
Metal-Catalysed Reactions of Hydrocarbons / 04-The Chemisorption of Hydrocarbons
.pdf
4
THE CHEMISORPTION
OF HYDROCARBONS
PREFACE
We have considered at some length the ways in which hydrogen can interact with metals and supported metal catalysts, and with itself when isotopic analogues are used, and because our main theme is the reactions that hydrogen undergoes with hydrocarbons it is logical that we should next examine how they interact with metal surfaces. The great variety of types of hydrocarbon (alkanes, alkenes, alkynes, aromatics etc.) make this potentially a much larger subject than that of the last Chapter, and a further complication is that the forms adopted by a specific hydrocarbon depend on the nature of the metal, the crystal plane, the particle size, and particularly the temperature. These factors have acted as a challenge to experimentalists and theoreticians, and there is a very extensive literature, on which it will be necessary to try to impose some order; selection and compression will also be called for.
Our aim will be to try to identify those adsorbed species most likely to be intermediates in the reactions we shall meet later, and to recognise species derived from the original molecule that may inhibit the desired reaction, or indeed be required for reaction under more forcing conditions. In some cases the structure of the relevant intermediate looks like that of the product, and, just as with hydrogen, the essential part of the process is accomplished in the chemisorption. The very precise and detailed information available on the structures of certain adsorbed species and their effects on the arrangement of surface metal atoms will excite our admiration.
153
154 |
CHAPTER 4 |
4.1. INTRODUCTION
4.1.1. Types of Alkane1
The principal structural types of alkane are (i) linear, (ii) branched, (iii) cyclic and (iv) polycyclic, and two or more of these may appear within a single molecule. Almost all the work done on their chemisorption has been with the lower members of the linear series, i.e. methane and ethane.
4.1.2. Types of Unsaturated Hydrocarbon
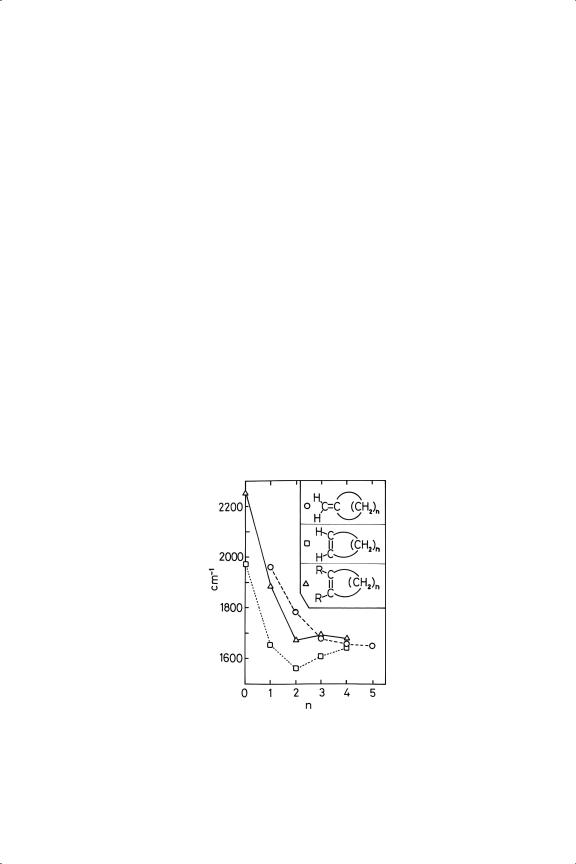
There are three basic types of carbon-carbon unsaturation, namely, (i) the double bond as in alkenes, (ii) the triple bond as in alkynes, and (iii) the aromatic bond as in benzene. If this were the end of the story, chemistry would be simpler but less interesting. The properties and reactivity of the double bond in particular are sensitive to its environment; when it is exocyclic, i.e. one of the sp2 carbons forms part of an alicyclic ring, its stretching frequency changes by about 130 cm−1 as the size of the ring is increased from three to six, while when endocyclic it hardly changes at all unless there are other alkyl substituents present (see Figure 4.1). These various differences are no doubt explicable in terms of electron delocalisation (which used to be called hyperconjugation). The carbon-carbon frequency of the triple bond increases markedly with alkyne substitution, but the vibrations in the aromatic ring are less dependent on substituents.
