
Practical Plastic Surgery
.pdf
522 |
Practical Plastic Surgery |
Pearls and Pitfalls
For obvious lacerations of nerves, primary repair and reconstruction should be undertaken in the acute stage. However, for traction or closed injuries, there is a necessary period of waiting for neuropraxia to resolve prior to surgical exploration. In this latter setting, neuromas may be encountered. Nerve conduction across neuromas may be one measure of determining whether simple neurolysis will suffice or neurotization and grafting is required (poor conduction being an indication for nerve transfer or grafting). Sural nerve will give a reasonable amount of graft material with modest donor site morbidity.
Notwithstanding primary reconstruction of injured nerves, secondary deformities may still become apparent over time. For instance, internal rotation and adduction deformities may result from a relative loss of upper root function. Partial release of contracted muscle may need to be performed in conjunction with latissimus dorsi and teres muscle transfers that alter vectors of action so that better external rotation is obtained. Other standard tendon transfers in the forearm and hand can help in specific situations of persistent ulnar, median and radial nerve palsy. If it is anticipated that a functional free muscle transfer may be needed for severe plexus injury, preserving nerves to act as future donors may be important.
Often, by the time a plexus injury patient has presented for reconstruction, a significant period of time has passed. Irreversible muscle atrophy occurs by 18-24 months; a calculus of time since injury and time until reinnervation of distal targets must be considered when evaluating feasibility of primary reconstruction.
86Suggested Reading
1.Chuang DC. Management of traumatic brachial plexus injuries in adults. Hand Clin 1999; 15(4):737-55.
2.Moran SL, Steinmann SP, Shin AY. Adult brachial plexus injuries: Mechanism, patterns of injury, and physical diagnosis. Hand Clin 2005; 21(1):13-24.
3.Shenaq S, Kim J. Repair and grafting of peripheral nerve. In: Mathes SJ, Hentz V, eds. Plastic Surgery. 2nd ed. (in press).
4.Shin AY, Spinner RJ. Clinically relevant surgical anatomy and exposures of the brachial plexus. Hand Clin 2005; 21(1):1-11.
5.Terzis JK, Papakonstantinou KC. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg 2000; 106(5):1097-1122.
524 Practical Plastic Surgery
Table 87.1. Classification of nerve injuries
Injury Classification |
Structure |
Functional |
|
(Seddon) |
(Sunderland) |
Injured |
Recovery |
Neurapraxia |
1st degree |
Axon only mildly injured |
Complete recovery |
|
|
|
in weeks-months |
Axontmesis |
2nd degree |
Axon severely injured, |
Complete recovery |
|
|
endoneurium intact |
in months |
Neurontmesis |
3rd degree |
Axon and endoneurium |
Near complete |
|
|
severed |
recovery |
|
4th degree |
Fascicles and perineurium |
Relatively good |
|
|
severed |
recovery |
|
5th degree |
Complete nerve |
Only partial |
|
|
transection |
recovery |
Diagnosis
Any person suspected of having a nerve injury should undergo a thorough sensory and motor examination. When the nerve injury is secondary to a laceration or penetrating trauma, the point of injury is relatively easy to locate. The extent of the injury may be difficult to diagnose since a muscle can retain function even if the nerve is partially transected. Open nerve injuries should therefore be explored. Closed injuries with suspected nerve involvement pose a more challenging situation. Locating the level of nerve injury can be difficult. In these cases, EMG and nerve conduction velocity studies should be obtained about 6 weeks after the injury to locate the
87level of injury. Nerve exploration should therefore be deferred if only partial nerve injury is suspected and there is reasonable prospect of spontaneous recovery.
Direct Nerve Repair
Several principles are important to consider in primary neurorrhaphy:
1.Timing of the repair. Primary repair is preferable to secondary repair. Most authors recommend repair as early as possible. In lengthy cases, such as replants, it may not be feasible to repair injured nerves, in which case the repair should be delayed several days to weeks. There are some surgeons who prefer to always wait 2-3 weeks after injury in order to time the repair with fibrosis of the nerve (for facilitating suturing) and maximal axonal sprouting across the gap.
2.Gap size. After resection of any devitalized or crushed nerve, the remaining gap should not exceed 2.0-2.5 cm. If the gap exceeds this distance, it should be bridged with a nerve graft or a conduit of some sort (e.g., vein graft or polyglycolic acid tube).
3.Tension. The elasticity and blood supply of peripheral nerves can tolerate mobilization of about 6-8 cm of nerve. Beyond this, damage to the axons and capillaries will occur.
