
- •TABLE OF CONTENTS
- •CHAPTER 1 Structure of Materials
- •CHAPTER 2 Composition of Materials
- •CHAPTER 3 Phase Diagram Sources
- •Compressive Strength
- •Yield Strength
- •Shear Strength
- •Hardness
- •Abrasion Resistance
- •Fracture Toughness
- •Tensile Modulus
- •Young’s Modulus
- •Elastic Modulus
- •Compression Modulus
- •Bulk Modulus
- •Torsion Modulus
- •Modulus of Rupture
- •Elongation
- •Area Reduction
- •Viscosity
- •Dissipation Factor
- •Dielectric Strength
- •Tangent Loss
- •Density
- •Heat of Fusion
- •Thermal Conductivity
- •Thermal Expansion
- •Compressive Strength
- •Yield Strength
- •Flexural Strength
- •Friction
- •Abrasion Resistance
- •Poisson’s Ratio
- •Elongation
- •Area Reduction
- •Dissipation Factor
- •Tangent Loss
- •Permittivity
- •Arc Resistance
- •Flammability

CHAPTER 3 Phase Diagram Sources
List of Tables |
Phase Diagram Sources |
©2001 CRC Press LLC
Phase Diagrams are especially useful tools for the field of materials science and engineering. In the last decade, a substantial effort has been made within the materials community to provide a comprehensive set of accurate phase equilibria information. Cooperative efforts involving academia, industry, and government have been coordinated through the professional societies, ASM International and the American Ceramic Society. As a result, the following references are available and new updates will become available on a regular basis.
|
Table 63. PHASE DIAGRAM SOURCES |
|
|
|
|
Society |
|
Source |
|
|
|
|
|
|
American Ceramic |
|
Phase Diagrams for Ceramists, Vols. 1-12, American Ceramic |
|
Society, Westerville, Ohio, 1964, 1969, 1975, 1981, 1983, 1987, |
|
Society |
|
|
|
1989, 1989, 1992, 1994, 1995, and 1996. |
|
|
|
|
|
|
Binary Alloy Phase Diagrams, Second Edition, Vols. 1, 2 and 3, |
ASM International |
|
T.B. Massalski, et.al., ed., ASM International, Materials Park, |
|
|
Ohio, 1990. |
ASM International |
|
ASM Handbook, Vol. 3, ASM International, Materials Park, |
|
Ohio, 1992. |
|
|
|
|
|
|
|
©2001 CRC Press LLC
Shackelford, James F. & Alexander, W. “Thermodynamic and Kinetic Data”
Materials Science and Engineering Handbook
Ed. James F. Shackelford & W. Alexander Boca Raton: CRC Press LLC, 2001

CHAPTER 4
Thermodynamic and
Kinetic Data
List of Tables |
Bond Strengths |
|
Bond Strengths in Diatomic Molecules |
|
Bond Strengths of Polyatomic Molecules |
|
Solubility of Copper and Copper Alloys |
|
Heat of Formation of Inorganic Oxides |
Phase Change
Phase Change Thermodynamic Properties for The Elements
Phase Change Thermodynamic Properties of Oxides
Melting Point
Melting Points of the Elements
Melting Points of Elements and Inorganic Compounds
Melting Points Of Ceramics
Heat of Fusion & Sublimation
Heat of Fusion For Elements and Inorganic Compounds
Heats of Sublimation of Metals and Their Oxides
Thermodynamic Coefficients
Key to Tables of Thermodynamic Coefficients
Thermodynamic Coefficients for Selected Elements
Thermodynamic Coefficients for Oxides
Entropy
Entropy of the Elements
©2001 CRC Press LLC
List of Tables
(Continued)
Vapor Pressure
Vapor Pressure of the Elements at Very Low Pressures Vapor Pressure of the Elements at Moderate Pressures Vapor Pressure of the Elements at High Pressures Vapor Pressure of Elements and Inorganic Compounds
Diffusion
Values of The Error Function
Diffusion in Metallic Systems
Diffusion of Metals into Metals
Diffusion in Semiconductors
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 1 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
H–H |
104.207 |
|
± 0.001 |
H–D |
105.030 |
|
± 0.001 |
D–D |
106.010 |
|
± 0.001 |
H–Li |
56.91 |
|
± 0.01 |
H–Be |
54 |
|
|
H–B |
79 |
|
± 1 |
H–C |
80.9 |
|
|
H–N |
75 |
|
± 4 |
H–O |
102.34 |
|
± 0.30 |
H–F |
135.9 |
|
± 0.3 |
H–Na |
48 |
|
± 5 |
H–Mg |
47 |
|
± 12 |
H–Al |
68 |
|
± 2 |
H–Si |
71.4 |
|
± 1.2 |
H–P |
82 |
|
± 7 |
H–S |
82.3 |
|
± 2.9 |
H–Cl |
103.1 |
|
|
H–K |
43.8 |
|
± 3.5 |
H–Ca |
40.1 |
|
|
H–Cr |
67 |
|
± 12 |
H–Mn |
56 |
|
± 7 |
H–Ni |
61 |
|
± 7 |
H –Cu |
67 |
|
± 2 |
H–Zn |
20.5 |
|
± 0.5 |
H –Ga |
68 |
|
± 5 |
H–Ge |
76.8 |
|
± 0.2 |
H–As |
65 |
|
± 3 |
H–Se |
73 |
|
± 1 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 2 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
H–Br |
87.4 |
|
± 0.5 |
H–Rb |
40 |
|
± 5 |
H–Sr |
39 |
|
± 2 |
H–Ag |
59 |
|
± 1 |
H–Cd |
16.5 |
|
± 0.1 |
H–In |
59 |
|
± 2 |
H–Sn |
63 |
|
± 1 |
H–Te |
64 |
|
± 1 |
H–I |
71.4 |
|
± 0.2 |
H–Cs |
42.6 |
|
± 0.9 |
H–Ba |
42 |
|
± 4 |
H–Yb |
38 |
|
± 1 |
H–Pt |
84 |
|
± 9 |
H–Au |
75 |
|
± 3 |
H–Hg |
9.5 |
|
|
H–Ti |
45 |
|
± 2 |
H–Pb |
42 |
|
± 5 |
H–Bi |
59 |
|
± 7 |
Li–Li |
24.55 |
|
± 0.14 |
Li–O |
78 |
|
± 6 |
Li– F |
137.5 |
|
± 1 |
Li–Cl |
111.9 |
|
± 2 |
Li–Br |
100.2 |
|
± 2 |
Li–I |
84.6 |
|
± 2 |
Be–Be |
17 |
|
|
Be–0 |
98 |
|
± 7 |
Be–F |
136 |
|
± 2 |
Be–S |
89 |
|
± 14 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 3 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Be–Cl |
92.8 |
|
± 2.2 |
Be–Au |
~ 67 |
|
|
B–B |
~ 67 |
|
± 5 |
B–N |
93 |
|
± 12 |
B–0 |
192.7 |
|
± 1.2 |
B–F |
180 |
|
± 3 |
B–S |
138.8 |
|
± 2.2 |
B–Cl |
119 |
|
|
B–Se |
110 |
|
± 4 |
B–Br |
101 |
|
± 5 |
B–Ru |
107 |
|
± 5 |
B–Rh |
114 |
|
± 5 |
B–Pd |
79 |
|
± 5 |
B–Te |
85 |
|
± 5 |
B–Ce |
~ 100 |
|
|
B–Ir |
123 |
|
± 4 |
B–Pt |
114 |
|
± 4 |
B–Au |
82 |
|
± 4 |
B–Th |
71 |
|
|
C–C |
144 |
|
± 5 |
C–N |
184 |
|
± 1 |
C–0 |
257.26 |
|
± 0.77 |
C–F |
128 |
|
± 5 |
C–Si |
104 |
|
± 5 |
C–P |
139 |
|
± 23 |
C–S |
175 |
|
± 7 |
C–Cl |
93 |
|
|
C–Ti |
~128 |
|
|
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 4 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
C–V |
133 |
|
|
C–Ge |
110 |
|
± 5 |
C–Se |
139 |
|
± 23 |
C–Br |
67 |
|
± 5 |
C–Ru |
152 |
|
± 3 |
C–Rh |
139 |
|
± 2 |
C–I |
50 |
|
± 5 |
C–Ce |
109 |
|
± 7 |
C–Ir |
149 |
|
± 3 |
C–Pt |
146 |
|
± 2 |
C–U |
111 |
|
± 7 |
N–N |
226.8 |
|
± 1.5 |
N–O |
150.8 |
|
± 0.2 |
N–F |
62.6 |
|
± 0.8 |
N–Al |
71 |
|
± 23 |
N–Si |
105 |
|
± 9 |
N–P |
148 |
|
± 5 |
N–S |
~ 120 |
|
± 6 |
N–Cl |
93 |
|
± 12 |
N–Ti |
111 |
|
|
N–As |
116 |
|
± 23 |
N–Se |
105 |
|
± 23 |
N–Br |
67 |
|
± 5 |
N–Sb |
72 |
|
± 12 |
N–I |
~.38 |
|
|
N–Xe |
55 |
|
|
N–Th |
138 |
|
± 1 |
N–U |
127 |
|
± 1 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 5 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
O–O |
118.86 |
|
± 0.04 |
O–F |
56 |
|
± 9 |
O–Na |
61 |
|
± 4 |
O–Mg |
79 |
|
± 7 |
O–Al |
116 |
|
± 5 |
O–Si |
184 |
|
± 3 |
O–P |
119.6 |
|
± 3 |
O–S |
124.69 |
|
± 0.03 |
O–Cl |
64.29 |
|
± 0.03 |
O–K |
57 |
|
± 8 |
O–Ca |
84 |
|
± 7 |
O–Sc |
155 |
|
± 5 |
O–Ti |
158 |
|
± 8 |
O–V |
154 |
|
± 5 |
O–Cr |
110 |
|
± 10 |
O–Mn |
96 |
|
± 8 |
O–Fe |
96 |
|
± 5 |
O–Co |
88 |
|
± 5 |
O–Ni |
89 |
|
± 5 |
O–Cu |
82 |
|
± 15 |
O–Zn |
≤ 66 |
|
|
O–Ga |
68 |
|
± 15 |
O–Ge |
158.2 |
|
± 3 |
O–As |
115 |
|
± 3 |
O–Se |
101 |
|
|
O–Br |
56.2 |
|
± 0.6 |
O–Rb |
(61) |
|
± 20 |
O–Sr |
93 |
|
± 6 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 6 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
O–Y |
162 |
|
± 5 |
O–Zr |
181 |
|
± 10 |
O–Nb |
189 |
|
± 10 |
O–Mo |
115 |
|
± 12 |
O–Ru |
115 |
|
± 15 |
O–Rh |
90 |
|
± 15 |
O–Pd |
56 |
|
± 7 |
O–Ag |
51 |
|
± 20 |
O–Cd |
≤ 67 |
|
|
O–In |
≤ 77 |
|
|
O–Sn |
127 |
|
± 2 |
O–Sb |
89 |
|
± 20 |
O–Fe |
93.4 |
|
± 2 |
O–I |
47 |
|
± 7 |
O–Xe |
9 |
|
± 5 |
O–Cs |
67 |
|
± 8 |
O–Ba |
131 |
|
± 6 |
O–La |
188 |
|
± 5 |
O–Ce |
188 |
|
± 6 |
O–Pr |
183.7 |
|
|
O–Nd |
168 |
|
± 8 |
O–Sm |
134 |
|
± 8 |
O–Eu |
130 |
|
± 10 |
O–Gd |
162 |
|
± 6 |
O–Tb |
165 |
|
± 8 |
O–Dy |
146 |
|
± 10 |
O–Ho |
149 |
|
± 10 |
O–Er |
147 |
|
± 10 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 7 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
O–Tm |
122 |
|
± 15 |
O–Yb |
98 |
|
± 15 |
O–Lu |
159 |
|
± 8 |
O–Hf |
185 |
|
± 10 |
O–Ta |
183 |
|
± 15 |
O–W |
156 |
|
± 6 |
O–Os |
< 142 |
|
|
O–Ir |
≤ 94 |
|
|
O–Pt |
83 |
|
± 8 |
O–Pb |
90.3 |
|
± 1.0 |
O–Bi |
81.9 |
|
± 1.5 |
O–Th |
192 |
|
± 10 |
O–U |
182 |
|
± 8 |
O–Np |
172 |
|
± 7 |
O–Pu |
163 |
|
± 15 |
O–Cm |
≤ 134 |
|
|
F–F |
37.5 |
|
± 2.3 |
F–Na |
114 |
|
± 1 |
F–Mg |
110 |
|
± 1 |
F–Al |
159: |
|
± 3 |