Figure 4.1. Dependence of C C vibration frequency in cyclic alkenes on ring size for the structures shown.
C vibration frequency in cyclic alkenes on ring size for the structures shown.

THE CHEMISORPTION OF HYDROCARBONS |
155 |
The double bond may also be conjugated with another, and in the series propadiene (allene), 1,3-butadiene, 1,4-pentadiene etc. the extent of interaction decreases as the number of single bonds between the points of unsaturation increases. So, as we shall see later, in catalysed reactions propadiene and 1,3-butadiene often resemble the corresponding alkynes. There is a conjugation between double and triple bonds in ethenylethyne, and between these bonds and the aromatic ring in, for example, styrene (phenylethene) and phenylethyne.
The cyclopropane ring represents an interesting intermediate between alkanes and alkenes. Theoreticians have had great fun with this molecule, for which no simple (i.e. non-wave mechanical) picture is satisfactory. There is certainly some electron density in the middle of the ring, and it can be hydrogenated to propane quite easily, although less easily than propene. Alkyland alkenylcyclopropanes react in interesting ways (Chapters 7 and 11).
4.1.3. The Literature
In view of the almost endless ways in which carbon-carbon unsaturation can manifest itself, it is perhaps fortunate that students of chemisorption have confined themselves in the main to looking at only a few molecules and only a few metals. Nevertheless the interactions of these few molecules especially with single crystal surfaces have proved an irresistible attraction,2,3 and a quite enormous literature developed, particularly in the late 1970s and throughout the 1980s. Interest has diminished since, although the lesser frequency of publication is compensated by the occasional use of more profound and informative experimental techniques. Nevertheless the fact that review articles4,5 published in 1995 and 1996 contain respectively 899 and 480 references underscores that magnitude of the task of summarising what is known in the space of a few pages.
It is worth noting the outstanding features of the relevant literature. Of the alkenes, at least 90% of papers deal only with ethene, and less than 10% with higher alkenes; the great simplicity of the ethene molecule, with only one kind of carbon-carbon and carbon-hydrogen bond, is at once its usefulness and limitation, because like so many first members of homologous series it is quite atypical. Similarly there are hardly any papers dealing with the higher alkynes, or for that matter with dienes. Of the metals, by far the most work has been done with platinum, presumably because of its importance in petroleum reforming: there is no obvious scientific explanation. Nickel, palladium and rhodium also feature substantially, but other metals of Groups 8 to10 have not had corresponding attention. In consequence there is a very good understanding of the chemisorption and reactions of a few hydrocarbon molecules with platinum, but with other metals of interest our knowledge is more sketchy. Nevertheless we are not short of material to discuss.

156 |
CHAPTER 4 |
4.2. THE CHEMISORPTION OF HYDROCARBONS: AN OVERVIEW6,7
The purpose of this section is to try to construct a map to guide us through the jungle that is the literature on the chemisorption of hydrocarbons, to evolve - if indeed it is possible - some general principles, to delineate the main features, and to outline the strategy to be adopted in later sections.
The study of the interactions of hydrocarbons with metal surfaces proceeds in the following way. At very low temperatures, molecules are physically adsorbed without significant effects on their structure: this state has very little relevance to catalysis, and may be passed over quickly. Unsaturated molecules are chemisorbed on metals of Groups 8 to 11 in many different ways: at low temperatures and higher pressures, forms are seen in which there is minimum disruption of the multiple bond, the bonding being due to overlap of π orbitals with orbitals projecting from the metal. Next there are forms in which one or more of the multiple bonds is undone, and σ-bonds between carbon and metal are formed. Both of these types are non-dissociative and are very probably those that are reactive in catalytic reactions such as hydrogenation.