4.Proper fascicular alignment. This is essential to ensure that motor and sensory fascicles will be properly aligned. In addition to visually matching the two ends, there are several intraoperative techniques for differentiating motor and sensory fascicles.
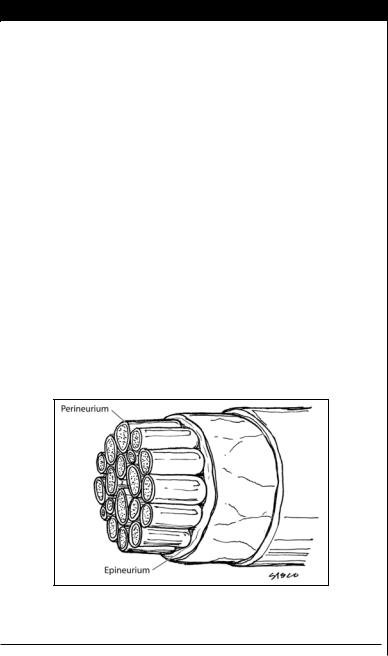
5.Epineurial vs. perineurial repair. Epineurial repair alone is adequate for digital nerves using several 9-0 or 10-0 nylon sutures. Advantages include its technical ease and minimal manipulation of internal nerve structures. Its main drawback is the lack of accurate fascicular alignment. Perineurial repair properly aligns the

Nerve Injuries |
525 |
fascicles either individually or in groups and is used for larger nerve repair in the hand and forearm. It is more time-consuming and technically demanding. It also increases the risk of scar formation at the repair site. There is still no clear guideline as to which type of repair should be used.
Median Nerve Injury
This should be suspected in deep lacerations of the wrist or distal humerus or in penetrating injuries on the medial side of the arm that injure the brachial artery. The most consistent sign of median nerve injury is loss of skin sensibility on the palmer surface of the first three digits and loss of thenar opponens function. Patients will make a fist without the thumb and index finger folded into the palm. If the FPL, FDS and FDP of the index and middle finger are flexing normally, then the nerve injury has occurred distal to the take off of the anterior interosseous nerve. Exposure of the median nerve depends on the level of injury and is outlined in Table 87.2.
Ulnar Nerve Injury
The ulnar nerve is at risk of injury primarily from deep lacerations of the ulnar side of the wrist or from penetrating trauma to the posterior medial epicondyle. A common sensory sign of ulnar nerve injury is the loss of sensibility to the small and ulnar side of the ring fingers. Motor signs include FCU paralysis, interosseous and thumb adduction loss with a weak “key pinch,” and FDP paralysis of the small and ring fingers. A long-standing injury can present with the classic “claw hand” deformity. Exposure of the ulnar nerve is outlined in Table 87.3.
Radial Nerve Injury |
|||
The radial nerve is at risk of injury primarily from fractures of the humerus or |
87 |
||
radius. Spiral, oblique humeral shaft fractures (especially open fractures) and frac- |
|
||
tures of the upper third of the radius are the ones that most commonly result in radial |
|||
nerve injury. Anterior dislocation of the radial head may stretch the nerve or pin it |
|||
between the radial head and the ulna. Signs of injury include loss of sensibility on the |
|||
dorsum of the hand and in the first web space. Patients will be unable to extend their |
|||
|
|
||
Table 87.2. Median nerve exposure |
|||
|
|
|
|
Location |
Key Points in Exposing |
||
of Injury |
the Median Nerve |
||
Arm |
• Medial incision anterior to intermuscular septum |
||
|
• Nerve runs adjacent to the brachial artery |
||
Elbow |
• Incision along medial border of biceps brachialis |
||
|
• Nerve is deep to antebrachial fascia and lacertus fibrosus |
||
Proximal forearm |
• Incision along inner border of pronator teres |
||
|
• Nerve passes between two heads of pronator teres |
||
|
• Ant. interosseous n. exits deep, proximal to pronator teres |
||
Distal forearm |
• Oblique incision between FCU and FCR tendons |
||
|
• Nerve lies between FDS and FDP tendons |
||
|
• Nerve runs deep to palmaris longus (when present) |
||
Wrist or palm |
• Incision parallel to skin crease at base of thenar eminence |
||
|
• Divide transverse carpal ligament |
||
|
• Watch for palmer cutaneous and recurrent motor branches |
|
|
526 Practical Plastic Surgery
Table 87.3. Ulnar nerve exposure
Location |
Key Points in Exposing |
of Injury |
the Ulnar Nerve |
Arm |
• Medial incision anterior to intermuscular septum |
|
• Nerve runs deep to the fascia of the septum |
Elbow |
• Incision is directly over the cubital tunnel |
|
• Incise between the olecranon and medial epicondyle |
Forearm |
• Incision along ulnar mid-axial line |
|
• The two head of FCU are split |
|
• Nerve runs deep to FCU on the flexor digitorum profundus |
Wrist or palm |
• Incision parallel to skin crease at base of thenar eminence |
