
Practical Plastic Surgery
.pdf
562 |
Practical Plastic Surgery |
The bilateral triangular advancement flap, also called the Kutler flap, was classically used for the transverse, or volar-oblique finger tip amputation with exposed bone. It is now rarely employed because (1) only about 3-4 mm advancement is obtained, (2) often it creates an insensate fingertip, and (3) it can create a sensitive sagittal scar at the finger tip.
The oblique triangular flap is used for the volar or oblique finger tip amputation with exposed bone. It combines the advantages of the volar V-Y advancement flap and bilateral triangular advancement flap. It can easily be converted to a neurovascular island pedicle flap if more elevation is required, in which case a skin graft is used to cover the donor site.
The cross-finger flap, also termed the trans-digital flap, is most often used for the volar pad injury that requires more tissue coverage than is possible with advancement-type flaps. Often there will be exposed bone, tendon, or distal interphalangeal (DIP) joint. There are multiple donor digits for this flap. Any adjacent finger can serve as a donor. The flap should be elevated superficial to the paratenon and slightly larger than the defect. The donor site is closed with a full-thickness skin graft (FTSG), and the two digits are held together with a splint or K-wires for 2 weeks. After this period, the flap is separated from the donor digit.
The reverse cross-finger flap is a variation of the traditional cross-finger flap. The papillary and reticular dermis are divided, and the reticular dermis is used to cover the dorsal defect on the adjacent finger. The papillary dermis is sewn back into the donor site to heal as a random flap.
The Hueston and Souquet flaps are both lateral palmar rotation-advancement flaps used to cover tip amputations with exposed bone. The Hueston flap includes only one neurovascular bundle at the base of the flap, whereas the Souquet flap includes both. In both flaps, a back cut is created that requires skin grafting.
The thenar flap is most often used to cover the index and long finger tip amputations. The donor site is found by placing the injured finger tip(s) over the thenar eminence, and an H-shaped incision is made to bury the stump in the thenar pad. The flap is separated after about 2 weeks.
The dorsal middle finger flap is a potentially sensate, neurovascular island
92flap that can be used to cover defects in all the fingers, except the thumb. The flap is similar to a cross-finger flap but extends more proximally to include a vascular bundle. The dissection often needs to progress as proximal as the common digital artery. A dorsal sensory branch that can be anastomosed with a recipient digital nerve is elevated with the flap. The donor site must be closed with a FTSG.
Flaps for Thumb Coverage
The Moberg flap is an advancement flap designed to preserve the amputated thumb’s length. It is a robust and sensate flap mobilizing the volar, proximal thumb tissue with both neurovascular bundles. The Moberg flap can easily be converted into a bipedicled flap if more advancement is needed. However, it has the potential of leaving the thumb metacarpophalangeal (MP) and interphalangeal (IP) joints in a flexed position.
The neurovascular island pedicle flap is used to provide padded, sensate skin to the thumb. The donor flap is from the ulnar side of the middle finger. The flap’s neurovascular bundle with its surrounding fat may need to be dissected proximally to the level of the superficial arch. A FTSG is used to close the donor site. A contraindication to the use of this flap is a middle finger that cannot be adequately

Soft Tissue Coverage |
563 |
perfused with only the digital artery on the radial side. This can be tested in a manner similar to the Allen test at the wrist.
The racquet flap, also called the Holevich flap, is used to provide sensate skin to the thumb, especially for chronic median nerve lesions. It is based on the second dorsal metacarpal artery. Dorsal sensory branches of the superficial radial nerve are included to provide sensation to the volar thumb. The racquet flap can also be used to provide pliable tissues to the first web space.
The kite flap, also termed the Foucher flap, is an extension of the racquet flap and is used to provide sensate skin to a scarred and denervated thumb pad. This flap includes the skin over the index MP joint. It is elevated from distal to proximal superficial to the paratenon, along with the first dorsal metacarpal artery. A skin tunnel is created, and the flap is passed through the tunnel to reach the recipient site. A FTSG is used to close the donor site.
The homodigital island flap, also called the annular flap or Goumain flap, is used to preserve thumb length and to provide sensate coverage to the amputated thumb tip, particularly at the proximal phalanx level. A circular incision is made around the thumb 2 cm proximal to the defect, and once the neurovascular bundles are freed, the circumferential flap advances distally to cover the defect. A FTSG is used to close the donor site.