The major difficulty in this field is the ease with which carbon-hydrogen bonds are broken, even at quite low temperatures, but progressively as temperatures are increased. Much is known about the routes followed in these thermal decompositions4,5,8−10 (Section 4.6); the species formed may be toxic to some catalytic reactions, but they may also be relevant intermediates in reactions such as hydrogenolysis that require higher temperatures. The ultimate fate of all adsorbed hydrocarbons is some type of carbon on the surface, and hydrogen in the gas phase (Section 4.8). With base metals, carbon atoms can easily dissolve to form a carbide phase; ethene seems likely to react at high temperature only with large palladium particles,11 and with Pd6 clusters it did not react at all at 323 K.12 As we shall see (for example in Chapter 9), small amounts of dissolved carbon have significant effects on catalytic reactions.
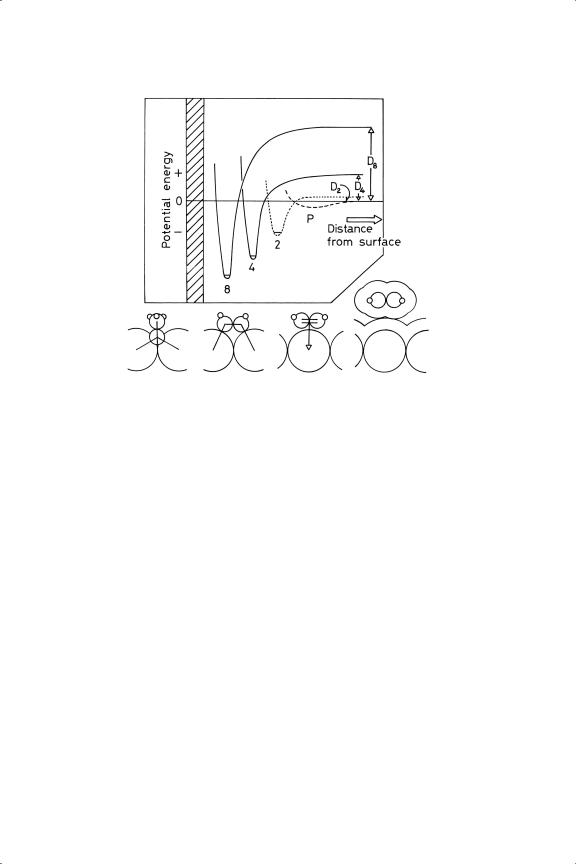
The motivation for these changes is of course thermodynamic in origin, and parallels those found in the gas phase, but in the presence of a metallic surface there are the additional factors of (i) the stability of the gaseous hydrogen molecules compared to adsorbed atoms at high temperature, and (ii) the lowering of the surface free energy by the formation of carbon-metal bonds. One guiding general principle may be therefore stated as follows. Provided sufficient thermal energy is available to overcome any activation barrier, those changes will occur to an adsorbed hydrocarbon species that lead to the formation of the greatest number of chemical bonds to the metal, and hence to the greatest reduction of surface free energy. This principle can be illustrated by a system of potential energy curves (Figure 4.2), and we shall encounter many examples of it in due course. Reactions within the chemisorbed layer may lead to gaseous products
THE CHEMISORPTION OF HYDROCARBONS |
157 |
Figure 4.2. A simplified schematic picture of potential energy curves describing the adsorption of ethene in a typical metal surface as being physically adsorbed P, π -adsorbed(2), σ -diadsorbed (4) or in the form of ethylidyne ( C––CH3 , 8). The numbers are those used in Table 4.2. The exact locations of the curves with respect to each other and to the zero of potential energy will differ from one system to another, and further intermediate forms may sometimes appear (see text).
C––CH3 , 8). The numbers are those used in Table 4.2. The exact locations of the curves with respect to each other and to the zero of potential energy will differ from one system to another, and further intermediate forms may sometimes appear (see text).
other than hydrogen: as has long been known,13,14 ethane may be a product of the chemisorption of ethene, but such reactions are not catalytic, because a dehydrogenated species is left behind blocking the surface. Reactions of a chemisorbed hydrocarbon by and with itself are for this reason treated in this chapter (Section 4.6).