F–Si |
116 |
|
± 12 |
F–P |
105 |
|
± 23 |
F–Cl |
59.9 |
|
± 0.1 |
F–K |
118.9 |
|
± 0.6 |
F–Ca |
125 |
|
± 5 |
F–Sc |
141 |
|
± 3 |
F–Ti |
136 |
|
± 8 |
F–Cr |
104.5 |
|
± 4.7 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 8 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
F–Mn |
101.2 |
|
± 3.5 |
F–Ni |
89 |
|
± 4 |
F–Cu |
88 |
|
± 9 |
F–Ga |
138 |
|
± 4 |
F–Ge |
116 |
|
± 5 |
F–Br |
55.9 |
|
|
F–Rb |
116.1 |
|
± 1 |
F–Sr |
129.5 |
|
± 1.6 |
F–Y |
144 |
|
± 5 |
Mg–I |
~.68 |
|
|
Mg–Au |
59 |
|
± 23 |
Al–Al |
44 |
|
|
Al–P |
52 |
|
± 3 |
Al–S |
79 |
|
|
Al–Cl |
119.0 |
|
± 1 |
Al–Br |
103.1 |
|
|
Al–I |
88 |
|
|
Al–Au |
65 |
|
|
Al–U |
78 |
|
± 7 |
Si–Si |
76 |
|
± 5 |
Si–S |
148 |
|
± 3 |
Si–Cl |
105 |
|
± 12 |
Si–Fe |
71 |
|
± 6 |
Si–Co |
66 |
|
± 4 |
Si–Ni |
76 |
|
± 4 |
Si–Ge |
72 |
|
± 5 |
Si–Se |
127 |
|
± 4 |
Si–Br |
82 |
|
± 12 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 9 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Si–Ru |
95 |
|
± 5 |
Si–Rh |
95 |
|
± 5 |
Si–Pd |
75 |
|
± 4 |
Si–Te |
121 |
|
± 9 |
Si–Ir |
110 |
|
± 5 |
Si–Pt |
120 |
|
± 5 |
Si–Au |
75 |
|
± 3 |
P–P |
117 |
|
± 3 |
F–Ag |
84.7 |
|
± 3.9 |
F–Cd |
73 |
|
± 5 |
F–In |
121 |
|
± 4 |
F–Sn |
111.5 |
|
± 3 |
F–Sb |
105 |
|
± 23 |
F–I |
67? |
|
|
F–Xe |
11 |
|
|
F–Cs |
119.6 |
|
± 1 |
F–Ba |
140.3 |
|
± 1.6 |
F–Nd |
130 |
|
± 3 |
F–Sm |
126.9 |
|
± 4.4 |
F–Eu |
126.1 |
|
± 4.4 |
F–Gd |
141. |
|
± 46.5 |
F–Hg |
31 |
|
± 9 |
F–Ti |
106.4 |
|
± 4.6 |
F–Pb |
85 |
|
± 2 |
F–Bi |
62 |
|
|
F–Pu |
129 |
|
± 7 |
Na–Na |
18.4 |
|
|
Na–Cl |
97.5 |
|
± 0.5 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 10 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Na–K |
15.2 |
|
± 0.7 |
Na–Br |
86.7 |
|
± 1 |
Na–Rb |
14 |
|
± 1 |
Na–I |
72.7 |
|
± 1 |
Mg–Mg |
8? |
|
|
Mg–S |
56? |
|
|
Mg–Cl |
76 |
|
± 3 |
Mg–Br |
75 |
|
± 23 |
P–S |
70 |
|
|
P–Ga |
56 |
|
|
P–W |
73 |
|
± 1 |
P–Th |
90 |
|
|
S–S |
101.9 |
|
± 2.5 |
S–Ca |
75 |
|
± 5 |
S–Sc |
114 |
|
± 3 |
S–Mn |
72 |
|
± 4 |
S–Fe |
78 |
|
|
S–Cu |
72 |
|
± 12 |
S–Zn |
49 |
|
± 3 |
S–Ge |
131.7 |
|
± 0.6 |
S–Se |
91 |
|
± 5 |
S–Sr |
75 |
|
± 5 |
S–Y |
127 |
|
± 3 |
S–Cd |
48 |
|
|
S–In |
69 |
|
± 4 |
S–Sn |
111 |
|
± 1 |
S–Te |
81 |
|
± 5 |
S–Ba |
96 |
|
± 5 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 11 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
S–La |
137 |
|
± 3 |
S–Ce |
137 |
|
± 3 |
S–Pr |
122.7 |
|
|
S– Nd |
113 |
|
± 4 |
S–Eu |
87 |
|
± 4 |
S–Gd |
126 |
|
± 4 |
S–Ho |
102 |
|
± 4 |
S–Lu |
121 |
|
± 4 |
S–Au |
100 |
|
± 6 |
S–Hg |
51 |
|
|
S–Pb |
82.7 |
|
± 0.4 |
S–Bi |
75.4 |
|
± 1.1 |
S–U |
135 |
|
± 2 |
Cl–Cl |
58.066 |
|
± 0.001 |
Cl–K |
101.3 |
|
± 0.5 |
Cl–Ca |
95 |
|
± 3 |
Cl–Sc |
79 |
|
|
Cl–Ti |
26 |
|
± 2 |
Cl–Cr |
87.5 |
|
± 5.8 |
Cl–Mn |
86.2 |
|
± 2.3 |
Cl–Fe |
84? |
|
|
Cl–Ni |
89 |
|
± 5 |
Cl–Cu |
84 |
|
± 6 |
Cl–Zn |
54.7 |
|
± 4.7 |
Cl–Ga |
114.5 |
|
|
Cl–Ge |
82? |
|
|
Cl–Br |
52.3 |
|
± 0.2 |
Cl–Rb |
100.7 |
|
± 1 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 12 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Cl–Sr |
97 |
|
± 3 |
Cl–Y |
82 |
|
± 23 |
Cl–Ag |
75 |
|
± 9 |
Cl–Cd |
49.9 |
|
|
Cl–In |
103.3 |
|
|
CI–Sn |
75? |
|
|
Cl–Sb |
86 |
|
± 12 |
Cl–I |
50.5 |
|
± 0.1 |
Cl–Cs |
106.2 |
|
± 1 |
Cl–Ba |
106 |
|
± 3 |
Cl–Au |
82 |
|
± 2 |
Cl–Hg |
24 |
|
± 2 |
Cl–Ti |
89.0 |
|
± 0.5 |
Cl–Pb |
72 |
|
± 7 |
Cl–Bi |
72 |
|
± 1 |
Cl–Ra |
82 |
|
± 18 |
Ar–Ar |
0.2 |
|
|
K–K |
12.8 |
|
|
K–Br |
90.9 |
|
± 0.5 |
K–I |
76.8 |
|
± 0.5 |
Ca–I |
70 |
|
± 23 |
Ca–Au |
18 |
|
|
Sc–Sc |
25.9 |
|
± 5 |
Ti–Ti |
34 |
|
± 5 |
V–V |
58 |
|
± 5 |
Cr–Cr |
<37 |
|
|
Cr –Cu |
37 |
|
± 5 |
Cr–Ge |
41 |
|
± 7 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 13 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Cr–Br |
78.4 |
|
± 5 |
Cr–I |
68.6 |
|
± 5.8 |
Cr–Au |
51.3 |
|
± 3.5 |
Mn–Mn |
4 |
|
± 3 |
Mn–Se |
48 |
|
± 3 |
Mn–Br |
75.1 |
|
± 23 |
Mn–I |
67.6 |
|
± 2.3 |
Mn–Au |
44 |
|
± 3 |
Fe–Fe |
24 |
|
± 5 |
Fe–Ge |
50 |
|
± 7 |
Fe–Br |
59 |
|
± 23 |
Fe–Au |
45 |
|
± 4 |
Co–Co |
40 |
|
± 6 |
Co–Cu |
39 |
|
± 5 |
Co–Ge |
57 |
|
± 6 |
Co–Au |
51 |
|
± 3 |
Ni–Ni |
55.5 |
|
± 5 |
Ni–Cu |
48 |
|
± 5 |
Ni–Ge |
67.3 |
|
± 4 |
Ni–Br |
86 |
|
± 3 |
Ni–I |
70 |
|
± 5 |
Ni–Au |
59 |
|
± 5 |
Cu–Cu |
46.6 |
|
± 2.2 |
Cu–Ge |
49 |
|
± 5 |
Cu–Se |
70 |
|
± 9 |
Cu–Br |
79 |
|
± 6 5 |
Cu–Ag |
41.6 |
|
± 2.2 |
Cu–Sn |
42.3 |
|
± 4 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 14 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Cu–Te |
42 |
|
± 9 |
Cu–I |
47? |
|
|
Cu–Au |
55.4 |
|
± 2.2 |
Zn–Zn |
7 |
|
|
Zn–Se |
33 |
|
± 3 |
Zn–Te |
49? |
|
|
Zn–I |
33 |
|
± 7 |
Ga–Ga |
3 |
|
± 3 |
Ga–As |
50.1 |
|
± 0.3 |
Ga–Br |
101 |
|
± 4 |
Ga–Ag |
4 |
|
± 3 |
Ga–Te |
60 |
|
± 6 |
Ga–I |
81 |
|
± 2 |
Ga–Au |
51 |
|
± 23 |
Ge–Ge |
65.8 |
|
± 3 |
Ge–Se |
114 |
|
± |
Ge–Br |
61 |
|
± 7 |
Ge–Te |
93 |
|
± 2 |
Ge–Au |
70 |
|
± 23 |
As–As |
91.7 |
|
|
As–Se |
23 |
|
|
Se–Se |
79.5 |
|
± 0.1 |
Se–Cd |
~75 |
|
|
Se–in |
59 |
|
± 4 |
Se–Sn |
95.9 |
|
± 1.4 |
Se–Te |
64 |
|
± 2 |
Se–La |
114 |
|
± 4 |
Se–Nd |
92 |
|
± 4 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 15 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Se–Eu |
72 |
|
± 4 |
Se–Gd |
103 |
|
± 4 |
Se–Ho |
80 |
|
± 4 |
Se–Lu |
100 |
|
± 4 |
Se–Pb |
72.4 |
|
± 1 |
Se–Bi |
67.0 |
|
± 1.5 |
Bi–Br |
46.336 |
|
± 0.001 |
Br–Rb |
90.4 |
|
± 1 |
Br–Ag |
70 |
|
± 7 |
Br–Cd |
~38 |
|
|
Br–In |
93 |
|
|
Bi–Sn |
47 |
|
± 23 |
Br–Sb |
75 |
|
± 14 |
Br–I |
42.8 |
|
± 0.1 |
Br–Cs |
96.5 |
|
± 1 |
Br–Hg |
17.3 |
|
|
Br–Ti |
79.8 |
|
± 0.4 |
Br–Pb |
59 |
|
± 9 |
Br–Bi |
63.9 |
|
± 1 |
Rb–Rb |
12.2 |
|
|
Rb–I |
76.7 |
|
± 1 |
Sr–Au |
63 |
|
± 23 |
Y–Y |
38.3 |
|
|
Y–La |
48.3 |
|
|
Pd–Pd |
33? |
|
|
Pd–Au |
34.2 |
|
± 5 |
Ag–Ag |
41 |
|
± 2 |
Ag–Sn |
32.5 |
|
± 5 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 16 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Ag–Te |
70 |
|
± 23 |
Ag–I |
56 |
|
± 7 |
Ag–Au |
48.5 |
|
± 2.2 |
Cd–Cd |
2.7 |
|
± 0.2 |
Cd–I |
33 |
|
± 5 |
In–In |
23.3 |
|
± 2.5 |
In–Sb |
36.3 |
|
± 2.5 |
In–Te |
52 |
|
± 4 |
In–I |
80 |
|
|
Sn–Sn |
46.7 |
|
± 4 |
Sn–Te |
76 |
|
± 1 |
Sn–Au |
58.4 |
|
± 4 |
Sb–Sb |
71.5 |
|
± 1.5 |
Sb–Te |
61 |
|
± 4 |
Sb–Bi |
60 |
|
± 1 |
Te–Te |
63.2 |
|
± 0.2 |
Te–La |
91 |
|
± 4 |
Te–Nd |
73 |
|
± 4 |
Te–Eu |
58 |
|
± 4 |
Te–Gd |
82 |
|
± 4 |
Te–Ho |
62 |
|
± 4 |
Te–Lu |
78 |
|
± 4 |
Te–Au |
59 |
|
± 16 |
Te–Pb |
60 |
|
± 3 |
Te–Bi |
56 |
|
± 3 |
I–I |
36.460 |
|
± 0.002 |
I–Cs |
82.4 |
|
± 1 |
I–Hg |
9 |
|
|
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC

Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 17 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
I–Ti |
65 |
|
± 2 |
I–Pb |
47 |
|
± 9 |
I–Bi |
52 |
|
± 1 |
Xe–Xe |
~ 0.7 |
|
|
Cs–Cs |
11.3 |
|
|
Ba–Au |
38 |
|
± 14 |
La–Ld |
58.6 |
|
|
La–Au |
80 |
|
± 5 |
Ce–Ce |
66 |
|
± 1 |
Ce–Au |
76 |
|
± 4 |
Pr–Au |
74 |
|
± 5 |
Nd–Au |
70 |
|
± 6 |
Au–Au |
52.4 |
|
± 2.2 |
Au–Pb |
31 |
|
± 23 |
Au–U |
76 |
|
± 7 |
Hg–Hg |
4.1 |
|
± 0.5 |
Hg–Tl |
1 |
|
|
Tl–Tl |
15? |
|
|
Pb–Pb |
24 |
|
± 5 |
Pb–Bi |
32 |
|
± 5 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
©2001 CRC Press LLC
Table 64. BOND STRENGTHS IN DIATOMIC MOLECULES*
(SHEET 18 OF 18)
Molecule |
|
kcal • mol-1 |
|
|
|
|
|
|
|
|
|
Bi–Bi |
45 |
|
± 2 |
Po–Po |
44.4 |
|
± 2.3 |
At–At |
19 |
|
|
Th–Th |
<69 |
|
|
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–204.
* |
Notes for Table of Bond Strengths in Diatomic Molecules |
|
The strength of a chemical bond, D (R–X), often known as the bond dissociation energy, is defined as the heat of the reaction: RX –> R + X. It is given by: D(R–X) = ΔΗf˚(R) +
Hf˚(X) – Hf˚(RX). Some authors list bond strengths for 0K, but here the values for 298K are given because more thermodynamic data are available for this temperature. Bond strengths, or bond dissociation energies, are not equal to, and may differ considerable from, mean bond energies derived solely from thermochemical data on molecules and atoms.
The values in this table have usually been measured spectroscopically or by mass spectrometric analysis of hot gases effusing from a Knudsen cell.