|
• Incise over the pisiform |
|
• Watch for the division into sensory and motor branches |
Table 87.4. Radial nerve exposure
|
|
Location |
Key Points in Exposing |
|
|
of Injury |
the Radial Nerve |
|
|
Proximal arm |
• Posterior incision between long and medial head of triceps |
|
|
|
• Nerve runs deep to the long head of triceps |
|
|
|
• Nerve runs in the spiral groove of the humerus |
|
|
Distal arm |
• Nerve runs between brachialis and brachioradialis |
|
|
|
• Accompanies the terminal branch of the profunda brachia |
|
|
Forearm |
• Nerve runs deep to brachioradialis |
87 |
|||
|
|
|
• Distal to brachialis tendon insertion, the nerve divides |
|
|
|
|
|
|
|
(superficial and posterior interosseous divisions) |
|
|
|
|
fingers. Injury to the radial nerve in the axilla will result in paralysis of the ECRL and ECRB, triceps and brachioradialis muscles. These muscles will atrophy in long-standing nerve injury. Exposure of the radial nerve is outlined in Table 87.4.
Alternatives to Direct Repair
When direct nerve repair is not possible, several alternatives should be considered. The following chart lists common reasons precluding direct repair and some surgical options:
Reason Direct Repair not Possible |
Solution |
Concomitant life-threatening |
Delay repair until patient |
injuries |
medically stable |
Gap greater than 2.5 cm or |
Mobilize nerve, use nerve graft |
excessive tension |
or conduit, shorten bone |
Inability to locate the proximal |
Nerve transfer (such as |
nerve stump |
intercostal n. to axillary n.) |
Inability to locate the distal |
Neurotization (imbed stump |
nerve ending |
into target muscle) |
End organs (skin or muscle) |
Neurovascular island flap |
irreversibly damaged |
or tendon transfer |

Nerve Injuries |
527 |
Nerve Grafting
Nerve grafting is appropriate whenever there is a gap greater than 2.5 cm that persists after other measures such as nerve mobilization or transposition have been performed. Many studies have demonstrated regrowth of axons through the nerve graft and excellent return of function. The following outline covers the basic steps of nerve grafting:
•The proximal and distal nerve ends are cut back until healthy nerve is evident
•The fascicular groups are identified and isolated at different levels along the nerve
•A schematic drawing of the nerve ending cross sections is made to enable proper matching of the fascicles
•The nerve grafts are harvested and are cut slightly longer than the defect size
•At least five nerve grafts should be used for each of the three major nerves of the upper extremity (median, ulnar and radial nerves)
•The direction of the graft is reversed relative to its normal anatomic direction
•Grafts are used to join corresponding fascicular groups
•One to two 10-0 stitches are used in a single bite to secure the perineurium and epineurium of the graft to those of the injured nerve
Nerve Transfers
Nerve transfers should be considered when the proximal stump of the injured nerve cannot be located. The idea behind nerve transfers is to redirect part or all of a functioning motor or sensory nerve to the dennervated muscle or skin, respectively. There are many clinical applications of nerve transfers. The majority of these
are for high level injuries to the nerves of the upper extremity.
87
Postoperative Considerations
The site of repair should be immobilized for 7-14 days. At about 3-4 weeks after surgery, range of motion exercises should be initiated. These should continue for at least 6 months. Strengthening activities should begin at about 3 months. The progress of the regenerating axons can be followed clinically with a Tinel’s sign. Sensory reeducation should commence when the Tinel’s sign indicates axonal regeneration has reached the target and should continue for the first year. Regenerating axons do not always make the appropriate connections with their targets. They can grow aberrantly and form a tangled mass of fibrotic nerve and scar termed a neuroma. Neuromas can range from subclinical and painless to exquisitely painful. There is no optimal treatment for symptomatic neuromas. Options include en bloc resection, nerve transposition, crushing, freezing, cauterization, steroid injections, and a slew of other physical and chemical treatments.