The next section will deal briefly with experimental techniques: many of these have been introduced already, but the use of vibrational spectroscopy and of sumfrequency generation call for some further description. Section 4.4.1 describes the principal types of adsorbed hydrocarbon structure that have been found with alkenes and alkynes (aromatic hydrocarbons and cyclic C6 species will be considered in Chapters 10 and 12 respectively); Section 4.4.2 discusses the conditions under which the several chemisorbed forms of alkenes make their appearance. In Section 4.5 we look at detailed structural studies of a few adsorbed molecules, and Section 4.6 deals somewhat briefly with interconversions and decompositions of adsorbed alkenes, and structures of species formed. Finally there are sections on theoretical approaches (4.7), on the chemisorption of alkanes (4.8), and carbonaceous deposits that are the ultimate product of the decomposition process (4.9).

158 |
CHAPTER 4 |
4.3. THE TECHNIQUES15
We have already met a number of the techniques that have been used to study the structure and reactivity of chemisorbed hydrocarbons. Table 4.1 lists most of the important ones together with a few references that will help the reader to access the wider literature, and to draw attention to the names of the scientists who have made major contributions to their use. It will be appreciated that many of them are only applicable to one class of material, i.e. either to single crystals or to finely divided metal particles: techniques that are equally applicable to both classes (e.g. vibrational spectroscopy) are particularly valuable in enabling comparisons between the two classes to be drawn.
Recalling that this is not a handbook to teach the theory and operation of the techniques used, it is necessary only to note and comment on certain constraints that apply to them: such constraints cover matters such as cost and availability of the equipment, theoretical limitations to the understanding of what is observed or what can be observed. Those factors are well illustrated by reference to the field of vibrational spectroscopy (see Further Reading section at the end of the chapter).
For a vibration to be infrared-active, it must produce a change in the molecule’s electric dipole moment: the intensity of the absorption band is proportional to the square of the size of the change that occurs between the extreme points of the motion. However at a flat metal surface, the very high polarisability of the conduction electrons leads to an image of opposite sign which offsets the effect of the dipole moment change, so only those fully symmetrical vibrations that have a component of the dipole moment normal to the surface will give an absorption band. The RAIRS method (Table 4.1) used for work with single crystals is therefore subject to this metal surface selection rule.16−19 If however the metal particle is sufficiently small (<2 nm), the rule is relaxed, and with organometallic complexes it does not apply at all. Whereas in infrared spectroscopy the molecule absorbs a quantum of radiation, in the Raman effect the photon is scattered but loses energy which is transferred to the molecule; for a vibration to be Raman active, the event has to produce a change in the polarisability of the molecule. Although widely used to study oxides of catalytic interest, Raman spectroscopy has contributed much less to the study of hydrocarbons on metals.9
HREELS (Table 4.1) has the advantage of detecting all types of vibration: this is because there are two excitation mechanisms, viz. dipole scattering and impact scattering. The former is subject to the same selection rules as RAIRS and gives strong features on-specular, but the latter excites all vibrational modes. There are however supplementary selection rules that apply to impact scattering in the onspecular direction.17 As noted earlier, this technique is not applicable to supported metal catalysts.