Flaps for Coverage of the Hand and Proximal Fingers
The dorsal island digital flap is an axial-type, island flap based on the dorsal digital artery. Its size can be up to 3 x 3 cm over the dorsal proximal phalanx. It is used to cover the ipsilateral or adjacent finger defect in the PIP region. The preservation of the dorsal digital veins and artery is crucial, and the defect is covered with a FTSG.
The fillet flap is used to cover a proximal digital amputation using the salvaged soft tissues from the amputated digit. The bone, tendon, and pulp tissues are filleted off the skin, and the resulting skin flap is used to cover the dorsal or palmar wound. Any attachments of the skin to the hand should be preserved.
The retrograde radial forearm flap is based on the radial artery with the intact palmar arch. It is a robust flap and has undergone several variations. A positive Allen 92 test is a contraindication for the use of this flap. Radial artery reconstruction after
the transfer is usually not required. The pedicle length is sufficient to cover almost any hand defect. The dissection begins at distal volar forearm radial to flexor carpi ulnaris (FCR) to expose the radial artery and its venae commitantes. The dissection continues between the FCR and brachioradialis, and when the distal flap edge is reached, the flap is elevated from its ulnar border until the septal perforators are reached. The pedicle and the septal perforators are preserved and kept contiguous with the flap. The dissection continues from the flap’s radial border until the septal perforators are reached. The branches off to the FCR and the proximal radial artery-venae commitante are ligated to elevate the flap. The donor site is closed with a split-thickness skin graft (STSG).
If the skin and subcutaneous tissues are not needed, it can be raised as an adipofascial flap. When harvested as an adipofascial flap, the skin is incised in a zigzag fashion, and the antebrachial fascia is exposed. The flap is designed on the fascia. The dissection is the same except the skin is spared, leaving the antebrachial fascia intact underneath. The donor site is closed primarily, and the flap is inset into the defect with a STSG covering the flap at the recipient site.

|
564 |
Practical Plastic Surgery |
|
|
|
The retrograde radial forearm fascial flap is a distally-based, random volar |
|
|
|
antebrachial fascia turnover flap. It differs from the retrograde radial forearm flap in |
|
|
|
that the radial artery is left in situ, and the vascular supply comes from the distal |
|
|
|
perforating branches of the radial artery. It can be used to cover both volar and |
|
|
|
dorsal hand defect. The dissection begins by making an S-shaped skin incision just |
|
|
|
deep to the hair follicles over the volar forearm. The branches of the radial antebra- |
|
|
|
chial nerves are protected. The flap is designed over the fascia at least 3-4 cm wide |
|
|
|
and dissected in a proximal to distal direction until a point 5 cm proximal to the |
|
|
|
radial styloid. The flap is turned over, and a STSG is used to cover the flap. |
|
|
|
The posterior interosseous forearm flap is another retrograde fasciocutaneous |
|
|
|
flap based on the posterior interosseous artery (PIA). It is used to cover defects in the |
|
|
|
following areas: first webspace-thumb, dorsal hand-dorsal PIP, and anterior |
|
|
|
wrist-palm. The flap is centered in the axis between the lateral epicondyle and ulnar |
|
|
|
styloid with elbow in full flexion. The PIA originates at the proximal and middle |
|
|
|
third junction of this axis. There are 7-14 fasciocutaneous perforators distal to this |
|
|
|
point along the axis, and the center of the flap should be distal to this point. The |
|
|
|
PIA terminates 2 cm proximal to the ulnar styloid by way of anastomoses with the |
|
|
|
dorsal wrist arcade. The dissection begins at this point in a distal to proximal direc- |
|
|
|
tion, and the PIA is identified. The posterior interosseous nerve is located radial to |
|
|
|
the artery and must be protected throughout the dissection. The flap dissection |
|
|
|
continues proximally to the main perforator with ligation of all muscular branches. |
|
|
|
The flap is elevated in an ulnar to radial direction with the pedicle being ligated |
|
|
|
proximal to the flap with and release of the septum from its ulnar shaft attachment. |