Outcomes
It may take over one year to reach maximal functional recovery after a peripheral nerve injury. All other things being equal, younger patients (especially teenagers and younger) do better. Children do the best. They usually regain superior two-point discrimination compared to older patients. Sensory recovery is usually superior to motor recovery. Another generalization is that the more proximal the injury, the worse the prognosis. The outcome is impossible to predict, but can be optimized by early repair with meticulous surgical technique and a motivated patient who will comply with the rigorous postoperative therapy that is required.

528 |
Practical Plastic Surgery |
Pearls and Pitfalls
•In replants, nerve repair is occasionally delayed. In such instances, the two ends of any divided nerve should be tagged with a suture so that they can easily retrieved at the time of repair.
•Patients may have a normal motor exam despite a nerve injury, such as partial transection. Exploration is warranted if the mechanism and anatomic site of injury place the nerve at risk.
•During direct nerve repair or nerve grafting, the adjacent joints should be flexed and extended to ensure that both sides of the repair are tension free.
•In regards to nerve regeneration, one suture line is always better for axons to have to cross than two suture lines. Therefore, direct repair should be undertaken whenever possible.
•The minimal numbers of sutures should be used to achieve proper fascicular alignment.
Suggested Reading
1.Chiu DTW. Nerve repair in the upper limb. Grabb and Smith’s Plastic Surgery. 5th ed. Philadelphia: Lippincott-Raven, 1997.
2.Sunderland S, ed. Nerves and Nerve Injuries. 2nd ed. Edinburgh: Churchill Livingstone, 1978:69-141.
3.Seddon HJ. Three types of nerve injury. Brain 1943; 66:237.
4.Frykman GK, Wolf A, Coyle T. An algorithm for management of peripheral nerve injuries. Orthop Clin North Am 1981; 12:239.
5.Grabb WC. Median and ulnar nerve suture. An experimental study comparing primary and secondary repair in monkeys. J Bone Joint Surg 1968; 50A:964.
6.Weber RA, Breidenbach WC, Brown RE et al. A randomized prospective study of
87polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg 2000; 106:1036.
7.Jabaley ME. Current concepts of nerve repair. Clin Plast Surg 1981; 8:33.
8.Millesi H. Fascicular nerve repair and interfascicular nerve grafting. In: Daniel, Terzis, eds. Reconstructive Microsurgery. Boston: Little Brown, 1977.
9.Nath RK, Mackinnon SE. Nerve transfers in the upper extremity. Hand Clin 2000; 16(1):131.
10.Wilgis EFS. Nerve repair and grafting. In: Green DP, ed. Operative Hand Surgery. 2nd ed. New York: Churchill Livingstone, 1988:1373-1404.
11.Mackinnon SE, Dellon AL. Algorithm for management of painful neuroma. Contemp Orthop 1986; 13:15.


530 |
Practical Plastic Surgery |
Diagnostic Imaging
Arteriography is the most accurate means of detecting a vascular injury when the clinical exam is equivocal. In one large series of brachial artery injuries, only 60% of patients had an absent pulse. Patients who have a distal pulse, but other signs suggesting arterial injury, should undergo angiographic evaluation. Studies have demonstrated that it is far superior to ultrasound (including color flow Doppler) at diagnosing intimal injuries and other smaller arterial defects. Unless the injury is due to a high velocity projectile, emergent arteriography is not needed for penetrating trauma that is more than 5 cm from a named vessel.
Recent evidence supports the use of helical computed tomographic angiography (CTA) as an alternative to traditional arteriography. It is as sensitive and specific as arteriography. The main advantages of CTA are that it is less invasive and does not require the presence of an interventional radiologist. It is not, however, available at all centers. Furthermore, arteriography can be therapeutic as well: in select cases, the interventionalist can attempt endoluminal repair, such as stent placement at the site of an intimal tear.
What should be done for those patients with angiographic evidence of small intimal flaps or other insignificant vascular injuries? Nonoperative management is safe as long as the patient can be followed clinically and the following signs are absent: active hemorrhage, absent distal pulse, distal ischemia, expanding or pulsatile hematoma, bruit or thrill; 90% of patients managed nonoperatively will never require surgery.
Operative Management
As in all vascular surgical procedures, the initial goal is to gain proximal and distal control of the injured vessel. Once this is achieved and the vessel ends have
88been trimmed back to normal appearing artery, primary anastomosis should be attempted. Large series have shown that direct repair is possible about a third of the time. The inability to perform primary anastomosis is a result of undue tension when the two ends of the vessel are approximated, or because a segment of artery is damaged beyond repair. The second choice to direct repair is to use a piece of vein as an interpositional graft. If there is not adequate vein available, PTFE grafts should be used. Synthetic grafts are a last resort, since their long-term patency is inferior to autogenous vein. Ligation of the axillary or brachial artery should only be done as damage control in the poly-trauma patient with life-threatening injuries.