THE CHEMISORPTION OF HYDROCARBONS |
159 |
TABLE 4.1. Methods Applicable to the Study of Chemisorbed Hydrocarbons
Acronym |
Name |
To single crystals etc.? |
To small particles? |
— |
(Micro) Calorimetry |
Yes23,24,310−314 |
Yes25,133,136,149,208 |
FTIR(S) |
Fourier-transform infrared |
Yes16,17 |
Yes9,17,209 |
|
(spectroscopy) |
|
|
RAIRS/IRAS |
Reflection - absorption |
Yes17,207,135 |
No |
|
infrared spectroscopy |
|
|
DRIFTS |
Diffuse-reflectance infrared |
No |
Yes17,209 |
|
Fourier-transform |
|
|
|
spectroscopy |
|
|
(HR)EELS/ |
(High resolution) or |
Yes17,74,210,211,316−320 |
No |
VEELS |
vibrational electron |
|
|
|
energy-loss spectroscopy |
|
|
SFG |
Sum-frequency generation |
Yes9,10,212−218 |
No |
LEED |
Low-energy electron |
Yes17,32,50,211,219,321 |
No |
|
diffraction |
|
|
NEXAFS |
Near-edge X-ray absorption |
Yes5,17,190,220−222 |
No |
(XANES) |
fine structure |
|
|
AES |
Auger electron spectroscopy |
Yes33,190,223−227 |
? |
PED |
Photoelectron diffraction |
Yes5,17,21,22,128,147 |
No |
UPS/XPS |
Ultraviolet (X-ray) |
Yes32−34,38,40,322,323 |
Possible |
|
photoelectron spectroscopy |
|
|
ARUPS |
Angle-resolved ultraviolet |
Yes5,41,232,324,325 |
No |
|
photoelectron spectroscopy |
|
|
INS |
Inelastic neutron scattering |
No |
Yes17,233,234 |
— |
Work function change |
Yes134,191,211,224,235−237 |
No |
— |
High-field magnetic methods |
No |
Yes238−240 |
TPD/LID/ CID |
Temperature-programmed |
Yes17,241,242 |
No |
|
(laser-induced, |
|
|
|
collision-induced) |
|
|
|
desorption |
|
|
(M)MB |
(Modulated) molecular beam |
Yes243,244 |
No |
STM |
Scanning-tunnelling |
Yes17,130,213,245−247,327,328 |
No |
|
microscopy |
|
|
— |
Stable and radioactive isotopes |
Yes329,330 |
Yes248−250 |
SIMS |
Secondary-ion |
Yes251 |
Yes142 |
|
mass-spectroscopy |
|
|
— |
Ellipsometry |
Yes192 |
No |
SERS |
Surface-enhanced Raman |
Yes331 |
Yes43,331 |
|
spectroscopy |
|
|
NMR |
Nuclear magnetic resonance |
Yes17,28,143,154,228−231,353 |
No |
(SEDOR) |
(spin-echo double |
|
|
|
resonance) |
|
|
— |
Chromatography |
No |
Yes274 |
Column 3 also covers foils and films; column 4, metal blacks and powders as well as supported metals. Only a few indicative references are given; for further sources of information, see for example 17, 25, 108, 254.

160 |
CHAPTER 4 |
A relatively new method for studying chemisorbed species is sum-frequency generation (SFG) (see Table 4.1 for references). This is a second-order non-linear process, requiring both a fixed visible and a tuneable laser: the selection rules determine that a vibrational mode must result in changes both to dipole moment and to polarisability for the effect to occur, and this limits it to a medium which lacks inversion symmetry, i.e. to the surface and not the gas phase. This, coupled with the fact that excitation is by photons, not electrons, leads to the inestimable benefit of being usable in the presence of a high gas pressure, and therefore enables in situ examination of the surface under reaction conditions.
It may be of interest to compare and contrast the usefulness of SFG and IR spectroscopy in this field. (i) IRS is experimentally much easier and the equipment needed is much cheaper: anybody can do it. (ii) It covers a wide range of frequencies whereas SFG is limited by the frequency range of the tuneable laser (originally to the C H stretch region). (iii) Not needing high-powered lasers, IRS employs very low energy radiation which does not affect surface structures, as SFG might. (iv) IR absorbance is usually easy to relate to surface concentration, whereas the relationship between SFG signal and concentration is much more complex. (v) While simple selection rules apply to IRS, while those for SFG are also more complex. (vi) On the other hand, signal intensity is sometimes higher with SFG than with IRS, low intensities of which can be limiting factors in examining single crystal surface. (vii) IRS detects both gaseous and surface species (subject to the selection rules), and so the gas pressure needs to be low, whereas as noted above SFG does not suffer this constraint. I am grateful for the assistance of Professor F. Zaera in drafting this paragraph.