©2001 CRC Press LLC

Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 1 OF 7)
|
|
Kcal • mol-1 |
|
|
|
|
|
Molecule |
Value |
|
Error |
|
|
|
|
|
|
|
|
H–CH |
102 |
|
± 2 |
H–CH2 |
110 |
|
± 2 |
H–CH3 |
104 |
|
± 1 |
H–ethynyl |
128 |
|
± 5 |
H–vinyl |
³ 108 |
|
± 2 |
H–C2H5 |
98 |
|
± 1 |
H–propargyl |
93.9 |
|
± 1.2 |
H–allyl |
89 |
|
± 1 |
H–cyclopropyl |
100.7 |
|
± 1 |
H–n–C3H7 |
98 |
|
± 1 |
H–i–C3H7 |
95 |
|
± 1 |
H–cyclobutyl |
96.5 |
|
± 1 |
H–cyclopropycarbinyl |
97.4 |
|
± 1.6 |
H–methdllyl |
83 |
|
± 1 |
H–s–C4H9 |
95 |
|
± 1 |
H–t–C4H9 |
92 |
|
± 1.2 |
H–cyclopentadien–1,3–yl–5 |
81.2 |
|
± 1.2 |
H–pentadien–1,4–yi–3 |
80 |
|
± 1 |
H–OH |
119 |
|
± 1 |
H–OCH3 |
103.6 |
|
± 1 |
H–OC2H5 |
103.9 |
|
± 1 |
H–OC(CH3)3 |
104.7 |
|
± 1 |
H–OC6H5 |
88 |
|
± 5 |
H–O2H |
90 |
|
± 2 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
©2001 CRC Press LLC
Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 2 OF 7)
|
|
Kcal • mol-1 |
|
|
|
Molecule |
Value |
Error |
H–O2CCH3 |
112 |
± 4 |
H–O2CC2H3 |
110 |
± 4 |
H–O2Cn–C3H7 |
103 |
± 4 |
H–ONO |
78.3 |
± 0.5 |
H–ONO2 |
101.2 |
± 0.5 |
H–SH |
90 |
± 2 |
H–SCH |
³ 88 |
|
H–SiH3 |
94 |
± 3 |
H–Si(CH3)3 |
90 |
± 3 |
BH3–BH3 |
35 |
|
HC=CH |
230 |
± 2 |
H2C=CH2 |
172 |
± 2 |
H3C–CH3 |
88 |
± 2 |
CH3–C(CH3)2CH:CH2 |
69.4 |
|
C6H5CH2–C2H5 |
69 |
± 2 |
C6H5CH(CH3)– CH3 |
71 |
|
C6H5CH2–n–C3H7 |
67 |
± 2 |
CH3–CH2CN |
72.7 |
± 2 |
CH3–C(CH3)2CN |
70.2 |
± 2 |
C6H5C(CH3 )(CN)–CH3 |
59.9 |
|
NC–CN |
128 |
± 1 |
C6H5CH2CO– CH2C6H5 |
65.4 |
|
C6H5CO– CF3 |
73.8 |
|
CH3CO– COCH3 |
67.4 |
± 2.3 |
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
©2001 CRC Press LLC

Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 3 OF 7)
|
|
Kcal • mol-1 |
|
|
|
|
|
Molecule |
Value |
|
Error |
|
|
|
|
|
|
|
|
C6H5CH2– COOH |
68.1 |
|
|
C6H5CH2– O2CCH3 |
67 |
|
|
C6H5CO– COC6H5 |
66.4 |
|
|
C6H5CH2– O2CC6H5 |
69 |
|
|
(C6H5CH2)2CH–COOH |
59.4 |
|
|
CH2F–CH2F |
88 |
|
± 2 |
CF2=CF2 |
76.3 |
|
± 3 |
CF3–CF3 |
96.9 |
|
± 2 |
C6H5CH2–NH2 |
71.9 |
|
± 1 |
C6H5NH–CH3 |
67.7 |
|
|
C6H5CH2–NHCH3 |
68.7 |
|
± 1 |
C6H5N(CH2)–CH3 |
65.2 |
|
|
C6H5CH2–N(CH3)2 |
60.9 |
|
± 1 |
CF3–NF2 |
65 |
|
± 2.5 |
CH2 = N2 |
≤ 41.7 |
|
± 1 |
CH3N:N–CH3 |
52.5 |
|
|
C2H5N:N–C2H5 |
50.0 |
|
|
i –C3H7N:N–i –C3H7 |
47.5 |
|
|
n –C4H9N:N–n –C4H9 |
50.0 |
|
|
i –C4H9N:N–i –C4H9 |
49.0 |
|
|
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
©2001 CRC Press LLC

Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 4 OF 7)
|
|
Kcal • mol-1 |
|
|
|
|
|
Molecule |
Value |
|
Error |
|
|
|
|
|
|
|
|
s –C4H9N:N–s –C4H9 |
46.7 |
|
|
t –C4H9N:N–t –C4H9 |
43.5 |
|
|
C6H5CH2N:N–C6H5CH2 |
37.6 |
|
|
CF3N:N–CF3 |
55.2 |
|
|
C2H5–NO2 |
62 |
|
|
O=CO |
127.2 |
|
± 0.1 |
CH3–O2SCH3 |
66.8 |
|
|
Allyl–O2SCH3 |
49.6 |
|
|
C6H5CH2–O2SCH3 |
52.9 |
|
|
C6H5S–CH3 |
60 |
|
|
C6H5CH2–SCH3 |
53.8 |
|
|
F–CH3 |
103 |
|
± 3 |
Cl–CN |
97 |
|
± 1 |
Cl–COC6H5 |
74 |
|
± 3 |
Cl–CF3 |
86.1 |
|
± 0.8 |
Cl–CCl2F |
73 |
|
± 2 |
Cl–C2F5 |
82.7 |
|
± 1.7 |
Br–CH3 |
70.0 |
|
± 1.2 |
Br–CN |
83 |
|
± 1 |
Br–COC6H5 |
64.2 |
|
|
Br–CF3 |
70.6 |
|
± 1.0 |
Br–CBr3 |
56.2 |
|
± 1.8 |
Br–C2F5 |
68.7 |
|
± 1.5 |
Br –n –C3F |
66.5 |
|
± 2.5 |
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
©2001 CRC Press LLC
Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 5 OF 7)
|
|
Kcal • mol-1 |
|
|
|
Molecule |
Value |
Error |
I–CH3 |
56.3 |
± 1 |
1–norbornyl |
62.5 |
± 2.5 |
I–CN |
73 |
± 1 |
I–CF3 |
53.5 |
± 2 |
CH3–Ga(CH3)2 |
59.5 |
|
CH3–CdCH3 |
54.4 |
|
CH3–HgCH3 |
57.5 |
|
C2H5–HgC2H5 |
43.7 |
± 1 |
n –C3H7–Hg n –C3H7 |
47.1 |
|
i –C3H7–Hg i –C3H7 |
40.7 |
|
C6H5–HgC6H5 |
68 |
|
CH3 –Tl(CH3)2 |
36.4 |
± 0.6 |
CH3–Pb(CH3)3 |
49.4 |
± 1 |
NH2–NH2 |
70.8 |
± 2 |
NH2–NHCH3 |
64.8 |
|
NH2 –N(CH3)2 |
62.7 |
|
NH2 –NHC6H5 |
51.1 |
|
NO–NO2 |
9.5 |
± 0.5 |
NO2–NO2 |
12.9 |
± 0.5 |
NF2–NF2 |
21 |
± 1 |
O–N2 |
40 |
|
O–NO |
73 |
|
HO–N:CHCH3 |
49.7 |
|
Cl–NF2 |
≈ 32 |
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
©2001 CRC Press LLC

Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 6 OF 7)
|
|
Kcal • mol-1 |
|
|
|
|
|
Molecule |
Value |
|
Error |
|
|
|
|
|
|
|
|
HO–OH |
51 |
|
± 1 |
CH3O–OCH3 |
36.9 |
|
± 1 |
HO–OC(CH3)3 |
42.5 |
|
|
C2H5O–OC2H5 |
37.3 |
|
± 1.2 |
n –C3H7O–O n –C3H7 |
37.2 |
|
± 1 |
i –C3H7O–O i –C3H7 |
37.0 |
|
± 1 |
s –C4H9O–O s –C4H9 |
36.4 |
|
± 1 |
t –C4H9O–O t –C4H9 |
37.4 |
|
± 1 |
(CH3)3CCH2O–OCH2C(CH3)3 |
36.4 |
|
± 1 |
O–O2CIF |
58.4 |
|
|
CH3CO2–O2CCH3 |
30.4 |
|
± 2 |
C2H5CO2–O2CC2H5 |
30.4 |
|
± 2 |
n –C3H7CO2–O2Cn –C3H7 |
30.4 |
|
± 2 |
O–SO |
132 |
|
± 2 |
F–OCF3 |
43.5 |
|
± 0.5 |
Cl–OH |
60 |
|
± 3 |
O–ClO |
59 |
|
± 3 |
Br–OH |
56 |
|
± 3 |
I–OH |
56 |
|
± 3 |
ClO3–ClO4 |
58.4 |
|
|
|
|
|
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
©2001 CRC Press LLC
Table 65. BOND STRENGTHS OF POLYATOMIC MOLECULES*
(SHEET 7 OF 7)
|
|
Kcal • mol-1 |
|
|
|
Molecule |
Value |
Error |
O = PF3 |
130 |
± 5 |
O = PCl3 |
122 |
± 5 |
O = PBr3 |
119 |
± 5 |
SiH3–SiH3 |
81 |
± 4 |
(CH3)3Si–Si(CH3)3 |
80.5 |
|
To convert kcal to KJ, multiply by 4.184.
Source: data from: Kerr, J. A., Parsonage, M. J., and Trotman–Dickenson, A. F., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, 1974, F–213.
*The values refer to a temperature of 298 K and have mostly been determined by kinetic methods. Some have been calculated from formation of the species involved according to equations:
D(R–X) = Hf˚ (R•) + Hf˚(X•) – Hf˚ (RX) or D(R–X) = 2 Hf˚ (R•) – Hf˚ (RR)
©2001 CRC Press LLC

Table 66. SOLUBILITY OF COPPER AND COPPER ALLOYS
|
|
|
Solid Solubility at 20 °C |
Family |
Wrought Alloys UNS Numbers |
Principal Alloying Element |
(at. %) |
|
|
|
|
|
|
|
|
Brasses |
C20000, C30000, C40000, C66400 to C69800 |
Zn |
37 |
Phosphor bronzes |
C50000 |
Sn |
9 |
Aluminum bronzes |
C60600 to C64200 |
Al |
19 |
Silicon bronzes |
C64700 to C66100 |
Si |
8 |
Copper nickels, nickel silvers |
C70000 |
Ni |
100 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p439, (1993).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 1 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 Ac(c) + 3/2 O2(g) = Ac2O3(c) |
298.16–1,000K |
–446,090 |
–16.12 |
– |
– |
+109.89 |
2 Al(c) + 1/2 O2(g) = Al2O(g) |
298.16–931.7K |
–31,660 |
+14.97 |
– |
– |
–72.74 |
2 Al(l) + 1/2 O2(g) = Al2O(g) |
931.7–2,000K |
–38,670 |
+10.36 |
– |
– |
–51.53 |
Al(c) + 1/2 O2(g) = AlO(g) |
298.16–931.7K |
+10,740 |
+5.76 |
– |
– |
–37.61 |
Al(l) + 1/2 O2(g) = AlO(g) |
931.7–2,000K |
+8,170 |
+5.76 |
– |
– |
–34.85 |
2 Al(c) + 3/2 O2(g) = Al2O3 (corundum) |
298.16–931.7K |
–404,080 |
–15.68 |
+2.18 |
+3.935 |
+123.64 |
2 Al(l) + 3/2 O2(g) = Al2O3 (corundum) |
931.7–2,000K |
–407,950 |
–6.19 |
–0.78 |
+3.935 |
+102.37 |
2 Sb(c) + 3/2 O2(g) = Sb2O3 (cubic) |
298.16–842K |
–169,450 |
+6.12 |
–6.01 |
–0.30 |
+52.21 |
2 Sb(c) + 3/2 O2(g) = Sb2O3 (orthorhombic) |
298.16–903K |
–168,060 |
+6.12 |
–6.01 |
–0.30 |
+50.56 |
2 As(c) + 3/2 O2(g) = As2O3 (orthorhombic) |
298.16–542K |
–154,870 |
+29.54 |
–21.33 |
–0.30 |
–8.83 |
2 As(c) + 3/2 O2(g) = As2O3 (monoclinic) |
298.16–586K |
–150,760 |
+29.54 |
–21.33 |
–0.30 |
–16.95 |
2 As(c) + 5/2 O2(g) = As2O5(c) |
298.16–883K |
–217,080 |
+12.32 |
–4.65 |
–0.50 |
+80.50 |
|
|
|
|
|
|
|
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 2 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ba(α) + 1/2 O2(g) = BaO(c) |
298.16–648K |
–134,590 |
–7.60 |
+0.87 |
+0.42 |
+45.76 |
Ba(β) + 1/2 O2(g) = BaO(c) |
648–977K |
–134,140 |
–3.34 |
–0.56 |
+0.42 |
+34.01 |
Be(c) + 1/2 O2(g) = BeO(c) |
298.16–1,556K |
–144,220 |
–1.91 |
–0.46 |
+1.24 |
+30.64 |
Bi(c) + 1/2 O2(g) = BiO(c) |
298.16–544K |
–50,450 |
–4.61 |
– |
– |
+35.51 |
Bi(l) + 1/2 O2(g) = BiO(c) |
544–1,600K |
–52,920 |
–4.61 |
– |
– |
+40.05 |
2 Bi(c) + 3/2 O2(g) = Bi2O3(c) |
298.16–544K |
–139,000 |
–11.56 |
+2.15 |
–0.30 |
+96.52 |
2 Bi(l) + 3/2 O2(g) = Bi2O3(c) |
544–1,090K |
–142,270 |
+2.30 |
–3.25 |
–0.30 |
+67.55 |
2 B(c) + 3/2 O2(g) = B2O(c) |
298.16–723K |
–304,690 |
+11.72 |
–7.55 |
+0.355 |
+34.25 |
2 B(c) + 3/2 O2(g) = B2O3(gl) |
298.16–723K |
–298,670 |
+26.57 |
–15.90 |
–0.30 |
–10.40 |
Cd(c) + 1/2 O2(g) = CdO(c) |
298.16–594K |
–62,330 |
–2.05 |
+0.71 |
–0.10 |
+29.17 |
Cd(l) + 1/2 O2(g) = CdO(c) |
594–1,038K |
–63,240 |
+2.07 |
–0.76 |
–0.10 |
+20.14 |
Ca(α) + 1/2 O2(g) = CaO(c) |
298.16–673K |
–151,850 |
–6.56 |
+1.46 |
+0.68 |
+43.93 |
Ca(β) + 1/2 O2(g) = CaO(c) |
673–1,124K |
–151,730 |
–4.14 |
+0.41 |
+0.68 |
+37.63 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 3 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C(graphite) + 1/2 O2(g) = CO(g) |
298.16–2,000K |
–25,400 |
+2.05 |
+0.27 |
–1.095 |
–28.79 |
C(graphite) + O2(g) = CO2(g) |
298.16–2,000K |
–93,690 |
+1.63 |
–0.7 |
–0.23 |
–5.64 |
2 Ce(c) + 3/2 O2(g) = Ce2O3(c) |
298.16–1,048K |
–435,600 |
–4.60 |
– |
– |
+92.84 |
2 Ce(l) + 3/2 O2(g) = Ce2O3(c) |
1,048–1,900K |
–440,400 |
–4.60 |
– |
– |
+97.42 |
Ce(c) + O2(g) = CeO2(c) |
298.16–1,048K |
–245,490 |
–6.42 |
+2.34 |
–0.20 |
+67.79 |
Ce(l) + O2(g) = CeO2(c) |
1,048–2,000K |
–247,930 |
+0.71 |
–0.66 |
–0.20 |
+51.73 |
2 Cs(c) + 1/2 O2(g) = Cs2O(c) |
298.16–301.5K |
–75,900 |
– |
– |
– |
+36.60 |
2 Cs(l) + 1/2 O2(g) = Cs2O(c) |
301.5–763K |
–76,900 |
– |
– |
– |
+39.92 |
2 Cs(l) + 1/2 O2(g) = Cs2O(l) |
763–963K |
–75,370 |
–9.21 |
– |
– |
+64.47 |
2 Cs(g) + 1/2 O2(g) = Cs2O(l) |
963–1,500K |
–113,790 |
–23.03 |
– |
– |
+145.60 |
2 Cs(c) + 3/2 O2(g) = Cs2O3(c) |
298.16–301.5K |
–112,690 |
–11.51 |
– |
– |
+110.10 |
2 Cs(l) + 3/2 O2(g) = Cs2O3(c) |
301.5–775K |
–113,840 |
–12.66 |
– |
– |
+116.77 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 4 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 Cs(l) + 3/2 O2(g) = Cs2O3(l) |
775–963K |
–110,740 |
–26.48 |
– |
– |
+152.70 |
2 Cs(g) + 3/2 O2(g) = Cs2O3(l) |
963–1,500K |
–148,680 |
–39.14 |
– |
– |
+229.87 |
Cl2(g) + 1/2 O2(g) = Cl2O(g) |
298.16–2,000K |
+17,770 |
–0.71 |
–0.12 |
+0.49 |
+16.81 |
1/2 Cl2(g) + 1/2 O2(g) = ClO(g) |
298.16–1,000K |
+33,000 |
– |
– |
– |
0.24 |
2 Cl2(g) + 3/2 O2(g) = ClO(g) |
298.16–500K |
+37,740 |
+5.76 |
– |
– |
+21.42 |
2 Cr(c) + 3/2 O2(g) = Cr2O3(β) |
298.16–1,823K |
–274,670 |
–14.07 |
+2.01 |
+0.69 |
+105.65 |
2 Cr(l) + 3/2 O2(g) = Cr2O3(β) |
1,823–2,000K |
–278,030 |
+2.33 |
–0.35 |
+1.57 |
+58.29 |
Cr(c) + O2(g) = CrO2 (c) |
298.16–1,000K |
–142,500 |
– |
– |
– |
+42.00 |
Cr(c) + 3/2 O2(g) = CrO3(c) |
298.16–471K |
–141,590 |
–13.82 |
– |
– |
+103.90 |
Cr(c) + 3/2 O2(g) = Cr2O3(l) |
471–600K |
–141,580 |
–32.24 |
– |
– |
+153.14 |
Co(α,β) + 1/2 O2(g) = CoO(c) |
298.16–1,400K |
–56,910 |
+0.69 |
– |
– |
+16.03 |
Co(γ) + 1/2 O2(g) = CoO(c) |
1,400–1,763K |
–58,160 |
–1.15 |
– |
– |
+22.71 |
2 Cu(c) + 1/2 O2(g) = Cu2O(c) |
298.16–1,357K |
+10,550 |
–1.15 |
–1.10 |
–0.10 |
+21.92 |
2 Cu(l) + 1/2 O2(g) = Cu2O(c) |
