and − |
Hfin the final heat (measured at high coverage). The species named in the first section are those formed first, the arrows printing to |
|
||||||||||||||||||||||||||||
species formed at progressively higher coverages. In some cases the final heat is that due to ethene chemisorption in the π -form (2) and the penultimate form to the di-σ form (4).
Only reversible adsorption occurs on Pd(100), so the adsorbed state cannot be identified: it may be either the π or di-σ form or a mixture of the two. On the nickel surfaces, the species CCH could not be identified certainly; it may be either 21 or 22. On Ni(110) and Rh(100) no further species could be determined.
4 CHAPTER
