
Английский язык от И. Ерохина / тысячи / Батарейка тысячи
.docxBattery,
Electric, a device that produces
electric current without the use of moving parts. Strictly speaking,
a battery consists of two or more electric cells connected
electrically to provide a single source of electricity. However, in
common usage, the term battery is also applied to single,
 cells
such as those used in flashlights and electronic watches.
cells
such as those used in flashlights and electronic watches.
Batteries
are a source of direct current (DC). They are widely used to supply
electricity for equipment ranging in size from hearing aids to
automobiles, and to provide power for portable equipment and
equipment at remote locations. Batteries are generally
 ,
however, for large-scale uses, such as lighting streets and houses.
,
however, for large-scale uses, such as lighting streets and houses.
Cells
that provide electricity by transforming chemical energy into
electrical energy are called galvanic, or voltaic, cells. There are
two major types: primary cells and secondary cells. A primary cell is
one that requires replacement once the materials it contains are used
by the chemical reactions that take place in the cell. With a
secondary cell, the chemical reactions can be
 reversed to restore the materials used up in the cell. Secondary
cells form the basis of storage batteries. A third type, called a
fuel cell, uses outside materials that are continuously supplied to
the cell.
reversed to restore the materials used up in the cell. Secondary
cells form the basis of storage batteries. A third type, called a
fuel cell, uses outside materials that are continuously supplied to
the cell.
Cells that convert the energy of visible light into electricity are called photovoltaic, or solar, cells. Thermoelectric cells produce electricity from heat energy; nuclear batteries produce electricity from the radiation emitted by radioactive substances.
The Primary Cell
All
galvanic cells consist
 of a negative electrode and a positive electrode in an electrolytic
solution. The solution contains ions (electrically charged atoms or
groups of atoms) that
of a negative electrode and a positive electrode in an electrolytic
solution. The solution contains ions (electrically charged atoms or
groups of atoms) that
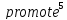 chemical changes in the electrode materials. When a cell is connected
to an electrical circuit, electrons flow from the negative electrode
through the circuit into the positive electrode. The flow of
electrons, or electric current, is produced by a difference in
potential between the electrodes. The difference in potential is
measured in volts and is generally
chemical changes in the electrode materials. When a cell is connected
to an electrical circuit, electrons flow from the negative electrode
through the circuit into the positive electrode. The flow of
electrons, or electric current, is produced by a difference in
potential between the electrodes. The difference in potential is
measured in volts and is generally
 to as voltage. It is caused by an electromotive force (emf) resulting
from the relative strength with which atoms of each electrode attract
electrons. The atoms of the positive electrode
to as voltage. It is caused by an electromotive force (emf) resulting
from the relative strength with which atoms of each electrode attract
electrons. The atoms of the positive electrode
 a stronger attraction for electrons than do the atoms of the negative
electrode. When a current is established between the two electrodes,
chemical reactions at each electrode proceed spontaneously, supplying
free electrons to the circuit from the negative electrode and, at the
same time, taking up free electrons from the circuit at the positive
electrode.
a stronger attraction for electrons than do the atoms of the negative
electrode. When a current is established between the two electrodes,
chemical reactions at each electrode proceed spontaneously, supplying
free electrons to the circuit from the negative electrode and, at the
same time, taking up free electrons from the circuit at the positive
electrode.
In chemical terms, the type of reaction that occurs at the negative electrode is called oxidation and the type of reaction that occurs at the positive electrode is called reduction. Together, they constitute an oxidation-reduction, or redox, reaction. The oxidized material loses electrons and the material that is reduced gains them. These processes can best be illustrated by using the zinc-mercury cell as an example. In this cell, zinc atoms make up the negative electrode and mercuric oxide molecules the positive electrode. When the two electrodes are connected electrically, the zinc is oxidized and the mercuric oxide reduced.
A simplified description of the cell's operation begins with the oxidation of an atom of zinc, which loses its two outer electrons. The resulting positive zinc ion combines with a negative oxygen ion in the electrolyte. The two free electrons left in the negative electrode enter the external circuit and pass to the positive electrode. There they combine with a molecule of mercuric oxide, reducing it. The mercury breaks away from the oxygen as a neutral metal atom and the oxygen enters the electrolyte as a negative oxygen ion.
The reactions in a cell continue until the materials in the cell are used up. In general, the larger a particular type of cell is, the more electrical energy it can deliver. However, such effects as the accumulation of waste products from the chemical reactions can decrease a cell's performance. Commercial cells are designed to lessen these effects, which are known collectively as polarization.
Most commercial primary cells use zinc as the negative electrode, a metallic oxide as the positive electrode, and an acidic or alkaline solution formed by water and an electrolyte as the electrolytic solution. Most primary cells today are dry cells—the electrolytic solution is in the form of a moist paste or is held in absorbent materials so that it is not free to flow.
History
The
first battery was made in about 1800 by Count Alessandro Volta, an
Italian scientist. It consisted of a
 of alternating zinc and silver discs with a
of alternating zinc and silver discs with a
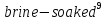 separating material after every second disc. The voltaic pile, as the
battery came to be known, has the disadvantage that its voltage
quickly drops because of waste products that accumulate near the
discs during discharge.
separating material after every second disc. The voltaic pile, as the
battery came to be known, has the disadvantage that its voltage
quickly drops because of waste products that accumulate near the
discs during discharge.
A
cell that overcame this problem was developed by an English
scientist, John F. Daniell, in 1836. An improved Daniell cell was a
 source of electrical energy for telegraphy through the end of the
19th century.
source of electrical energy for telegraphy through the end of the
19th century.
The
first storage battery was built by Gaston Plant in 1859. The battery,
called an accumulator, used lead plates
 in a solution of
in a solution of
 ,
basically the same system as that used in the lead-acid batteries of
today. Another storage battery system, using plates of iron and
nickel in an alkaline solution, was invented by Thomas A. Edison in
1901.
,
basically the same system as that used in the lead-acid batteries of
today. Another storage battery system, using plates of iron and
nickel in an alkaline solution, was invented by Thomas A. Edison in
1901.
The
carbon-zinc cell was developed by Georges Leclanche, a French
chemist, in the late 1860's. The original cell was a wet cell—one
that used a free-flowing electrolytic solution. The carbon-zinc dry
cell, in which the electrolytic solution is
 ,
was developed in the 1880's.
,
was developed in the 1880's.
In the mid-1900's, several types of cells were developed and improved. The mercury cell was developed during World War II. In 1954 the first practical solar battery was demonstrated. The first nuclear batteries were made in the late 1950's.
During the 1970's, a growing number of battery-powered electronic items for consumer use stimulated the production of numerous types of cells in many shapes and sizes. By the early 1980's lithium cells had been developed for consumer use.