The technique of choice therefore depends upon the nature of the questions posed, and on the limitations of cost and theory, which have been outlined above for one particular field. Very precise structural information is obtainable by LEED, and particularly by photoelectron diffraction (PED, Table 4.1): this last method is, unlike LEED, applicable to disordered adlayers.17,20 The effect is due to the diffraction of locally generated photoelectrons by other atoms within the same adsorbed species: variation of the diffraction intensity with the photoelectron energy using synchrotron radiation provides structural information.17,21,22
A major advance in technique has permitted the calorimetric determination of heats of adsorption of hydrocarbons on thin single-crystal surfaces of metals.23,24 Films of about 200 nm thickness are grown epitaxially by vapour deposition on the surface of a sodium chloride crystal cut to expose the desired face; it is then dissolved in water, and the film, the surface of which mimics that of the crystal, is then rescued. Its small thermal mass means that the heat liberated is rapidly equilibrated throughout the metal, and the temperature rise can be measured by a remote infrared detector. By using a pulsed molecular beam, simultaneous estimates of sticking probability can be made. Heats of adsorption of hydrocarbons on supported metals at various temperatures have also been reported25 (Section 4.5).

THE CHEMISORPTION OF HYDROCARBONS |
161 |
Direct structural information on hydrocarbon species on supported metals (e.g. C––C bond distances) is hard to come by, but can be obtained with some difficulty using NMR in the SEDOR mode17,26−28 (Section 2.42 and Table 4.1). NEXAFS, through the polarisation dependence of absorption resonances and selection rules for photoabsorption, can determine the average orientation of adsorbed chromophores with respect to the surface5,17 (see also Section 2.4.2 and Table 4.1).
In the early days of investigating the structures of chemisorbed hydrocarbons on metal single crystals, much use was made of ultraviolet photoelectron spectroscopy (UPS), especially by Demuth and his associates.29−36 UV photoemission spectra of valence orbitals were obtained using He I (24.4 eV) or He II (40.8 eV) as the exciting radiation, and comparison with the spectre of the free molecules, combined with calculation of the Hartree-Fock ground-state orbital energies by the self-consistent-field linear-combination-of-atomic-orbitals (SCF-LCAO) method, enabled structural information to be obtained for the principal adsorbed species.29 The limitation of this technique lay in the fact that the observed spectra were the sum of those due to all species present, and for this reason the combination of UPS with its X-ray cousin XPS,37−39 and with LEED,33,40 was more informative. In a further development, the angle of incidence of the exciting radiation was changed, giving angle-resolved UPS (ARUPS);41 this helped to identify the orbitals from whence the photoemitted electrons came. Additional references to these techniques are given in Table 4.1.
While little use has been made of Raman spectroscopy,42 somewhat more has been done with Surface-Enhanced Raman Spectroscopy (SERS),43 especially in the study of adsorbed aromatic hydrocarbons.9
4.4. IDENTIFICATION OF ADSORBED HYDROCARBON SPECIES
4.4.1. The Catalogue – or ‘The Organometallic Zoo’8,16,17,44
We noted when discussing hydrogen chemisorption that the old idea of a hydrogen atom forming only a single covalent bond to some other atom was inadequate to describe its interaction with metallic surfaces. A similar problem arises when trying to formulate the structures of chemisorbed hydrocarbons: simpleminded notions of tetravalent carbon and hybridisation of s and p orbitals as sp3 and sp2 and sp are simply not sufficient. The difficulty however is not entirely new, as it will certainly have been met when studying organometallic chemistry. We have to face the fact that a molecule such as ethene is able to bond to metals in a large variety of ways, depending on the nature and the extent of the interaction of its orbitals with those of the surface metal atoms. Analogies between chemisorption and co-ordination chemistry have in fact been very helpful,9,17,40,45−50 although they should not be pushed too far (see later).