|
|
|
The dorsal ulnar artery flap is a fourth retrograde fasciocutaneous flap of the |
|
|
|
forearm. It is used to cover defects of the ulnar-volar or dorsal hand. It is based on |
|
|
|
the dorsal branches of the ulnar artery that originates 2-5 cm proximal to the pisi- |
|
|
|
form bone. The dissection begins 2 cm proximal to the pisiform, and with ulnar |
|
|
|
retraction of flexor carpi ulnaris, the dorsal ulnar branch is seen arising from the |
|
|
|
ulnar artery. The flap is centered along the ulnar axis with palmaris longus forming |
|
|
|
the volar border and the fourth extensor digitorum communis tendon forming the |
|
|
|
dorsal border. The proximal and middle third junction of the ulnar forearm forms |
|
92 |
|
the distal flap border. |
|
|
|
Rotation flaps are useful for coverage of the dorsal fingers and the dorsum of the |
|
|
|
hand, where the skin is more lax. They are designed to redistribute the tension over |
|
|
|
the larger radian of the flap edges. The use of a back cut, also called Burow’s triangle, |
|
|
|
further helps to reduce the tension at the tip of the flap. Transposition flaps, such as |
|
|
|
the Limberg or rhomboid flap, are also useful on the dorsal hand. The design of |
|
|
|
these flaps is discussed in greater detail in Chapter 11. |
|
Pearls and Pitfalls
There are numerous options available for soft tissue coverage of the hand. Whenever possible the simplest technique, namely direct closure, should be used. The next more complex option is the local random flap, followed by the pedicle flap. At the top of the reconstructive ladder is the microvascular free tissue transfer to the hand. Split-thickness skin grafts and the use of tissue expanders are also good options for coverage when there is an adequate tissue bed without exposed tendon, artery, nerve or bone, and the adjacent skin is healthy. The choice of reconstructive technique depends on the wound geometry, the amount of soft tissue required, and the local wound conditions, such as the immediate necessity to cover exposed bone

Soft Tissue Coverage |
565 |
or tendon. Conditions that limit joint motion, (e.g., arthritis and Dupuytren’s disease), or impair circulation, (e.g., Raynaud’s or heavy smoking), should always be taken into account when planning the reconstruction.
Suggested Reading
1.Atasoy E, O’Neill E. Local flap coverage about the hand. Atlas Hand Clin 1998; 3(2):179.
2.Chase RA. Historical review of skin and soft tissue coverage of the upper extremity. Hand Clin 1985; 1:599.
3.Germann G. Principles of flap design for surgery of the hand. Atlas Hand Clin 1998; 3(2):33.
4.Gilbert A. Pedicle flaps of the upper limb. Philadelphia: Lippincott, 1992.
5.Lister G. The theory of the transposition flap and its practical application in the hand. Clin Plast Surg 1981; 8:115.
6.Martin D, Bakhach J, Casoli V et al. Reconstruction of the hand with forearm island flaps. Br J Plast Surg 1990; 43:290.
7.Weinzweig N, Chen L, Chen ZW. The distally based radial forearm fasciocutaneous flap with preservation of the radial artery: An anatomic and clinical approach. Plast Reconstr Surg 1994; 94:675.
8.Zancolli EA, Angrigiani C. Posterior interosseous island forearm flap. J Hand Surg {Br} 1988; 13B:130.
92


Carpal Tunnel Syndrome |
567 |
I.Idiopathic/spontaneous
IIa. Intrinsic factors outside the nerve that increase CT volume
A.Conditions altering fluid balance
1.Pregnancy
2.Renal failure
3.Chronic hemodialysis
4.Myxedema
5.Acromegaly
6.Menopause
7.Oral Contraceptives
8.Congestive heart failure
9.Thyroid disease
B.Inflammatory conditions
1.Rheumatoid arthritis
2.Gout
3.Amyloidosis
4.Pseudogout
5.Lupus erythematosis
6.Scleroderma
7.Nonspecific tenosynovitis
8.Dematomyositis
9. Infection: – Pyogenic (bacterial)
–Mycobacterium
–Fungal
–Lyme disease
–Viral
–Parasitic (guinea worm)
C.Tumor and tumor-like masses
1.Ganglion
2.Lipoma
3.Fibroma
4.Pigmented villonodular tenosynovitis
D.Anatomical abnormalities
1.Vascular malformations
2.Anomalous muscles: – Proximal origin of lumbrical muscles
–Distal muscle of flexor digitorum superficialis muscle
–Abnormal insertion of palmaris longus
–Anomalous slip of flexor pollicis longus
–Palmaris profundus
E.Hemorrhagic disorders-hemorrhage within the carpal tunnel
1.Hemophilia
2.von Willebrand disease
3.Acute leukemia
4.Anticoagulants