1,357–1,502K |
–43,880 |
+8.47 |
–2.60 |
–0.10 |
–3.72 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 5 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 Cu(l) + 1/2 O2(g) = Cu2O(l) |
1,502–2,000K |
–37,710 |
–12.48 |
+0.25 |
–0.10 |
+54.44 |
Cu(c) + 1/2 O2(g) = CuO(c) |
298.16–1,357K |
–37,740 |
–0.64 |
–1.40 |
–0.10 |
+24.87 |
Cu(l) + 1/2 O2(g) = CuO(c) |
1,357–1,720K |
–39,410 |
+4.17 |
–2.15 |
–0.10 |
+12.05 |
Cu(l) + 1/2 O2(g) = CuO(l) |
1,720–2,000K |
–41,060 |
–11.35 |
+0.25 |
–0.10 |
+59.09 |
2 Au(c) + 3/2 O2(g) = Au2O3(c) |
298.16–500K |
–2,160 |
–10.36 |
– |
– |
+95.14 |
Hf(c) + O2(g) = HfO2 (monoclinic) |
298.16–2,000K |
–268,380 |
–9.74 |
–0.28 |
+1.54 |
+78.16 |
H2(g) + 1/2 O2(g) = H2O(l) |
298.16–373.16K |
–70,600 |
–18.26 |
+0.64 |
–0.04 |
+91.67 |
H2(g) + 1/2 O2(g) = H2O(g) |
298.16–2,000K |
–56,930 |
+6.75 |
–0.64 |
–0.08 |
–8.74 |
D2(g) + 1/2 O2(g) = D2O(l) |
298.16–374.5K |
–72,760 |
–18.10 |
– |
– |
+93.59 |
D2(g) + 1 /2 O2(g) = D2O(g) |
298.16–2,000K |
–58,970 |
+5.50 |
–0.75 |
+0.085 |
–3.74 |
0.947 Fe(α) + 1/2 O2(g) = Fe0.9470(c) |
298.16–1,033K |
–65,320 |
–11.26 |
+2.61 |
+0.44 |
+48.60 |
0.947 Fe(α) + 1/2 O2(g) = Fe0.9470(c) |
1,033–1,179K |
–62,380 |
+4.08 |
–0.75 |
+0.235 |
+3.00 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 6 OF 16)
|
|
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
0.947 |
Fc(β) + 1/2 O2(g) = Fe0.9470(c) |
1,179–1,650K |
–66,750 |
–8.04 |
+0.67 |
–0.10 |
+42.28 |
|
0.947 |
Fe(γ) + 1/2 O2(g) = Fe0.9470(l) |
1,650–1,674K |
–64,200 |
–18.72 |
+1.67 |
–0.10 |
+73.45 |
|
0.947 |
Fe(γ) + 1/2 O2(g) = Fe0.9470(l) |
1,647–1,803K |
–59,650 |
–6.84 |
+0.25 |
–0.10 |
+34.81 |
|
0.947 |
Fe(δ) + 1/2 O2(g) = Fe0.9470(l) |
1,803–2,000K |
–63,660 |
–7.48 |
+0.25 |
–0.10 |
+39.12 |
|
3 |
Fe(α) + 2 O2(g) = Fe3O4(magnetite) |
298.16–900K |
–268,310 |
+5.87 |
–12.45 |
+0.245 |
+73.11 |
|
3 |
Fe(α) + 2 O2(g) = Fe3O4(β) |
900–1,033K |
–272,300 |
–54.27 |
+11.65 |
+0.245 |
+233.52 |
|
3 |
Fe(β) + 2 O2(g) = Fe3O4(β) |
1,033–1,179K |
–262,990 |
–5.71 |
+1.00 |
–0.40 |
+89.19 |
|
3 |
Fe(γ) + 2 O2(g) = Fe3O4(β) |
1,179–1,674K |
–276,990 |
~4.05 |
+5.50 |
–0.40 |
+213.52 |
|
2 |
Fe(α) + 3/2 O2(g) = Fe2O3(hematite) |
298.16–950K |
–200,000 |
–13.84 |
–1.45 |
+1.905 |
+108.26 |
|
2 |
Fe(α) + 3/2 O2(g) = Fe2O3(β) |
950–1,033K |
–202,960 |
–42.64 |
+7.85 |
+0.13 |
+188.48 |
|
2 |
Fe(β) + 3/2 O2(g) = Fe2O3(β) |
1,033–1,050K |
–196,740 |
–10.27 |
+0.75 |
–0.30 |
+92.26 |
|
2 |
Fe(β) + 3/2 O2(g) = Fe2O3(γ) |
1,050–1,179K |
–193,200 |
–0.39 |
–0.13 |
–0.30 |
+59.96 |
|
2 |
Fe(γ) + 3/2 O2(g) = Fe2O3(γ) |
1,179–1,674K |
–202,540 |
–25.95 |
+2.87 |
–0.30 |
+142.85 |
|
|
|
|
|
|
|
|
|
|
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 7 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
2 Fe(α) + 3/2 O2(g) = Fe2O3(γ) |
1,674–1,800K |
–192,920 |
–0.85 |
–0.13 |
–0.30 |
+61.21 |
Pb(c) + 1/2 O2(g) = PbO (red) |
298.16–600.5K |
–52,800 |
–2.76 |
–0.80 |
–0.10 |
+32.49 |
Pb(l) + 1/2 O2(g) = PbO (red) |
600.5–762K |
–53,780 |
–0.51 |
–1.75 |
–0.10 |
+28.44 |
Pb(c) + 1/2 O2(g) = PbO (yellow) |
298.16–600.5K |
–52,040 |
+0.81 |
–2.00 |
–0.10 |
+22.13 |
Pb(l) + 1/2 O2(g) = PbO (yellow) |
600.5–1,159K |
–53,020 |
+3.06 |
–2.95 |
–0.10 |
+18.08 |
I2(c) + 5/2 O2(g) = I2O5(c) |
298.16–386.8K |
–42,040 |
+2.30 |
– |
– |
+113.71 |
I2(l) + 5/2 O2(g) = I2O5(c) |
386.8–456K |
–43,490 |
+16.12 |
– |
– |
+81.70 |
I2(g) + 5/2 O2(g) = I2O5(c) |
456–500K |
–58,020 |
–6.91 |
– |
– |
+174.79 |
Ir(c) + O2(g) = IrO2(c) |
298.16–1,300K |
–39,480 |
+8.17 |
–6.39 |
–0.20 |
+20.33 |
3 Pb(c) + 2 O2(g) = Pb3O4(c) |
298.16–600.5K |
–174,920 |
+8.82 |
–8.20 |
–0.40 |
+72.78 |
Pb(c) + O2(g) = PbO2(c) |
298.16–600.5K |
–66,120 |
+0.64 |
–2.45 |
–0.20 |
+45.58 |
2 Li(c) + 1/2 O2(g) = Li2O(c) |
298.16–452K |
–142,220 |
–3.06 |
+5.77 |
–0.10 |
+34.19 |
|
|
|
|
|
|
|
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 8 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mg(c) + 1/2 O2(g) = MgO (periclase) |
298.16–923K |
–144,090 |
–1.06 |
+0.13 |
+0.25 |
+29.16 |
Mg(l) + 1/2 O2(g) = MgO (periclase) |
923–1,393K |
–145,810 |
+1.84 |
–0.62 |
+0.64 |
+23.07 |
Mg(g) + 1/2 O2(g) = MgO (periclase) |
1,393–2,000K |
–180,700 |
–3.75 |
–0.62 |
+0.64 |
+65.69 |
Mn(α) + 1/2 O2(g) = MnO(c) |
298.16–1,000K |
–92,600 |
–4.21 |
+0.97 |
+0.155 |
+29.66 |
Mn(β) + 1/2 O2(g) = MnO(c) |
1,000–1,374K |
–91,900 |
+1.84 |
–0.39 |
+0.34 |
+12.15 |
Mn(γ) + 1/2 O2(g) = Mno(c) |
1,374–1,410K |
–89,810 |
+7.30 |
–0.72 |
+0.34 |
–6.05 |
Mn(δ) + 1/2 O2(g) = MnO(c) |
1,410–1,517K |
–89,390 |
+8.68 |
–0.72 |
+0.34 |
–10.70 |
Mn(l) + 1/2 O2(g) = MnO(c) |
1,517–2,000K |
–93,350 |
+7.99 |
–0.72 |
+0.34 |
–5.90 |
3 Mn(α) + 2 O2(g) = Mn3O4(α) |
298.16–1,000K |
–332,400 |
–7.41 |
+0.66 |
+0.145 |
+106.62 |
2 Mn(α) + 3/2 O2(g) = Mn2O3(c) |
298.16–1,000K |
–230,610 |
–5.96 |
–0.06 |
+0.945 |
+80.74 |
Mn(α) + O2(g) = MnO2(c) |
298.16–1,000K |
–126,400 |
–8.61 |
+0.97 |
+1.555 |
+70.14 |
2 Hg(l) + 1/2 O2(g) = Hg2O(c) |
298.16–629.88K |
–22,400 |
–4.61 |
– |
– |
+43.29 |
Hg(l) + 1/2 O2(g) = HgO (red) |
298.16–629.88K |
–21,760 |
+0.85 |
–2.47 |
–0.10 |
+24.81 |
Mo(c) + O2(g) = MoO2(c) |
298.16–2,000K |
–132,910 |
–3.91 |
– |
– |
+47.42 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 9 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mo(c) + 3/2 O2(g) = MoO3(c) |
298.16–1,068K |
–182,650 |
–8.86 |
–1.55 |
+1.54 |
+90.07 |
Ni(α) + 1/2 O2(g) = NiO(c) |
298.16–633K |
–57,640 |
–4.61 |
+2.16 |
–0.10 |
+34.41 |
Ni(β) + 1/2 O2(g) = NiO(c) |
633–1,725K |
–57,460 |
–0.14 |
–0.46 |
–0.10 |
+23.27 |
2 Nb(c) + 2 O2(g) = Nb2O4(c) |
298.16–2,000K |
–382,050 |
–9.67 |
– |
– |
+116.23 |
2 Nb(c) + 5/2 O2(g) = Nb2O5(c) |
298.16–1,785K |
–458,640 |
–16.14 |
–0.56 |
+1.94 |
+157.66 |
2 Nb(c) + 5/2 O2(g) = Nb2O5(l) |
1,785–2,000K |
–463,630 |
–66.04 |
+2.21 |
–0.50 |
+317.84 |
N2(g) + 1/2 O2(g) = N2O(g) |
298.16–2,000K |
18,650 |
–1.57 |
–0.27 |
+0.92 |
+23.47 |
3/2 O2(g) = O3(g) |
298.16–2,000K |
+33,980 |
+2.03 |
–0.48 |
+0.36 |
+11.45 |
P (white) + 1/2 O2(g) = PO(g) |
298.16–317.4K |
–9,370 |
+2.53 |
– |
– |
–25.40 |
P(l) + 1/2 O2(g) = PO(g) |
317.4–553K |
–9,390 |
+3.45 |
– |
– |
–27.63 |
4 P (white) + 5 O2(g) = P4H10 (hexagonal) |
298.16–317.4K |
–711,520 |
+95.67 |
–51.50 |
–1.00 |
–28.24 |
2 K(c) + 1/2 O2(g) = K2O(c) |
298.16–336.4K |
–86,400 |
– |
– |
– |
+33.90 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 10 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 K(l) + 1/2 O2(g) = K2O(c) |
336.4–1,049K |
–87,380 |
+1.15 |
– |
– |
+33.90 |
2 K(g) + 1/2 O2(g) = K2O(c) |
1,049–1,500K |
–133,090 |
–16.12 |
– |
– |
+129.64 |
Ra(c) + 1/2 O2(g) = RaO(c) |
298.16–1,000K |
–130,000 |
– |
– |
– |
+23.50 |
Re(c) + 3/2 O2(g) = ReO3(c) |
298.16–433K |
–149,090 |
–16.12 |
– |
– |
+110.49 |
Re(c) + 3/2 O2(g) = ReO3(l) |
433–1,000K |
–146,750 |
–31.32 |
– |
– |
+145.16 |
2Re(c) + 7/2 02(g) = Re2O7(c) |
298.16–569K |
–301,470 |
–34.64 |
– |
– |
+250.57 |
2 Re(c) + 7/2 02(g) = Re2O7(l) |
569–635.5K |
–295,810 |
–73.68 |
– |
– |
+348.45 |
2 Re(c) + 4 O2(g) = Re2O8(l) |
420–600K |
–318,470 |
–87.50 |
– |
– |
+425.32 |
2 Rb(c) + 1/2 O2(g) = Rb2O(c) |
298.16–312.2K |
–78,900 |
– |
– |
– |
+32.20 |
2 Rb(l) + 1/2 O2(g) = Rb2O(c) |
312.2–750K |
–79,950 |
– |
– |
– |
+35.56 |
Se(c) + 1/2 O2(g) = SeO(g) |
298.16–490K |
+9,280 |
–3.04 |
+4.40 |
+0.30 |
–14.78 |
Se(l) + 1/2 O2(g) = SeO(g) |
490–1,027K |
+9,420 |
+8.70 |
– |
+0.30 |
–44.50 |
1/2 Se2(g) + 1/2 O2(g) = SeO(g) |
1,027–2,000K |
–7,400 |
–0.37 |
– |
+0.19 |
–0.80 |
Si(c) + 1/2 O2(g) = SiO(g) |
298.16–1,683K |
–21,090 |
+3.84 |
–0.16 |
–0.295 |
–33.14 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 11 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Si(l) + 1/2 O2(g) = SiO(g) |
1,683–2,000K |
–30,170 |
–7.78 |
–0.12 |
+0.25 |
–40.01 |
Si(c) + O2(g) = SiO2(α–quartz) |
298.16–848K |
–210,070 |
+3.98 |
–3.32 |
+0.605 |
+34.59 |
Si(c) + O2(g) = SiO2(β–quartz) |
848–1,683K |
–209,920 |
–3.36 |
–0.19 |
–0.745 |
+53.44 |
Si(l) + O2(g) = SiO2(l) |
1,883–2,000K |
–228,590 |
–15.66 |
– |
– |
+103.97 |
Si(c) + O2(g) = SiO2(α–cristobalite) |
298.16–523K |
–207,330 |
+19.96 |
–9.75 |
–0.745 |
–9.78 |
Si(c) + O2(g) = SiO2(β–cristobalite) |
523–1,683K |
–209,820 |
–3.34 |
–0.24 |
–0.745 |
+53.35 |
Si(c) + 02(g) = SiO2(α–tridymite) |
298.16–390K |
–207,030 |
+22.29 |
–11.62 |
–0.745 |
–15.64 |
Si(c) + O2(g) = SiO2(β–tridymite) |
390–1,683K |
–209,350 |
–1.59 |
–0.54 |
–0.745 |
+47.86 |
2 Ag(c) + 1/2 O2(g) = Ag2O2(c) |
298.16–1,000K |
–7,740 |
–4.14 |
– |
– |
+27.84 |
2 Ag(c) + O2(g) = Ag2O2(c) |
298.16–500K |
–6,620 |
–3.22 |
– |
– |
+52.17 |
2 Na(c) + 1/2 O2(g) = Na2O(c) |
298.16–371K |
–99,820 |
–7.51 |
+5.47 |
–0.10 |
+50.43 |
2 Na(l) + 1/2 O2(g) = Na2O(c) |
371–1,187K |
–100,150 |
+4.97 |
–2.45 |
–0.10 |
+22.19 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 12 OF 16)
Reaction |
|
|
|
Temperature range of validity |
|
H0 |
2.303a |
b |
|
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 Na(c) + O2(g) = Na2O2(c) |
|
|
|
298.16–371K |
|
–122,500 |
–2.30 |
– |
|
– |
+57.51 |
Sr(c) + 1/2 O2(g) = SrO(c) |
|
|
|
298.16–1,043K |
|
–142,410 |
–6.79 |
+0.305 |
|
+0.675 |
+44.33 |
S(rhombohedral) + 1/2 O2(g) = SO(g) |
298.16–368.6K |
|
+19,250 |
–1.24 |
+2.95 |
|
+0.225 |
–18.84 |
|||
S(monoclmic) + 1/2 O2(g) = SO(g) |
|
368.6–392K |
|
+19,200 |
–1.29 |
+3.31 |
|
+0.225 |
–18.72 |
||
S(lλ,μ) + 1/2 O2(g) = SO(g) |
|
|
|
392–718K |
|
+20,320 |
+10.22 |
–0.17 |
|
+0.225 |
–50.05 |
1/2 S2 (g) + 1/2 O2(g) = SO(g) |
|
|
|
298.16–2,000K |
|
+3,890 |
+0.07 |
– |
|
– |
–1.50 |
S(rhombohedral) + O2(g) = SO2(g) |
|
298.16–368.6K |
|
–70,980 |
+0.83 |
+2.35 |
|
+0.51 |
–5.85 |
||
S(monoclinic) + O2(g) = SO2(g) |
|
|
|
368.6–392K |
|
–71,020 |
+0.78 |
+2.71 |
|
+0.51 |
–5.74 |
S(lλ,μ) + O2(g) = SO2(g) |
|
|
|
392–718K |
|
–69,900 |
+12.30 |
–0.77 |
|
+0.51 |
–37.10 |
1/2 S2(g) + O2(g) = SO2(g) |
|
|
|
298.16–2,000K |
|
–86,330 |
+2.42 |
–0.70 |
|
+0.31 |
+10.71 |
S(rhombohedral) + 3/2 O2(g) = SO3(c–I) |
298.16–335.4K |
|
–111,370 |
–6.45 |
– |
|
– |
+88.32 |
|||
S(rhombohedral) + 3/2 O2(g) = SO3(c–II) |
298.16–305.7K |
|
–108,680 |
–11.97 |
– |
|
– |
+94.95 |
|||
S(rhombohedral) + 3/2 O2(g) = SO3(l) |
298.16–335.4K |
|
–107,430 |
–21.18 |
– |
|
– |
+113.76 |
|||
|
|
||||||||||
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the |
F and S values by use |
||||||||||
of the following equations: |
F |
t |
= ΔΗ + 2.303aT log T + b x 10–3 T2 + c x l05 |
T–1 + IT |
|
|
|
|
|
||
|
|
o |
|
|
|
|
|
|
|
|
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 13 OF 16)
|
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S(rhombohedral) + 3/2 O2(g) = SO3(g) |
298.16–368.6K |
–95,070 |
+1.43 |
+0.66 |
+1.26 |
+16.81 |
|
S(monoclinic) + 3/2 O2(g) = SO3(g) |
368.6–392K |
–95,120 |