If concomitant injuries are present, arterial repair takes precedence. Fractures should be repaired following this, and the vascular anastomosis must be examined following any orthopedic procedure to ensure that the anastomotic site is intact and free from tension. Any injured vein that is not easily repairable should be ligated. Finally, any damaged nerves should be repaired using microsurgical technique. Nerve repair is discussed in detail in a separate chapter.
Axillary Artery
When the point of penetration is in the shoulder region and the pulse in the arm is absent, one may consider performing preoperative angiography in order to determine the precise point of vascular injury. This will help determine the surgical approach. When the external injury is more distal on the extremity, it is usually evident roughly where the vascular damage has occurred.
Vascular Trauma |
531 |
|
|
|
The approach for axillary artery injuries is with the arm abducted 90˚ and exter- |
|
|||
|
||||
nally rotated. The incision should extend from the lower, midclavicle to the |
|
|||
anteromedial upper arm. The axillary fascia is opened, and the pectoralis major and |
|
|||
minor are divided near their insertion. After the segment of injured artery is identi- |
|
|||
fied and vascular control obtained, the other critical structures such as the axillary |
|
|||
vein and adjacent nerves are evaluated. If the limb is ischemic from an occlusive |
|
|||
thrombus, proximal and distal balloon angioplasty is performed. Before restoration |
|
|||
of flow, the artery should be flushed with a heparin-containing saline solution. |
|
|
|
|
Brachial Artery |
|
|
|
|
The approach for brachial artery injuries is via an incision along the medial arm, |
|
|||
between the biceps and triceps muscles. The median nerve should be identified and |
|
|||
retracted away. If the injury is near the elbow, A “lazy S” type incision over the |
|
|||
antecubital fossa should be made with the horizontal limbs oriented longitudinally |
|
|||
on the arm and forearm. The brachial artery bifurcation should be exposed. If an |
|
|||
interpositional vein graft is needed, a segment of cephalic vein can be used. |
|
|
|
|
Radial and Ulnar Artery |
|
|
|
|
The proximal radial artery is exposed via an incision extending from the an- |
|
|||
tecubital fossa along the radial side of the forearm. The middle and distal seg- |
|
|||
ments are approached via an incision along the medial border of the brachioradialis |
|
|||
muscle. The ulnar artery is approached by making an incision extending from |
|
|||
below the medial epicondyle along the lateral border of flexor carpi ulnaris. Inju- |
|
|||
ries of the radial and ulnar arteries at the wrist should be explored through longi- |
|
|||
tudinal incisions. |
|
|
|
|
Outcomes |
|
|
|
|
|
|
88 |
||
Good upper limb function will be more likely the more distal in the extremity |
||||
|
||||
the vascular injury. Hence, isolated radial artery injuries do much better than |
|
|||
axillary arterial trauma. The more proximal injuries have a higher likelihood of |
|
|||
associated nerve injuries. This is especially true of blunt traumatic injuries. For |
|
|||
instance, over 60% of brachial artery injuries have a concomitant nerve injury, |
|
|||
usually the median nerve. Most limbs can ultimately be salvaged, with the length |
|
|||
of ischemia prior to reperfusion being the primary determinant. Amputation rates |
|
|||
range from 5-15%. |
|
|
|
|
Isolated radial or ulnar artery injuries almost never result in hand ischemia. In |
|
|||
fact, in the absence of an ischemic hand, the injured vessel can be safely ligated |
|
|||
without any significant long-term complications such as hand claudication. Minor |
|
|||
complications can occur; however the incidence is no greater than in patients who |
|
|||
have undergone operative repair. |
|
|
|
|
Complications |
|
|
|
|
Early complications necessitating emergent reexploration of the site include |
|
|||
thrombosis, hematoma and hemorrhage. Another serious complication requiring |
|
|||
surgical intervention is compartment syndrome. It is due to the edema of reperfused |
|
|||
ischemic muscle. Diagnosing compartment syndrome can be difficult; the hallmark |
|
|||
is severe pain. The management of compartment syndrome is discussed in a separate |
|
|||
chapter. A limb that has been ischemic for greater than 6-8 hours prior to reperfusion |
|
|||
is at risk for muscle necrosis. In severe cases, amputation is often the only option. |
|
|||
|
|
|
|
|