162 |
CHAPTER 4 |
Description of the chemisorbed state of a hydrocarbon proceeds through stages, the number that can be accomplished depending on the type of surface and the techniques deployed. There are fewer methods available to the study of molecules on small particles than to single crystal surfaces, and so the information available is more restricted. The first stage requires the composition of the species to be specified, e.g. if the molecule starts as ethene, is its formula still given by C2H4? If some C––H bond breaking has occurred, do we have C2H3 or C2H2 or something else? The second stage enquires into the organisation of electrons within the adsorbed species. Has there been some degree of rehybridisation of the carbon atoms? Or has there just been some engagement of the π orbital lobes with metal orbitals, with little change to the formal sp2 hybridisation of the carbons? The third and final stage, applicable only to single crystal surfaces, is to establish the new structure in terms of metal-carbon and carbon-hydrogen bond lengths and angles, and to locate its position with respect to the lattice of surface metal atoms. In this last stage in particular we must be ready to find different techniques giving discordant results: a value judgement on the reliability of the method, aided by some chemical common sense and theoretical calculation, then has to be applied.
Table 4.2 sets out the names and structures of a number of adsorbed C2 species, for the existence of which there is some evidence. To simplify reference to these and other structures and compounds in the text, the following symbolism is introduced; M C1 species; E
C1 species; E C2 species; P
C2 species; P C3 species, and the pre-superscript gives the number of hydrogen atoms. At the foot of each box there are noted examples of organometallic complexes containing structures of the same type, and which have been used to identify the adsorbed species by their vibrational spectra. The numbering, and much of the other information that follows, have been provided by Sheppard and de la Cruz in their epic reviews.9,17,51 It is interesting to see how frequently osmium52−54 and cobalt44 appear in the cited complexes, the former in particular exhibiting a great variety of coordinated hydrocarbon structures. Higher alkenes form analogous structures, some of which, derived from propene, are shown in Table 4.3, but note that alkylidynes can only be formed from terminal alkenes. With propene and the butenes another adsorbed state becomes possible, namely, the π -alkenyl structure (usually called π -allylic), in which loss of a hydrogen atom results in a delocalised π -bond over three carbon atoms: such structures are often found in co-ordination complexes containing in particular either cobalt or palladium (see also Table 4.4, where some of the structures derivable from the 2-butenes are shown).
C3 species, and the pre-superscript gives the number of hydrogen atoms. At the foot of each box there are noted examples of organometallic complexes containing structures of the same type, and which have been used to identify the adsorbed species by their vibrational spectra. The numbering, and much of the other information that follows, have been provided by Sheppard and de la Cruz in their epic reviews.9,17,51 It is interesting to see how frequently osmium52−54 and cobalt44 appear in the cited complexes, the former in particular exhibiting a great variety of coordinated hydrocarbon structures. Higher alkenes form analogous structures, some of which, derived from propene, are shown in Table 4.3, but note that alkylidynes can only be formed from terminal alkenes. With propene and the butenes another adsorbed state becomes possible, namely, the π -alkenyl structure (usually called π -allylic), in which loss of a hydrogen atom results in a delocalised π -bond over three carbon atoms: such structures are often found in co-ordination complexes containing in particular either cobalt or palladium (see also Table 4.4, where some of the structures derivable from the 2-butenes are shown).
Higher alkynes may form structures analogous to numbers 11 to 16 in Table 4.2 but analogues of 16A to 21 are only formed by terminal alkynes. Molecules containing more than one multiple bond, e.g. 1,2-propadiene, 1,2- and 1,3-butadiene, etc. can have their adsorbed states formulated in many different ways, but in general both C C bonds are connected to the surface in the same way, i.e. both are either π or di-σ . It will be evident that species containing an odd number of hydrogen
C bonds are connected to the surface in the same way, i.e. both are either π or di-σ . It will be evident that species containing an odd number of hydrogen