+1.38 |
+1.02 |
+1.26 |
+16.93 |
|
S(lλ,μ) + 3/2 O2(g) = SO3(g) |
392–718K |
–94,010 |
+12.89 |
–2.46 |
+126 |
–14.40 |
|
1/2 S2(g) + 3/2 O2(g) = SO3(g) |
298.16–1,500K |
–110,420 |
+3.02 |
–2.39 |
+106 |
+33.41 |
|
2 Ta(c) + 5/2 O2(g) = Ta2O5(c) |
298.16–2,000K |
–492,790 |
–17.18 |
–1.25 |
+2.46 |
+161.68 |
|
Te(c) + 1/2 O2(g) = TeO(g) |
298.16–723K |
+43,110 |
+1.91 |
+0.84 |
+0.315 |
–27.22 |
|
Te(l) + 1/2 O2(g) = TeO(g) |
723–1,360K |
+39,750 |
+6.08 |
+0.09 |
+0.315 |
–33.94 |
|
2 |
Tl(α) + O2(g) = Tl2rO(c) |
298.16–505.5K |
–44,110 |
–6.91 |
– |
– |
+42.30 |
2 |
Tl(β) + O2(g) = Tl2rO(c) |
505.5–573K |
–44,260 |
–6.91 |
– |
– |
+42.60 |
2 |
Tl(α) + 3/2 O2(g) = Tl2rO3(c) |
298.16–505.5K |
–99,410 |
–16.12 |
– |
– |
+119.09 |
Th(c) + O2(g) = ThO2(c) |
298.16–2,000K |
–294,350 |
–5.25 |
+0.59 |
+0.775 |
+62.81 |
|
Sn(c) + 1/2 O2(g) = SnO(c) |
298.16–505K |
–68,600 |
–3.57 |
+1.65 |
–0.10 |
+32.59 |
|
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 14 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sn(l) + 1/2 O2(g) = SnO(c) |
505–1,300K |
–69,670 |
+3.06 |
–1.50 |
–0.10 |
+18.39 |
Sn(c) + O2(g) = SnO2(c) |
298.16–505K |
–0,142 |
–14.00 |
+2.45 |
+2.38 |
+90.74 |
Ti(α) + 1/2 O2(g) = TiO(α) |
298.16–1,150K |
–125,010 |
–4.01 |
–0.29 |
+0.83 |
+36.28 |
Ti(α) + 1/2 O2(g) = TiO(α) |
1,150–1,264K |
–125,040 |
+1.17 |
–1.55 |
+0.83 |
+21.90 |
2 Ti(α) + 3/2 O2(g) = Ti2O3(α) |
298.16–473K |
–360,660 |
+32.08 |
–23.49 |
–0.30 |
–10.66 |
2 Ti(α) + 3/2 O2(g) = Ti2O3(β) |
473–1,150K |
–369,710 |
–30.95 |
+2.62 |
+4.80 |
+162.79 |
Ti(α) + O2(g) = TiO2 (rutile) |
298.16–1,150K |
–228,360 |
–12.80 |
+1.62 |
+1.975 |
+82.81 |
Ti(α) + O2(g) = TiO2 (rutile) |
1,150–2,000K |
–228,380 |
–7.62 |
+0.36 |
+1.975 |
+68.43 |
W(c) + O2(g) = WO2(c) |
298.16–1,500K |
–137,180 |
–1.38 |
– |
– |
+45.56 |
4W(c) + 11/2 O2(g) = W4O11(c) |
298.16–1,700K |
–745,730 |
–32.70 |
– |
– |
+321.84 |
W(c) + 3/2 O2(g) = WO3(c) |
298.16–1,743K |
–201,180 |
–2.92 |
–1.81 |
–0.30 |
+70.89 |
W(c) + 3/2 O2(g) = WO3(l) |
1,743–2,000K |
–203,140 |
–35.74 |
+1.13 |
–0.30 |
+173.27 |
U(α) + O2(g) = UO2(c) |
298.16–935K |
–262,880 |
–19.92 |
+3.70 |
+2.13 |
+100.54 |
U(β) + O2(g) = UO2(c) |
935–1,045K |
–260,660 |
–4.28 |
–0.31 |
+1.78 |
+55.50 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 15 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
U(γ) + O2(g) = UO2(c) |
1,045–1,405K |
–262,830 |
–6.54 |
–0.31 |
+1.78 |
+64.41 |
U(l) + O2(g) = UO2(l) |
1,405–1,500K |
–264,790 |
–5.92 |
– |
– |
+63.50 |
3 U(α) + 4 O2(g) = U3O8(c) |
298.16–935K |
–863,370 |
–56.57 |
+10.68 |
+5.20 |
+330.19 |
3 U(β) + 4 O2(g) = U3O8(c) |
935–1,045K |
–856,720 |
–9.67 |
–1.35 |
+4.15 |
+195.12 |
3 U(γ) + 4 O2(g) = U3O8(c) |
1,045–1,405K |
–863,230 |
–16.44 |
–1.35 |
+4.15 |
+221.79 |
3 U(l) + 4 O2(g) = U3O8(c) |
1,405–1,500K |
–869,460 |
–10.91 |
–1.35 |
+4.15 |
+208.82 |
U(α) + 3/2 O2(g) = UO3 (hexagonal) |
298.16–935K |
–294,090 |
–18.33 |
+3.49 |
+1.535 |
+114.94 |
U(β) + 3/2 O2(g) = UO3 (hexagonal) |
935–1,045K |
–291,870 |
–2.69 |
–0.52 |
+1.185 |
+69.90 |
U(γ) + 3/2 O2(g) = UO3 (hexagonal) |
1,045–1,400K |
–294,040 |
–4.95 |
–0.52 |
+1.185 |
+78.80 |
V(c) + 1/2 O2(g) = VO(c) |
298.16–2,000K |
–101,090 |
–5.39 |
–0.36 |
+0.53 |
+38.69 |
V(c) + 1/2 O2(g) = VO(g) |
298.16–2,000K |
+52,090 |
+1.80 |
+1.04 |
+0.35 |
–28.42 |
2 V(c) + 3/2 O2(g) = V2O3(c) |
298.16–2,000K |
–299,910 |
–17.98 |
+0.37 |
+2.41 |
+118.83 |
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 67. HEAT OF FORMATION OF INORGANIC OXIDES
(SHEET 16 OF 16)
Reaction |
Temperature range of validity |
H0 |
2.303a |
b |
c |
I |
|
|
|
|
|
|
|
2 V(c) + 2 O2(g) = V2O4(α) |
209.16–345K |
–342,890 |
–11.03 |
+3.00 |
–0.40 |
+117.38 |
2 V(c) + 2 O2(g) = V2O4(β) |
345–1,818K |
–345,330 |
–24.36 |
+1.30 |
+3.545 |
+155.55 |
6 V(c) + 13/2 O2(g) = V6O13(c) |
298.16–1,000K |
–1,076,340 |
–95.33 |
– |
– |
+557.61 |
2 V(c) + 5/2 O2(g) = V2O5(c) |
298.16–943K |
–381,960 |
–41.08 |
+5.20 |
+6.11 |
+228.50 |
2 Y(c) + 3/2 O2(g) = Y2O3(c) |
298.16–1,773K |
–419,600 |
+2.76 |
–1.73 |
–0.30 |
+66.36 |
Zn(c) + 1/2 O2(g) = ZnO(c) |
298.16–692.7K |
–84,670 |
–6.40 |
+0.84 |
+0.99 |
+43.25 |
Zr(α) + O2(g) = ZrO2(α) |
298.16–1,135K |
–262,980 |
–6.10 |
+0.16 |
+1.045 |
+65.00 |
Zr(β) + O2(g) = ZrO2(α) |
1,135–1,478K |
–264,190 |
–5.09 |
–0.40 |
+1.48 |
+63.58 |
Zr(β) + O2(g) = ZrO2(β) |
1.478–2,000K |
–262,290 |
–7.76 |
+0.50 |
–0.20 |
+69.50 |
|
|
|
|
|
|
|
The Ho values are given in gram calories per mole. The a, b, and I values listed here make it possible for one to calculate the F and S values by use
of the following equations: |
F |
= ΔΗ + 2.303aT log T + b x 10–3 T2 |
+ c x l05 T–1 + IT |
|
t |
o |
|
St = – a – 2.303a log T – 2b x 10–3T + c x l05 T–2 – I
Source: data from CRC Handbook of Materials Science, Vol I, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 1 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Ac |
solid |
(1090) |
(2.5) |
(2.3) |
|
liquid |
(2750) |
(70) |
(25) |
Ag |
solid |
1234 |
2.855 |
2.313 |
|
liquid |
2485 |
60.72 |
24.43 |
Al |
solid |
931.7 |
2.57 |
2.76 |
|
liquid |
2600 |
67.9 |
26 |
Am |
solid |
(1200) |
(2.4) |
(2.0) |
|
liquid |
2733 |
51.7 |
18.9 |
As |
solid |
883 |
3.25 |
35.25 |
Au |
solid |
1336.16 |
3.03 |
2.27 |
|
liquid |
2933 |
74.21 |
25.30 |
B |
solid |
2313 |
(3.8) |
(1.6) |
|
liquid |
2800 |
75 |
27 |
Ba |
solid, α |
648 |
0.14 |
0.22 |
|
solid, β |
977 |
1.83 |
1.87 |
|
liquid |
1911 |
35.665 |
18.63 |
Be |
solid |
1556 |
2.919 |
1.501 |
|
liquid |
– |
|
|
Bi |
solid |
544.2 |
2.63 |
4.83 |
|
liquid |
1900 |
41.1 |
21.6 |
C |
solid |
– |
– |
– |
Ca |
solid, α |
723 |
0.24 |
0.33 |
|
solid, β |
1123 |
2.2 |
1.96 |
|
liquid |
1755 |
38.6 |
22.0 |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 2 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Cd |
solid |
594.1 |
1.46 |
2.46 |
|
liquid |
1040 |
23.86 |
22.94 |
Ce |
solid |
1048 |
2.1 |
2.0 |
|
liquid |
2800 |
73 |
26 |
Cl2 |
gas |
– |
– |
– |
Co |
solid, α |
723 |
0.005 |
0.007 |
|
solid, β |
1398 |
0.095 |
0.068 |
|
solid, γ |
1766 |
3.7 |
2.1 |
|
liquid |
3370 |
93 |
28 |
Cr |
solid |
2173 |
3.5 |
1.6 |
|
liquid |
2495 |
72.97 |
29.25 |
Cs |
solid |
301.9 |
0.50 |
1.7 |
|
liquid |
963 |
16.32 |
17.0 |
Cu |
solid |
1356.2 |
3.11 |
2.29 |
|
liquid |
2868 |
72.8 |
25.4 |
F2 |
gas |
– |
– |
– |
Fe |
solid, α |
1033 |
0.410 |
0.397 |
|
solid, β |
1180 |
0.217 |
0.184 |
|
solid, γ |
1673 |
0.15 |
0.084 |
|
solid, δ |
1808 |
3.86 |
2.14 |
|
liquid |
3008 |
84.62 |
28.1 |
Ga |
solid |
302.94 |
1.335 |
4.407 |
|
liquid |
2700 |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 3 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Ge |
solid |
1232 |
8.3 |
6.7 |
|
liquid |
2980 |
68 |
23 |
H2 |
gas |
– |
– |
– |
Hf |
solid |
(2600) |
(6.0) |
(2.3) |
Hg |
liquid |
629.73 |
13.985 |
22.208 |
In |
solid |
430 |
0.775 |
1.80 |
|
liquid |
2440 |
53.8 |
22.0 |
Ir |
solid |
2727 |
6.6 |
2.4 |
K |
solid |
336.4 |
0.5575 |
1.657 |
|
liquid |
1052 |
18.88 |
17.95 |
La |
solid |
1153 |
(2.3) |
(2.0) |
|
liquid |
3000 |
80 |
27 |
Li |
solid |
459 |
0.69 |
1.5 |
|
liquid |
1640 |
32.48 |
19.81 |
Mg |
solid |
923 |
2.2 |
2.4 |
|
liquid |
1393 |
31.5 |
22.6 |
Mn |
solid, α |
1000 |
0.535 |
0.535 |
|
solid, β |
1374 |
0.545 |
0.397 |
|
solid, γ |
1410 |
0.430 |
0.305 |
|
solid, δ |
1517 |
3.5 |
2.31 |
|
liquid |
2368 |
53.7 |
22.7 |
Mo |
solid |
2883 |
(5.8) |
(2.0) |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 4 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
N2 |
gas |
– |
– |
– |
Na |
solid |
371 |
0.63 |
1.7 |
|
liquid |
1187 |
23.4 |
20.1 |
Nb |
solid |
2760 |
(5.8) |
(2.1) |
Nd |
solid |
1297 |
(2.55) |
(197) |
|
liquid |
(2750) |
(61) |
(22) |
Ni |
solid α |
626 |
0.092 |
0.15 |
|
solid β |
1728 |
4.21 |
2.44 |
|
liquid |
3110 |
90.48 |
29.0 |
Np |
solid |
913 |
(2.3) |
(2.5) |
|
liquid |
(2525) |
(55) |
(22) |
O2 |
gas |
– |
– |
– |
Os |
solid |
2970 |
(6.4) |
(2.2) |
P4 |
solid, white |
317.4 |
0.601 |
1.89 |
|
liquid |
553 |
11.9 |
21.5 |
Pa |
solid |
(18.25) |
(4.0) |
(2.2) |
|
liquid |
(4500) |
(115) |
(26) |
Pb |
solid |
600.6 |
1.141 |
1.900 |
|
liquid |
2023 |
42.5 |
21.0 |
Pd |
solid |
1828 |
4.12 |
2.25 |
|
liquid |
3440 |
89 |
26 |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 5 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Po |
solid |
525 |
(2.4) |
(4.6) |
|
liquid |
(1235) |
(24.6) |
(19.9) |
Pr |
solid |
1205 |
(25) |
(2.1) |
|
liquid |
3563 |
– |
– |
Pt |
solid |
2042.5 |
5.2 |
25 |
|
liquid |
4100 |
122 |
29.8 |
Pu |
solid |
913 |
(2.26) |
(2.48) |
|
liquid |
– |
|
|
Ra |
solid |
1233 |
(2.3) |
(1.9) |
|
liquid |
(1700) |
(35) |
(21) |
Rb |
solid |
312.0 |
0.525 |
1.68 |
|
liquid |
952 |
18.11 |
19.0 |
Re |
solid |
3440 |
(7.9) |
(2.3) |
Rh |
solid |
2240 |
(5.2) |
(2.3) |
|
liquid |
4150 |
127 |
30.7 |
Ru |
solid, α |
1308 |
0.034 |
0.026 |
|
solid, β |
1473 |
0 |
– |
|
solid, γ |
1773 |
0.23 |
0.13 |
|
solid, δ |
2700 |
(6.1) |
(2.3) |
S |
solid, α |
368.6 |
0.088 |
0.24 |
|
solid, β |
392 |
0.293 |
0.747 |
|
liquid |
717.76 |
2.5 |
3.5 |
Sb |
solid (α, β, γ) |
903.7 |
4.8 |
5.3 |
|
liquid |
1713 |
46.665 |
27.3 |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 6 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Sc |
solid |
1670 |
(4.0) |
(2.4) |
|
liquid |
3000 |
80 |
27 |
Se |
solid |
490.6 |
1.25 |
2.55 |
|
liquid |
1000 |
14.27 |
14.27 |
Si |
solid |
1683 |
11.1 |
6.60 |
|
liquid |
2750 |
71 |
26 |
Sm |
solid |
1623 |
3.7 |
2.3 |
|
liquid |
(2800) |
(70) |
(25) |
Sn |
solid, α, β |
505.1 |
1.69 |
335 |
|
liquid |
2473 |
(55) |
(22) |
Sr |
solid |
1043 |
2.2 |
2.1 |
|
liquid |
1657 |
33.61 |
20.28 |
Ta |
solid |
3250 |
7.5 |
2.3 |
Tc |
solid |
(2400) |
(5.5) |
(2.3) |
|
liquid |
(3800) |
(120) |
(32) |
Te |
solid, α |
621 |
0.13 |
0.21 |
|
solid, β |
723 |
4.28 |
5.92 |
|
liquid |
1360 |
11.9 |
8.75 |
Th |
solid |
2173 |
(4.6) |
(2.1) |
|
liquid |
4500 |
(130) |
(29) |
Ti |
solid, α |
1155 |
0.950 |
0.822 |
|
solid, β |
2000 |
(4.6) |
(23) |
|
liquid |
3550 |
(101) |
(28) |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 68. PHASE CHANGE THERMODYNAMIC PROPERTIES FOR
THE ELEMENTS
(SHEET 7 OF 7)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Element |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Tl |
solid, α |
508.3 |
0.082 |
0.16 |
|
solid, β |
576.8 |
1.03 |
1.79 |
|
liquid |
1730 |
38.81 |
22.4 |
U |
solid, α |
938 |
0.665 |
0.709 |
|
solid, β |
1049 |
1.165 |
1.111 |
|
solid, γ |
1405 |
(3.0) |
(2.1) |
|
liquid |
3800 |
– |
– |
V |
solid |
2003 |
(4.0) |
(2.0) |
|
liquid |
3800 |
– |
– |
W |
solid |
3650 |
8.42 |
2.3 |
Y |
solid |
1750 |
(4.0) |
(2.3) |
|
liquid |
3500 |
(90) |
(26) |
Zn |
solid |
692.7 |
1.595 |
2.303 |
|
liquid |
1180 |
27.43 |
23.24 |
Zr |
solid, α |
1135 |