5.Rupture of aneurysm of a persistent median artery
F. Traumatic injuries |
93 |
||
1. |
Posttraumatic scarring within the tunnel-traction neuropathy |
||
|
|||
2. |
Trauma causing hemorrhage within the CT |
|
|
IIb. Intrinsic factors inside the nerve that increase CT volume
A.Neurilemmoma
B.Lipofibroma
C.Neurofibroma
D.Neuroma
III. Extrinsic factors that alter tunnel contour
A.Acute fractures
B.Acute carpal dislocations/subluxations
1.Lunate dislocation
2.Rotatory subluxation of scaphoid
C.Intercarpal arthritis
IV. Exertional/overuse conditions
V.Neuropathic factors
A.Diabetes mellitus
B.Multiple myeloma
C.Alcoholism
D.Vitamin toxicity
E.Nutritional deficiency
F.Exposure to industrial solvents
G.Hand-arm vibration syndrome
H.Medication-lithium, beta blockers, ergot overdose

568 |
Practical Plastic Surgery |
Anatomy
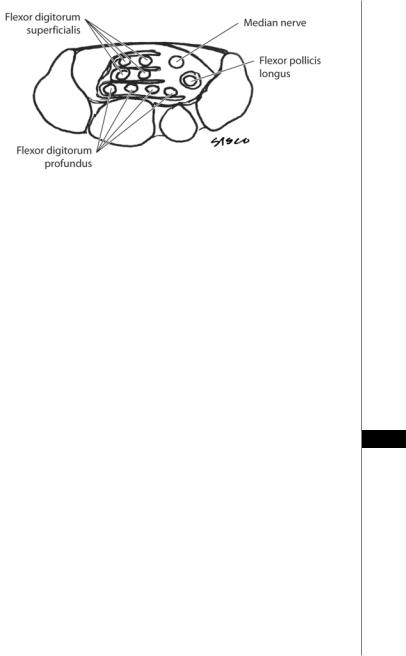
The carpal tunnel is a space defined by the concave arch of the carpus enclosed by the transverse carpal ligament (TCL). The scaphoid, trapezium, and sheath of the flexor carpi radialis (FCR) make up its radial margin. The ulnar boundary consists of the triquetrum, the hook of the hamate, and pisiform. Ten structures course through the carpal tunnel. These include the median nerve, four flexor digitorum superficialis (FDS) and four flexor digitorum profundus (FDP) tendons (Fig. 93.1), all ensheathed by the ulnar bursa, and the flexor pollicis longus, found at the radial side of the canal and surrounded by the radial bursa. The flexor carpi radialis tendon (FCR) travels through its own separate osteofibrous tunnel. The TCL itself attaches medially to the pisiform and laterally to the tuberosity of the scaphoid bone. It is thickest (0.6 to 2 mm) at the junction of the mid and distal thirds and thin at its proximal and distal ends. In individuals with CTS the ligament is much thicker and can be up to 6 mm wide at its thickest portion.
The median nerve is the most superficial structure within the carpal tunnel and is covered by a layer of cellulo-adipose tissue. The nerve lies directly under the TCL, in the radiopalmar portion of the canal. The superficialis and profundus tendons of the index finger lie immediately dorsal to the median nerve. The nerve divides distally into five sensory branches and the recurrent motor branch. In 80% of cases, the motor branch arises from the radiopalmar region of the median nerve. The remainder of patients has the origin in the central location, and a small percentage take-off from the ulnar aspect. Ten percent of patients have multiple motor branches. The palmar sensory cutaneous branches (PSCB) of the median nerve branches off 5-7 cm proximal to the wrist crease, often found between the palmaris longus and FCR tendons. The PSCB terminate in the subcutaneous tissues overlying the thenar musculature.
A sensory communicating branch between the median and ulnar nerves in the palm is present in 80% of patients, with roughly half lying within millimeters of the TCL. Injuries to this nerve have been reported during carpal tunnel release, leading to paresthesias or dysesthesias in the ulnar digits.
Physical Examination
Clinical history and physical examination including provocative testing are more
93easily performed than electrodiagnostic evaluation, and they are more appropriate tools for the ambulatory setting. Because patients suffering from CTS frequently report symptoms of numbness and tingling in the radial three digits, a thorough sensory exam should be done on initial assessment. The most common tests for sensibility utilize static or moving two-point discrimination, Semmes-Weinstein monofilament testing, and vibrometry. Sensibility testing is conceptually divided into innervation density testing and threshold testing. Static and moving two-point discrimination are density tests. Static testing measures slowly adapting neuron receptors (those mediating the sensation of constant touch or pressure) and moving two-point discrimination measures quickly adapting fibers (which mediate transient touch or movement). Innervation density measures several overlapping peripheral receptor fields. Testing for innervation density is a significant part of the exam because it may be perceived as normal in the presence of mild or even moderate compression of a peripheral nerve due to the presence of multiple overlapping fields.