0.920 |
0.811 |
|
solid, β |
2125 |
(4.9) |
(2.3) |
|
liquid |
(3900) |
(100) |
(26) |
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 1 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Ac2O3 |
Solid |
(2250) |
(20) |
(8.9) |
|
Liquid |
– |
– |
– |
Ag2O |
Solid |
dec. 460 |
– |
– |
Ag2O2 |
Solid |
dec. |
– |
– |
Al2O3 |
Solid |
2300 |
26 |
11 |
|
Liquid |
dec. |
– |
– |
Am2O3 |
Solid |
(2225) |
(17) |
(7.6) |
|
Liquid |
(3400) |
(85) |
(25) |
AmO2 |
Solid |
dec. |
– |
– |
As2O3 |
Solid, α |
503 |
4.1 |
8.2 |
|
Solid, β |
586 |
4.4 |
7.5 |
|
Liquid |
730 |
7.15 |
9.79 |
AsO2 |
Solid |
(1200) |
(9.0) |
(7.5) |
|
Liquid |
(dec.) |
– |
– |
As2O5 |
Solid |
dec. >1100 |
– |
– |
Au2O3 |
Solid |
dec. |
– |
– |
B2O3 |
Solid |
723 |
5.27 |
7.29 |
|
Liquid |
2520 |
(55) |
(22) |
Ba2O |
Solid |
(880) |
(5.2) |
(5.9) |
|
Liquid |
(1040) |
(20) |
(19) |
BaO |
Solid |
2196 |
13.8 |
6.28 |
|
Liquid |
3000 |
(62) |
(21) |
BaO2 |
Solid |
723 |
(5.7) |
(7.9) |
|
Liquid |
dec. 1110 |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 2 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
BeO |
Solid |
dec. |
– |
– |
BiO |
Solid |
(1175) |
(3.7) |
(3.1) |
|
Liquid |
(1920) |
(54) |
(28) |
Bi2O3 |
Solid |
1090 |
6.8 |
6.2 |
|
Liquid |
(dec.) |
– |
– |
CO |
Gas |
– |
– |
– |
CO2 |
Gas |
– |
– |
– |
CaO |
Solid |
2860 |
(18) |
(6.3) |
CdO |
Solid |
dec. |
– |
– |
Ce2O3 |
Solid |
1960 |
(20) |
(10) |
|
Liquid |
(3500) |
(80) |
(23) |
CeO2 |
Solid |
3000 |
(19) |
(6.3) |
CoO |
Solid |
2078 |
(12) |
(5.8) |
|
Liquid |
(2900) |
(61) |
(21) |
Co3O4 |
Solid |
dec. 1240 |
– |
– |
Cr2O3 |
Solid |
2538 |
(25) |
(10) |
CrO2 |
Solid |
dec. 700 |
– |
– |
CrO3 |
Solid |
460 |
(6.1) |
(13) |
|
Liquid |
(1000) |
(25) |
(25) |
Cs2O |
Solid |
763 |
(4.58) |
(6.0) |
|
Liquid |
dec. |
– |
– |
Cs2O2 |
Solid |
867 |
(5.5) |
(6.3) |
|
Liquid |
dec. |
– |
– |
Cs2O3 |
Solid |
775 |
(7.75) |
(10) |
|
Liquid |
dec. |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 3 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Cu2O |
Solid |
1503 |
13.4 |
8.92 |
|
Liquid |
dec. |
– |
– |
CuO |
Solid |
1609 |
(8.9) |
(5.5) |
|
Liquid |
dec. |
– |
– |
FeO |
Solid |
1641 |
7.5 |
4.6 |
|
Liquid |
(2700) |
(55) |
(20) |
Fe3O4 |
Solid, α |
900 |
(0) |
(0) |
|
Solid, β |
dec. |
– |
– |
Fe2O3 |
Solid, α |
950 |
0.16 |
0.17 |
|
Solid, β |
1050 |
0 |
0 |
|
Solid, γ |
dec. |
– |
– |
Ga2O |
Solid |
(925) |
(8.5) |
(9.2) |
|
Liquid |
(1000) |
(20) |
(20) |
Ga2O3 |
Solid |
2013 |
(22) |
(11) |
|
Liquid |
(2900) |
(75) |
(26) |
GeO |
Solid |
983 |
(50) |
(51) |
GeO2 |
Solid (α,β) |
1389 |
10.5 |
7.56 |
|
Liquid |
(2625) |
(61) |
(23) |
In2O |
Solid |
(600) |
(4.5) |
(7.5) |
|
Liquid |
(800) |
(16) |
(20) |
InO |
Solid |
(1325) |
(4.0) |
(3.0) |
|
Liquid |
(2000) |
(60) |
(30) |
In2O3 |
Solid |
(2000) |
(20) |
(10) |
|
Liquid |
(3600) |
(85) |
(24) |
Ir2O3 |
Solid |
(1450) |
(10) |
(6.8) |
|
Liquid |
(2250) |
(50) |
(22) |
IrO2 |
Solid |
dec. 1373 |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 4 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
K2O |
Solid |
(980) |
(6.8) |
(6.9) |
|
Liquid |
dec. |
– |
– |
K2O2 |
Solid |
763 |
(7.0) |
(9.2) |
|
Liquid |
(1800) |
(45) |
(25) |
K2O3 |
Solid |
703 |
(6.1) |
(8.7) |
|
Liquid |
(975) |
(25) |
(26) |
KO2 |
Solid |
653 |
(4.9) |
(7.5) |
|
Liquid |
dec. |
– |
– |
La2O3 |
Solid |
2590 |
(18) |
(7) |
Li2O |
Solid |
2000 |
(14) |
(7) |
|
Liquid |
2600 |
(56) |
(22) |
Li2O2 |
Solid |
dec.470 |
– |
– |
MgO |
Solid |
3075 |
18.5 |
5.8 |
MgO2 |
Solid |
dec. 361 |
– |
– |
MnO |
Solid |
2058 |
13.0 |
6.32 |
|
Liquid |
dec. |
– |
– |
Mn3O4 |
Solid, α |
1445 |
4.97 |
3.44 |
|
Solid, β |
1863 |
(33) |
(18) |
|
Liquid |
(2900) |
(75) |
(26) |
Mn2O3 |
Solid |
dec. 1620 |
– |
– |
MnO2 |
Solid |
dec. 1120 |
– |
– |
MoO2 |
Solid |
(2200) |
(16) |
(7.3) |
|
Liquid |
dec. 2250 |
– |
– |
MoO3 |
Solid |
1068 |
12.54 |
11.74 |
|
Liquid |
1530 |
33 |
22 |
N2O |
Gas |
– |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 5 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Na2O |
Solid |
1193 |
(7.1) |
(6.0) |
|
Liquid |
dec. |
– |
– |
Na2O2 |
Solid |
dec. 919 |
– |
– |
NaO2 |
Solid |
(825) |
(6.2) |
(7.5) |
|
Liquid |
(1300) |
(28) |
(22) |
NbO |
Solid |
(2650) |
(16) |
(6.0) |
NbO2 |
Solid |
(2275) |
(16) |
(7.0) |
|
Liquid |
(3800) |
(85) |
(22) |
Nb2O5 |
Solid |
1733 |
(28) |
(16) |
|
Liquid |
(3200) |
(80) |
(25) |
Nd2O3 |
Solid |
2545 |
(22) |
(8.8) |
NiO |
Solid |
2230 |
(12.1) |
(5.43) |
|
Liquid |
dec. |
– |
– |
NpO2 |
Solid |
(2600) |
(15) |
(5.7) |
Np2O5 |
Solid |
dec. |
– |
– |
|
|
800–900 K |
|
|
OsO2 |
Solid |
dec. 923 |
– |
– |
OsO4 |
Solid |
313.3 |
3.41 |
10.9 |
|
Liquid |
403 |
9.45 |
23.4 |
P2O3 |
Liquid |
448.5 |
4.5 |
10 |
PO2 |
Solid |
(350) |
(2.7) |
(7.7) |
|
Liquid |
(dec.) |
– |
– |
P2O5 |
Solid |
631 |
8.8 |
13.9 |
PaO2 |
Solid |
(2560) |
(20) |
(7.8) |
Pa2O5 |
Solid |
(2050) |
(26) |
(13) |
|
Liquid |
(3350) |
(95) |
(28) |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 6 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
PbO |
Solid, red |
762 |
(0.4) |
(0.5) |
|
Solid, yellow |
1159 |
2.8 |
2.4 |
|
Liquid |
1745 |
51 |
29 |
Pb2O4 |
Solid |
dec. |
– |
– |
PbO2 |
Solid |
dec. |
– |
– |
PdO |
Solid |
dec. 1150 |
– |
– |
PoO2 |
Solid |
(825) |
(5.5) |
(6.7) |
|
Liquid |
(dec.) |
– |
– |
Pr2O3 |
Solid |
(2200) |
(22) |
(10) |
|
Liquid |
(4000 |
(90) |
(23) |
PrO2 |
Solid |
dec. 700 |
– |
– |
PtO |
Solid |
dec. 780 |
– |
– |
Pt3O4 |
Solid |
(dec.) |
– |
– |
PtO2 |
Solid |
723 |
(4.6) |
(6.4) |
|
Liquid |
dec. 750 |
– |
– |
PuO |
Solid |
(1290) |
(7.2) |
(5.6) |
|
Liquid |
(2325) |
(47) |
(20) |
Pu2O3 |
Solid |
(1880) |
(16) |
(8.5) |
|
Liquid |
(3250) |
(75) |
(23) |
PuO2 |
Solid |
(2400) |
(15) |
(6.2) |
|
Liquid |
(3500) |
(90) |
(26) |
RaO |
Solid |
(>2500) |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 7 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
Rb2O |
Solid |
(910) |
(5.7) |
(6.3) |
|
Liquid |
dec. |
– |
– |
Rb2O2 |
Solid |
843 |
(7.3) |
(8.7) |
|
Liquid |
(dec.) |
– |
– |
Rb2O3 |
Solid |
762 |
(7.6) |
(10) |
|
Liquid |
dec. |
– |
– |
RbO2 |
Solid |
685 |
(4.1) |
(6.0) |
|
Liquid |
dec. |
– |
– |
ReO2 |
Solid |
(1475) |
(12) |
(8.1) |
|
Liquid |
(3250) |
(80) |
(25) |
ReO3 |
Solid |
433 |
5.2 |
12 |
|
Liquid |
dec. |
– |
– |
Re2O7 |
Solid |
569 |
15.8 |
27.8 |
|
Liquid |
635.5 |
17.7 |
27.9 |
ReO4 |
Solid |
420 |
(4.2) |
(10) |
|
Liquid |
(460) |
(9.3) |
(20) |
Rh2O |
Solid |
dec. 1400 |
– |
– |
RhO |
Solid |
dec. 1394 |
– |
– |
Rh2O3 |
Solid |
dec. 1388 |
– |
– |
RuO2 |
Solid |
dec. 1400 |
– |
– |
RuO4 |
Solid |
300 |
(3.2) |
(11) |
|
Liquid |
dec. |
– |
– |
SO2 |
Gas |
– |
– |
– |
Sb2O3 |
Solid |
928 |
14.74 |
15.88 |
|
Liquid |
1698 |
8.92 |
5.25 |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 8 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
SbO2 |
Solid |
dec. |
– |
– |
Sb2O5 |
Solid |
dec. |
– |
– |
Sc2O3 |
Solid |
(2500) |
(23) |
(9.3) |
SeO |
Solid |
(1375) |
(7.6) |
(5.5) |
|
Liquid |
(2075) |
(45) |
(22) |
SeO2 |
Solid |
603 |
(24.5) |
(40.6) |
SiO |
Solid |
(2550) |
(12) |
(4.7) |
SiO2 |
Solid, β |
856 |
0.15 |
0.18 |
|
Solid, α |
1883 |
2.04 |
1.08 |
|
Liquid |
dec. 2250 |
– |
– |
Sm2O3 |
Solid |
(2150) |
(20) |
(9.3) |
|
Liquid |
(3800) |
(80) |
(21) |
SnO |
Solid |
(1315) |
(6.4) |
(4.9) |
|
Liquid |
(1800) |
(60) |
(33) |
SnO2 |
Solid |
1898 |
(11.39) |
(5.95) |
|
Liquid |
(3200) |
(75) |
(23) |
SrO |
Solid |
2703 |
16.7 |
6.2 |
SrO2 |
Solid |
dec.488 |
– |
– |
Ta2O5 |
Solid |
2150 |
(16) |
(7.4) |
|
Liquid |
– |
– |
– |
TcO2 |
Solid |
(2400) |
(18) |
(7.5) |
|
Liquid |
(4000) |
(105) |
(26) |
TcO3 |
Solid |
(dec. <1200) |
– |
– |
Tc2O7 |
Solid |
392.7 |
(11) |
(28) |
|
Liquid |
583.8 |
(14) |
(24) |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 9 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
TeO |
Solid |
(1020) |
(7.1) |
(7.0) |
|
Liquid |
(1775) |
(50) |
(28) |
TeO3 |
Solid |
1006 |
3.2 |
3.2 |
|
Liquid |
dec. |
– |
– |
TeO2 |
Solid |
(2150) |
(13) |
(6.0) |
|
Liquid |
(3250) |
(65) |
(20) |
ThO2 |
Solid |
3225 |
(18) |
(5.6) |
TiO |
Solid, α |
1264 |
0.82 |
0.65 |
|
Solid, β |
dec. 2010 |
– |
– |
Ti2O3 |
Solid, α |
473 |
0.215 |
0.455 |
|
Solid, β |
2400 |
(24) |
(10) |
|
Liquid |
3300 |
|
|
Ti3O5 |
Solid, α |
450 |
2.24 |
4.98 |
|
Solid, β |
(2450) |
(50) |
(20) |
|
Liquid |
(3600) |
(85) |
(24) |
TiO2 |
Solid |
2128 |
(16) |
(7.5) |
|
Liquid |
dec. 3200 |
|
|
Ti2O |
Solid |
573 |
(5.0) |
(8.7) |
|
Liquid |
773 |
(17) |
(22) |
Tl2O3 |
Solid |
990 |
(12.4) |
(13) |
|
Liquid |
(dec.) |
– |
– |
UO |
Solid |
(2750) |
(14) |
(5.1) |
UO2 |
Solid |
3000 |
– |
– |
U3O8 |
Solid |
dec. |
– |
– |
UO3 |
Solid |
dec. 925 |
– |
– |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 69. PHASE CHANGE THERMODYNAMIC PROPERTIES
OF OXIDES (SHEET 10 OF 10)
|
|
Transition |
Heat of |
Entropy of |
|
|
Transition |
||
|
|
Temperature |
Transition |
|
|
|
(kcal • g mole-1) |
||
Oxide |
Phase |
(K) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
VO |
Solid |
(2350) |
(15) |
(6.4) |
|
Liquid |
(3400) |
(70) |
(21) |
V2O3 |
Solid |
2240 |
(24) |
(11) |
|
Liquid |
dec. 3300 |
– |
– |
V3O4 |
Solid |
(2100) |
(42) |
(20) |
|
Liquid |
(dec.) |
– |
– |
VO2 |
Solid, α |
345 |
1.02 |
2.96 |
|
Solid, β |
1818 |
13.60 |
7.48 |
|
Liquid |
dec. 3300 |
– |
– |
V2O5 |
Solid |
943 |
15.56 |
16.50 |
|
Liquid |
(2325) |
(63) |
(27) |
WO2 |
Solid |
(1543) |
(11.5) |
(7.45) |
|
Liquid |
dec. 2125 |
– |
– |
WO3 |
Solid |
1743 |
(17) |
(9.8) |
|
Liquid |
(2100) |
(43) |
(20) |
Y2O3 |
Solid |
(2500) |
(25) |
(10) |
ZnO |
Solid |
dec. |
– |
– |
ZrO2 |
Solid, α |
1478 |
1.420 |
0.961 |
|
Solid, β |
2950 |
20.8 |
7.0 |
|
|
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 70. MELTING POINTS OF THE ELEMENTS
(SHEET 1 OF 4)
|
|
|
Melting |
At. |
|
|
Point |
No. |
Element |
Symbol |
(˚C) |
|
|
|
|
|
|
|
|
1 |
Hydrogen |
H |
-259.14 |
2 |
Helium |
He |
-272.2 |
3 |
Lithium |
Li |
180.54 |
4 |
Beryllium |
Be |
1278 |
5 |
Boron |
B |
2300 |
6 |
Carbon |
C |
~3550 |
7 |
Nitrogen |
N |
-209.86 |
8 |
Oxygen |
O |
-218.4 |
9 |
Fluorine |
F |
-219.62 |
10 |
Neon |
N |
-248.67 |
11 |
Sodium |
Na |
97.81 |
12 |
Magnesium |
Mg |
648.8 |
13 |
Aluminum |
Al |
660.37 |
14 |
Silicon |
Si |
1410 |
15 |
Phosphorus |
P |
44.1 |
|
(White) |
|
|
16 |
Sulfur |
S |
112.8 |
17 |
Chlorine |
Cl |
-100.98 |
18 |
Argon |
Ar |
-189.2 |
19 |
Potassium |
K |
63.65 |
20 |
Calcium |
Ca |
839 |
21 |
Scandium |
Sc |
1539 |
22 |
Titanium |
Ti |
1660 |
23 |
Vanadium |
V |
1890 |
24 |
Chromium |
Cr |
1857 |
25 |
Manganese |
Mn |
1244 |
26 |
Iron |
Fe |
1535 |
27 |
Cobalt |
Co |
1495 |
|
|
|
|
Source: data from James F. Shackelford, Introduction to Materials Science for Engineers, Second Edition, Macmillan Publishing Company, New York, pp.686-688, (1988).
©2001 CRC Press LLC

Table 70. MELTING POINTS OF THE ELEMENTS
(SHEET 2 OF 4)
|
|
|
Melting |
At. |
|
|
Point |
No. |
Element |
Symbol |
(˚C) |
|
|
|
|
|
|
|
|
28 |
Nickel |
Ni |
1453 |
29 |
Copper |
Cu |
1083.4 |
30 |
Zinc |
Zn |
419.58 |
31 |
Gallium |
Ga |
29.78 |
32 |
Germanium |
Ge |
937.4 |
33 |
Arsenic |
As |
817 |
34 |
Selenium |
Se |
217 |
35 |
Bromine |
Br |
-7.2 |
36 |
Krypton |
Kr |
-156.6 |
37 |
Rubidium |
Rb |
38.89 |
38 |
Strontium |
Sr |
769 |
39 |
Yttrium |
Y |
1523 |
40 |
Zirconium |
Zr |
1852 |
41 |
Niobium |
Nb |
2408 |
42 |
Molybdenum |
Mo |
2617 |
43 |
Technetium |
Tc |
2172 |
44 |
Ruthenium |
Ru |
2310 |
45 |
Rhodium |
Rh |
1966 |
46 |
Palladium |
Pd |
1552 |
47 |
Silver |
Ag |
961.93 |
48 |
Cadmium |
Cd |
320.9 |
49 |
Indium |
In |
156.61 |
50 |
Tin |
Sn |
231.9681 |
51 |
Antimony |
Sb |
630.74 |
52 |
Tellurium |
Te |
449.5 |
53 |
Iodine |
I |
113.5 |
54 |
Xenon |
Xe |
-111.9 |
55 |
Cesium (-10˚) |
Ce |
28.4 |
|
|
|
|
Source: data from James F. Shackelford, Introduction to Materials Science for Engineers, Second Edition, Macmillan Publishing Company, New York, pp.686-688, (1988).
©2001 CRC Press LLC

Table 70. MELTING POINTS OF THE ELEMENTS
(SHEET 3 OF 4)
|
|
|
Melting |
At. |
|
|
Point |
No. |
Element |
Symbol |
(˚C) |
|
|
|
|
|
|
|
|
56 |
Barium |
Ba |
7.25 |
57 |
Lantium |
La |
920 |
58 |
Cerium |
Ce |
798 |
59 |
Praseodymium |
Pr |
931 |
60 |
Neodymium |
Nd |
1010 |
61 |
Promethium |
Pm |
~1080 |
62 |
Samarium |
Sm |
1072 |
63 |
Europium |
Eu |
822 |
64 |
Gadolinium |
Gd |
1311 |
65 |
Terbium |
Tb |
1360 |
66 |
Dysprosium |
Dy |
1409 |
67 |
Holmium |
Ho |
1470 |
68 |
Erbium |
Er |
1522 |
69 |
Thulium |
Tm |
1545 |
70 |
Ytterbium |
Yb |
824 |
71 |
Lutetium |
Lu |
1659 |
72 |
Hafnium |
Hf |
2227 |
73 |
Tantalum |
Ta |
2996 |
74 |
Tungsten |
W |
3410 |
75 |
Rhenium |
Re |
3180 |
76 |
Osmium |
Os |
3045 |
77 |
Iridium |
Ir |
2410 |
78 |
Platinum |
Pt |
1772 |
79 |
Gold |
Au |
1064.43 |
80 |
Mercury |
Hg |
-38.87 |
81 |
Thallium |
Tl |
303.5 |
82 |
Lead |
Pb |
327.502 |
83 |
Bismuth |
Bi |
271.3 |
|
|
|
|
Source: data from James F. Shackelford, Introduction to Materials Science for Engineers, Second Edition, Macmillan Publishing Company, New York, pp.686-688, (1988).
©2001 CRC Press LLC

Table 70. MELTING POINTS OF THE ELEMENTS
(SHEET 4 OF 4)
|
|
|
Melting |
At. |
|
|
Point |
No. |
Element |
Symbol |
(˚C) |
|
|
|
|
|
|
|
|
84 |
Polonium |
Po |
254 |
85 |
Asatine |
At |
302 |
86 |
Radon |
Rn |
-71 |
87 |
Francium |
Fr |
~27 |
88 |
Radium |
Ra |
700 |
89 |
Actinium |
Ac |
1050 |
90 |
Thorium |
Th |
1750 |
91 |
Protoactinium |
Pa |
<1600 |
92 |
Uranium |
U |
1132 |
93 |
Neptunium |
Np |
640 |
94 |
Plutonium |
Pu |
641 |
95 |
Americium |
Am |
994 |
96 |
Curium |
Cm |
1340 |
|
|
|
|
Source: data from James F. Shackelford, Introduction to Materials Science for Engineers, Second Edition, Macmillan Publishing Company, New York, pp.686-688, (1988).
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 1 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Actinium227 |
Ac |
1050±50 |
Aluminum |
Al |
658.5 |
Aluminum bromide |
Al2Br6 |
87.4 |
Aluminum chloride |
Al2Cl6 |
192.4 |
Aluminum iodide |
Al2I6 |
190.9 |
Aluminum oxide |
Al2O3 |
2045.0 |
Antimony |
Sb |
630 |
Antimony pentachloride |
SbCl5 |
4.0 |
Antimony tribromide |
SbBr3 |
96.8 |
Antimony trichloride |
SbCl3 |
73.3 |
Antimony trioxide |
Sb4O6 |
655.0 |
Antimony trisulfide |
Sb4S6 |
546.0 |
Argon |
Ar |
190.2 |
Arsenic |
As |
816.8 |
Arsenic pentafluoride |
AsF5 |
80.8 |
Arsenic tribromide |
AsBr3 |
30.0 |
Arsenic trichloride |
AsCl3 |
–16.0 |
Arsenic trifluoride |
AsF3 |
–6.0 |
Arsenic trioxide |
As4O6 |
312.8 |
Barium |
Ba |
725 |
Barium bromide |
BaBr2 |
846.8 |
Barium chloride |
BaCl2 |
959.8 |
Barium fluoride |
BaF2 |
1286.8 |
Barium iodide |
BaI2 |
710.8 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 2 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Barium nitrate |
Ba(NO3)2 |
594.8 |
Barium oxide |
BaO |
1922.8 |
Barium phosphate |
Ba3(PO4)2 |
1727 |
Barium sulfate |
BaSO4 |
1350 |
Beryllium |
Be |
1278 |
Beryllium bromide |
BeBr2 |
487.8 |
Beryllium chloride |
BeCl2 |
404.8 |
Beryllium oxide |
BeO |
2550.0 |
Bismuth |
Bi |
271 |
Bismuth trichloride |
BiCl3 |
223.8 |
Bismuth trifluoride |
BiF3 |
726.0 |
Bismuth trioxide |
Bi2O3 |
815.8 |
Boron |
B |
2300 |
Boron tribromide |
BBr3 |
–48.8 |
Boron trichloride |
BCl3 |
–107.8 |
Boron trifluoride |
BF3 |
–128.0 |
Boron trioxide |
B2O3 |
448.8 |
Bromine |
Br2 |
–7.2 |
Bromine pentafluoride |
BrF5 |
–61.4 |
Cadmium |
Cd |
320.8 |
Cadmium bromide |
CdBr2 |
567.8 |
Cadmium chloride |
CdCl2 |
567.8 |
Cadmium fluoride |
CdF2 |
1110 |
Cadmium iodide |
CdI2 |
386.8 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 3 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Cadmium sulfate |
CdSO4 |
1000 |
Calcium |
Ca |
851 |
Calcium bromide |
CaBr2 |
729.8 |
Calcium carbonate |
CaCO3 |
1282 |
Calcium chloride |
CaCl2 |
782 |
Calcium fluoride |
CaF2 |
1382 |
Calcium metasilicate |
CaSiO3 |
1512 |
Calcium nitrate |
Ca(NO3)2 |
560.8 |
Calcium oxide |
CaO |
2707 |
Calcium sulfate |
CaSO4 |
1297 |
Carbon dioxide |
CO2 |
–57.6 |
Carbon monoxide |
CO |
–205 |
Cyanogen |
C2N2 |
–27.2 |
Cyanogen chloride |
CNCl |
–5.2 |
Cerium |
Ce |
775 |
Cesium |
Cs |
28.3 |
Cesium chloride |
CsCl |
38.5 |
Cesium nitrate |
CsNO3 |
406.8 |
Chlorine |
Cl2 |
–103±5 |
Chromium |
Cr |
1890 |
Chromium (II) chloride |
CrCl2 |
814 |
Chromium (III) sequioxide |
Cr2O3 |
2279 |
Chromium trioxide |
CrO3 |
197 |
Cobalt |
Co |
1490 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 4 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Cobalt (II) chloride |
CoCl2 |
727 |
Copper |
Cu |
1083 |
Copper (II) chloride |
CuCl2 |
430 |
Copper (I) chloride |
CuCl |
429 |
Copper(l) cyanide |
Cu2(CN)2 |
473 |
Copper (I) iodide |
CuI |
587 |
Copper (II) oxide |
CuO |
1446 |
Copper (I) oxide |
Cu2O |
1230 |
Copper (I) sulfide |
Cu2S |
1129 |
Dysprosium |
Dy |
1407 |
Erbium |
Er |
1496 |
Europium |
Eu |
826 |
Europium trichloride |
EuCl3 |
622 |
Fluorine |
F2 |
–219.6 |
Gadolinium |
Gd |
1312 |
Gallium |
Ga |
29 |
Germanium |
Ge |
959 |
Gold |
Au |
1063 |
Hafnium |
Hf |
2214 |
Holmium |
Ho |
1461 |
Hydrogen |
H2 |
–259.25 |
Hydrogen bromide |
HBr |
–86.96 |
Hydrogen chloride |
HCl |
–114.3 |
Hydrogen fluoride |
HF |
83.11 |
Hydrogen iodide |
HI |
–50.91 |
Hydrogen nitrate |
HNO3 |
–47.2 |
Hydrogen oxide (water) |
H2O |
0 |
Deuterium oxide |
D2O |
3.78 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 5 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Hydrogen peroxide |
H2O2 |
–0.7 |
Hydrogen selenate |
H2SeO4 |
57.8 |
Hydrogen sulfate |
H2SO4 |
10.4 |
Hydrogen sulfide |
H2S |
–85.6 |
Hydrogen sulfide, di– |
H2S2 |
–89.7 |
Hydrogen telluride |
H2Te |
–49.0 |
Indium |
In |
156.3 |
lodine |
I2 |
112.9 |
lodine chloride (α) |
ICl |
17.1 |
lodine chloride (β) |
ICl |
13.8 |
Iron |
Fe |
1530.0 |
Iron carbide |
Fe3C |
1226.8 |
Iron (III) chloride |
Fe2Cl6 |
303.8 |
Iron (II) chloride |
FeCl2 |
677 |
Iron (II) oxide |
FeO |
1380 |
Iron oxide |
Fe3O4 |
1596 |
Iron pentacarbonyl |
Fe(CO)5 |
–21.2 |
Iron (II) sulfide |
FeS |
1195 |
Lanthanum |
La |
920 |
Lead |
Pb |
327.3 |
Leadbromide |
PbBr2 |
487.8 |
Lead chloride |
PbCl2 |
497.8 |
Lead fluoride |
PbF2 |
823 |
Lead iodide |
PbI2 |
412 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 6 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Lead molybdate |
PbMoO4 |
1065 |
Lead oxide |
PbO |
890 |
Lead sulfate |
PbSO4 |
1087 |
Lead sulfide |
PbS |
1114 |
Lithium |
Li |
178.8 |
Lithium bromide |
LiBr |
552 |
Lithium chloride |
LiCl |
614 |
Lithium fluoride |
LiF |
896 |
Lithium hydroxide |
LiOH |
462 |
Lithium iodide |
LiI |
440 |
Lithium metasilicate |
Li2SiO3 |
1177 |
Lithium molybdate |
Li2MoO4 |
705 |
Lithium nitrate |
LiNO3 |
250 |
Lithium orthosilicate |
Li4SiO4 |
1249 |
Lithium sulfate |
Li2SO4 |
857 |
Lithium tungstate |
Li2WO4 |
742 |
Lutetium |
Lu |
1651 |
Magnesium |
Mg |
650 |
Magnesium bromide |
MgBr2 |
711 |
Magnesium chloride |
MgCl2 |
712 |
Magnesium fluoride |
MgF2 |
1221 |
Magnesium oxide |
MgO |
2642 |
Magnesium silicate |
MgSiO3 |
1524 |
Magnesium sulfate |
MgSO4 |
1327 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 7 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Manganese |
Mn |
1220 |
Manganese dichloride |
MnCl2 |
650 |
Manganese metasilicate |
MnSiO3 |
1274 |
Manganese (II) oxide |
MnO |
1784 |
Manganese oxide |
Mn3O4 |
1590 |
Mercury |
Hg |
–39 |
Mercury bromide |
HgBr2 |
241 |
Mercury chloride |
HgCl2 |
276.8 |
Mercury iodide |
HgI2 |
250 |
Mercury sulfate |
HgSO4 |
850 |
Molybdenum |
Mo |
2622 |
Molybdenum dichloride |
MoCl2 |
726.8 |
Molybdenum hexafluoride |
MoF6 |
17 |
Molybdenum trioxide |
MoO3 |
795 |
Neodymium |
Nd |
1020 |
Neon |
Ne |
– 248.6 |
Nickel |
Ni |
1452 |
Nickel chloride |
NiCl2 |
1030 |
Nickel subsulfide |
Ni3S2 |
790 |
Niobium |
Nb |
2496 |
Niobium pentachloride |
NbCl5 |
21 l |
Niobium pentoxide |
Nb2O5 |
1511 |