Threshold tests such as vibrometry or Semmes-Weinstein monofilaments evaluate single nerve fibers innervating a single receptor or group of receptor cells. Threshold testing is more likely to show a gradual and progressive change in value as a
Carpal Tunnel Syndrome |
569 |
|
|
|
|
|
|
|
Figure 93.1. A schematic cross-section of the carpal tunnel.
greater and greater number of nerve fibers are affected, as seen in nerve compression. For CTS, threshold testing is the preferred method for evaluating hand sensibility. Although vibrometry is more sensitive, the nylon Semmes-Weinstein monofilaments are more user-friendly, and therefore more often used.
Provocative testing also assists in the diagnosis of CTS. These tests are based on the principle that stressing an already injured median nerve will exacerbate the symptoms of pain, paresthesias and numbness. Three tests are most common: Tinel’s test (median nerve percussion), Phalen’s test (wrist-flexion) and the median nerve compression test. Phalen’s test is performed with the forearms upright, allowing the elbows to rest on a hard surface. The wrists are allowed to drop into flexion for 30-60 seconds, causing reproduction of symptoms. Eliciting Tinel’s sign involves tapping gently along the course of the median nerve at the wrist from proximal to distal. A positive response is recorded if the patient perceives tingling in the median nerve distribution. The median nerve compression test is performed with the examiner gently applying sustained pressure with their thumb over the patient’s carpal
canal. Paresthesias in the distribution of the median nerve, which resolve when the 93 pressure is released, within 30 seconds, are indicative of a positive test.
Motor testing of the thenar eminence can be a useful adjunct to the exam, especially in patients with long-standing symptoms. Compare the profiles of the thenar eminences of both hands, and check the strength of the abductor pollicis brevis by testing resistance to palmar abduction, while using a simultaneous comparison to the opposite hand. This test is less sensitive in patients with pain from basilar joint arthritis.
Many hand surgeons feel that electrodiagnostic testing should be obtained in any patient with symptoms of CTS. There is argument over this issue, but valid electrodiagnostic testing is regarded as the “gold standard” for CTS. In order to make the diagnosis of CTS, electrodiagnostic testing must show slowing of median sensory or motor nerve conduction velocity at the wrist, prolonged distal motor or sensory latency, or denervation of the abductor pollicis brevis muscle. Electromyography (EMG) may also demonstrate that a patient is suffering from other conditions such as pronator teres syndrome or cervical radiculopathy. But is EMG absolutely necessary to make the diagnosis? Most articles conclude that in a classical CTS presentation there is no need for electrodiagnostic testing, but in atypical symptoms

570 |
Practical Plastic Surgery |
EMG is mandatory. There have been reports of successful treatment of CTS despite initially normal EMG findings, and in a U.S. survey only 33% of surgeons routinely use electrophysiolgic studies. EMG itself does not have prognostic value, and there is no good correlation between electrophysiology and symptoms.
Management
In treating patients with CTS, the objectives are to ameliorate symptoms, optimize physical performance, and improve function. Nonsurgical management includes minimizing work and leisure exposures which aggravate symptoms, neutral wrist splinting in the evening, nonsteroidal anti-inflammatory drugs (NSAIDS) and/ or steroid injections. Steroid injection into the carpal tunnel provides temporary relief in about 40-80% of patients, lasting several months. Steroid injections which provide favorable relief after treatment are associated with patients having a positive surgical outcome. Occupational and physical therapy can also be of benefit. Indications for surgical intervention include:
•Lack of response to conservative management over a 3-6 month period
•Moderate/severe symptoms associated with significantly prolonged distal sensory and/or motor latencies
•Slowed sensory conduction velocities
•Denervation of the abductor pollicus brevis muscle on EMG
Surgical Release of the Carpal Tunnel
Local anesthesia with sedation is often used with carpal tunnel release, but the optimal method of anesthesia must be decided upon between the surgeon and the anesthesiologist. The patient is placed supine on the operating table and the forearm is supinated on a hand table. A well padded pneumatic tourniquet is placed on the upper arm and antibiotic prophylaxis is given. The tourniquet is inflated to 250 mm Hg (except in patients with hemodialysis shunts).
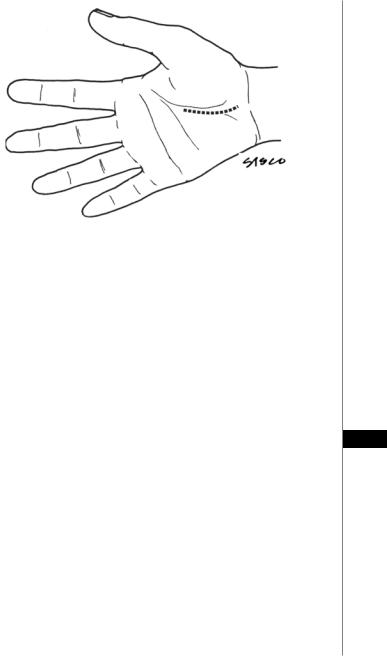
Prior to administration of the local anesthetic, surface markings should be made to assist in designing an incision that avoids injury to the deep and superficial arches, recurrent motor branch, palmar cutaneous branch and ulnar neurovascular bundle (Fig. 93.2).
The incision is started at Kaplan’s cardinal line (a line parallel with the palmar
93wrist crease, beginning at the apex of the thumb-index web and ending slightly distal to the hook of the hamate. Aim toward the ulnar border of the palmaris longus and continue the incision until reaching the proximal wrist crease. The incision may be continued 2-3 cm into the distal forearm in an ulnar/oblique direction to aid in localization of the median nerve and ulnar neurovascular bundle. The dissection is continued bluntly through subcutaneous tissue allowing identification of the palmar fascia. This fascia is incised longitudinally in a direction parallel to the ulnar border of the palmaris longus. The borders of the transverse carpal ligament are visualized, including the perivascular tissue protecting the superficial arch distally. The ulnar neurovascular bundle is identified and protected. Proximally, the skin and soft tissues are retracted volarly to expose the antebrachial fascia. Blunt dissection proceeds above and below the fascia to protect the palmar cutaneous and median nerves. Division of the TCL is continued into the antebrachial fascia for 1-2 cm. The distal median nerve and recurrent motor branch are then inspected. Residual distal fibers of the flexor retinaculum and any fascia constricting the motor branch are carefully divided. The tourniquet is deflated, careful hemostasis is obtained, the wound is irrigated, and the
Carpal Tunnel Syndrome |
571 |
|
|
|
|
|
|
|
Figure 93.2. Surface markings for the incisions in open carpal tunnel release.
skin closed with 4-0 nylon. A bulky, compressive hand dressing is applied with a short-arm volar splint to maintain the wrist in slight extension.
Endoscopic Carpal Tunnel Release
The endoscopic carpal tunnel release (ECTR) was first described by Okutsu in 1987. Since then, several modifications to the technique have been made. The majority of the literature in ECTR deals with the Chow and Agee technique. The indications for ECTR and traditional CT release are similar. There are several situations where ECTR is not the optimal choice. These include cases where the patient has undergone a previous CT release, patients with prior wrist fusion, cases where the surgeon suspects proliferative tenosynovitis, and any suspicion of a mass within the CT.
The dual portal ECTR, described by Chow, involves making a small incision just 93 1 cm proximal to the wrist flexion crease in the midline of the forearm (Fig. 93.3). A tourniquet is applied after positioning the patient similar to the manner of a traditional CTR. The volar antebrachial fascia is entered and a curved dissector is passed
into the CT, hugging the hook of the hamate. A “washboard” sensation is felt as the tip of the dissector passes over the ridges in the dorsal surface of the TCL. If this “washboard” sensation is not felt, the dissector is either palmar to the TCL or in Guyon’s canal. The distal edge of the TCL is identified and marked on the overlying skin. A Ragnell retractor is used to retract the antebrachial fascia and open the proximal port. The slotted cannula/obturator is placed into the CT after removing the dissector. The cannula is safely placed, the wrist and fingers are extended and the hand is placed in the hand-holder. A distal port is established by making a 1 cm transverse incision over the tip of the cannula. The cannula system is passed from the distal port. A 30˚, 4 mm endoscope is passed into the proximal end of the cannula system.
Once the surgeon is confident there are no intervening structures in the operative field, the probe blade is inserted through the proximal port. It is advanced until it engages the distal end of the TCL and then pushed proximally, cutting the distal