Nitric oxide |
NO |
–163.7 |
Nitrogen |
N2 |
–210 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 8 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Nitrogen tetroxide |
N2O4 |
–13.2 |
Nitrous oxide |
N2O |
–90.9 |
Osmium |
Os |
2700 |
Osmium tetroxide (white) |
OsO4 |
41.8 |
Osmium tetroxide (yellow) |
OsO4 |
55.8 |
Oxygen |
O2 |
–218.8 |
Palladium |
Pd |
1555 |
Phosphoric acid |
H3PO4 |
42.3 |
Phosphoric acid. hypo– |
H4P2O6 |
54.8 |
Phosphorus acid, hypo– |
H3PO2 |
17.3 |
Phosphorus acid, ortho– |
H3PO3 |
73.8 |
Phosphorus oxychloride |
POCl3 |
1.0 |
Phosphorus pentoxide |
P4O10 |
569.0 |
Phosphorus trioxide |
P4O6 |
23.7 |
Phosphorus, yellow |
P4 |
44.1 |
Platinum |
Pt |
1770 |
Potassium |
K |
63.4 |
Potassium borate, meta– |
KBO2 |
947 |
Potassium bromide |
KBr |
742 |
Potassium carbonate |
K2CO3 |
897 |
Potassium chloride |
KCl |
770 |
Potassium chromate |
K2CrO4 |
984 |
Potassium cyanide |
KCN |
623 |
Potassium dichromate |
K2Cr2O7 |
398 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 9 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Potassium fluoride |
KF |
875 |
Potassium hydroxide |
KOH |
360 |
Potassium iodide |
Kl |
682 |
Potassium nitrate |
KNO3 |
338 |
Potassium peroxide |
K2O2 |
490 |
Potassium phosphate |
K3PO4 |
1340 |
Potassium pyro– phosphate |
K4P2O7 |
1092 |
Potassium sulfate |
K2SO4 |
1074 |
Potassium thiocyanate |
KSCN |
179 |
Praseodymium |
Pr |
931 |
Rhenium |
Re |
3167±60 |
Rhenium heptoxide |
Re2O7 |
296 |
Rhenium hexafluoride |
ReF6 |
19.0 |
Rubidium |
Rb |
38 .9 |
Rubidium bromide |
RbBr |
677 |
Rubidium chloride |
RbCl |
717 |
Rubidium fluoride |
RbF |
833 |
Rubidium iodide |
Rbl |
638 |
Rubidium nitrate |
RbNO3 |
305 |
Samarium |
Sm |
1072 |
Scandium |
Sc |
1538 |
Selenium |
Se |
217 |
Seleniumoxychloride |
SeOCl3 |
9.8 |
Silane, hexaHuoro– |
Si2F6 |
–28.6 |
Silicon |
Si |
1427 |
Silicon dioxide (Cristobalite) |
SiO2 |
1723 |
Silicon tetrachloride |
SiCl4 |
–67.7 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 10 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Silver |
Ag |
961 |
Silver bromide |
AgBr |
430 |
Silver chloride |
AgCl |
455 |
Silver cyanide |
AgCN |
350 |
Silver iodide |
Agl |
557 |
Silver nitrate |
AgNO3 |
209 |
Silver sulfate |
Ag2SO4 |
657 |
Silver sulfide |
Ag2S |
841 |
Sodium |
Na |
97.8 |
Sodium borate, meta– |
NaBO2 |
966 |
Sodium bromide |
NaBr |
747 |
Sodium carbonate |
Na2CO3 |
854 |
Sodium chlorate |
NaClO3 |
255 |
Sodium chloride |
NaCl |
800 |
Sodium cyanide |
NaCN |
562 |
Sodium fluoride |
NaF |
992 |
Sodium hydroxide |
NaOH |
322 |
Sodium iodide |
Nal |
662 |
Sodium molybdate |
Na2MoO4 |
687 |
Sodium nitrate |
NaNO3 |
310 |
Sodium peroxide |
Na2O2 |
460 |
Sodium phosphate, meta– |
NaPO3 |
988 |
Sodium pyrophosphate |
Na4P2O7 |
970 |
Sodiumsilicate,aluminum– |
NaAlSi3O8 |
1107 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 11 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Sodium silicate, di– |
Na2Si2O5 |
884 |
Sodium silicate, meta– |
Na2SiO3 |
1087 |
Sodium sulfate |
Na2SO4 |
884 |
Sodium sulfide |
Na2S |
920 |
Sodium thiocyanate |
NaSCN |
323 |
Sodium tungstate |
Na2WO4 |
702 |
Strontium |
Sr |
757 |
Strontium bromide |
SrBr2 |
643 |
Strontium chloride |
SrCl2 |
872 |
Strontium fluoride |
SrF2 |
1400 |
Strontium oxide |
SrO |
2430 |
Sulfur (monatomic) |
S |
119, |
Sulfur dioxide |
SO2 |
– 73.2 |
Sulfur trioxide (α) |
SO3 |
16.8 |
Sulfur trioxide (β) |
SO3 |
32.3 |
Sulfur trioxide (γ) |
SO3 |
62.1 |
Tantalum |
Ta |
2996 ± 50 |
Tantalum pentachloride |
TaCl5 |
206.8 |
Tantalum pentoxide |
Ta2O5 |
1877 |
Tellurium |
Te |
453 |
Terbium |
Tb |
1356 |
Thallium |
Tl |
302.4 |
Thallium bromide, mono– |
TlBr |
460 |
Thallium carbonate |
Tl2CO3 |
273 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 12 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Thallium chloride, mono– |
TICl |
427 |
Thallium iodide, mono– |
TlI |
440 |
Thallium nitrate |
TINO3 |
207 |
Thallium sulfate |
Tl2SO4 |
632 |
Thallium sulfide |
Tl2S |
449 |
Thorium |
Th |
1845 |
Thorium chloride |
ThCl4 |
765 |
Thorium dioxide |
ThO2 |
2952 |
Thulium |
Tm |
1545 |
Tin |
Sn |
231.7 |
Tin bromide, di– |
SnBr2 |
231.8 |
Tin bromide, tetra– |
SnBr4 |
29.8 |
Tin chloride, di– |
SnCl2 |
247 |
Tinchloride,tetra– |
SnCl4 |
–33.3 |
Tin iodide, tetra– |
SnI4 |
143.4 |
Tin oxide |
SnO |
1042 |
Titanium |
Ti |
1800 |
Titanium bromide, tetra– |
TiBr4 |
38 |
Titanium chloride, tetra– |
TiCl4 |
–23.2 |
Titanium dioxide |
TiO2 |
1825 |
Titanium oxide |
TiO |
991 |
Tungsten |
W |
3387 |
Tungsten dioxide |
WO2 |
1270 |
Tungsten hexafluoride |
WF6 |
–0.5 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 71. MELTING POINTS OF ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 13 OF 13)
|
|
Melting Point |
Compound |
Formula |
˚C |
|
|
|
|
|
|
Tungsten tetrachloride |
WCl4 |
327 |
Tungsten trioxide |
WO3 |
1470 |
Uranium235 |
U |
~1133 |
Uranium tetrachloride |
UCl4 |
590 |
Vanadium |
V |
1917 |
Vanadium dichloride |
VCl2 |
1027 |
Vanadium oxide |
VO |
2077 |
Vanadium pentoxide |
V2O5 |
670 |
Xenon |
Xe |
–111.6 |
Ytterbium |
Yb |
823 |
Yttrium |
Y |
1504 |
Yttrium oxide |
Y2O3 |
2227 |
Zinc |
Zn |
419.4 |
Zincchloride |
ZnCl2 |
283 |
Zinc oxide |
ZnO |
1975 |
Zinc sulfide |
ZnS |
1745 |
Zirconium |
Zr |
1857 |
Zirconium dichloride |
ZrCl2 |
727 |
Zirconium oxide |
ZrO2 |
2715 |
|
|
|
Source: data from: Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973), p.479 .
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 1 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
AgBr |
|
703 |
AgCl |
|
728 |
AgF |
|
708 |
AgI |
|
831 |
AgNO3 |
|
483 |
Ag2O |
|
573 |
Ag2SO4 |
|
933 |
Ag2S |
|
1098 |
AlBr3 |
|
371 |
Al4C3 |
|
2000 |
AlCl3 |
|
465 |
AlF3 |
|
1564 |
AlI |
|
464 |
AlN |
|
>2475 |
Al2O3 |
|
2322 |
Al2(SO4)3 |
|
1043 |
Al2S3 |
|
1373 |
BBr3 |
|
227 |
B4C |
|
2720 |
BCl3 |
|
166 |
BF3 |
|
146 |
BN |
|
3000 |
B2O3 |
|
723 |
BS4 |
|
663 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 2 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
BaB4 |
|
2543 |
BaBr2 |
|
1123 |
BaCl2 |
|
1235 |
BaF2 |
|
1627 |
BaI2 |
|
1013 |
Ba(NO3)2 |
|
865 |
BaO |
|
2283 |
BaSO4 |
|
1853 |
BaS |
|
1473 |
BeB2 |
|
>2243 |
BeBr2 |
|
793 |
Be2C |
|
>2375 |
BeCl2 |
|
713 |
BeF2 |
|
813 |
BeI2 |
|
783 |
Be3N2 |
|
2513 |
BeO |
|
2725 |
BeSO4 |
|
848 |
BiBr3 |
|
491 |
BiCl3 |
|
507 |
BiF3 |
|
1000 |
BiI3 |
|
681 |
B2O3 |
|
1098 |
Bi(SO4)3 |
|
678 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 3 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
Bi2S3 |
|
1020 |
CaBr2 |
|
1003 |
CaCl2 |
|
1055 |
CaF2 |
|
1675 |
CaI2 |
|
848 |
Ca(NO3)2 |
|
623 |
Ca3N2 |
|
1468 |
CaO |
|
3183 |
CaSO4 |
|
1723 |
CdBr2 |
|
841 |
CdCl2 |
|
841 |
CdF2 |
|
1373 |
CdI2 |
|
423 |
Cd(NO3)2 |
|
834 |
CdO |
|
1773 |
CdSO4 |
|
1273 |
CdS |
|
2023 |
CeB6 |
|
2463 |
CeCl3 |
|
1095 |
CeF2 |
|
1710 |
CeI3 |
|
1025 |
CeO2 |
|
>2873 |
CeS |
|
2400 |
Ce(SO4)2 |
|
468 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 4 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
CrB2 |
|
2123 |
Cr3C2 |
|
2168 |
CrN |
|
1770 |
Cr2O3 |
|
>2603 |
CrSi2 |
|
1843 |
CuBr |
|
777 |
CuCl |
|
695 |
CuF2 |
|
1129 |
CuI |
|
878 |
Cu3N |
|
573 |
Cu2O |
|
1508 |
Cu4Si |
|
1123 |
Cu2S |
|
1400 |
FeBr2 |
|
955 |
Fe3C |
|
2110 |
FeCl2 |
|
945 |
FeF3 |
|
>1275 |
Fe2O3 |
|
1864 |
Fe2(SO4)3 |
|
753 |
FeS |
|
1468 |
InBr3 |
|
709 |
InCl |
|
498 |
InF3 |
|
1443 |
InI3 |
|
483 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 5 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
In2O3 |
|
2183 |
In2S3 |
|
1323 |
KBr |
|
1008 |
KCl |
|
1043 |
KF |
|
1131 |
KI |
|
958 |
KNO3 |
|
610 |
K2O3 |
|
703 |
K2SO4 |
|
1342 |
K2S |
|
1113 |
LiBr |
|
823 |
LiCl |
|
883 |
LiF |
|
1119 |
LiI |
|
722 |
LiNO3 |
|
527 |
Li3N |
|
1118 |
Li2O |
|
>1975 |
Li2SO4 |
|
1132 |
Li2S |
|
1198 |
MgBr2 |
|
984 |
MgCl2 |
|
987 |
MgF2 |
|
1535 |
MgI2 |
|
<910 |
MgO |
|
3098 |
Mg2Si |
|
1375 |
MgS |
|
>2275 |
MgSO4 |
|
1397 |
MnCl2 |
|
923 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 6 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
MnF2 |
|
1129 |
MnO |
|
1840 |
MoB |
|
2625 |
Mo2C |
|
2963 |
MoF6 |
|
290 |
MoI4 |
|
373 |
MoO3 |
|
1068 |
MoSi2 |
|
2553 |
MoS2 |
|
1458 |
NaBr |
|
1023 |
NaC2 |
|
973 |
NaCl |
|
1073 |
NaF |
|
1267 |
NaI |
|
935 |
NaNO3 |
|
583 |
Na2N |
|
573 |
Na2SO4 |
|
1157 |
Na2S |
|
1453 |
NbB |
|
>2270 |
NbC |
|
3770 |
NbN |
|
2323 |
Nb2O5 |
|
1764 |
NbSi2 |
|
2203 |
NiBr2 |
|
1236 |
NiCl3 |
|
1274 |
NiF2 |
|
1273 |
NiI2 |
|
1070 |
NiO |
|
2257 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 7 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
NiSO4 |
|
1121 |
NiS |
|
1070 |
PbBr2 |
|
643 |
PbCl2 |
|
771 |
PbF2 |
|
1095 |
PbI2 |
|
675 |
Pb(NO3)2 |
|
743 |
PbO |
|
1159 |
PbSO4 |
|
1443 |
PbS |
|
1387 |
PtBr2 |
|
523 |
PtCl2 |
|
854 |
PtI2 |
|
633 |
PtS2 |
|
508 |
SbBr3 |
|
370 |
SbCl3 |
|
346 |
SbF3 |
|
565 |
SbI3 |
|
443 |
Sb2O3 |
|
928 |
SbS3 |
|
820 |
SiC |
|
2970 |
SiF4 |
|
183 |
Si3N4 |
|
2715 |
SiO2 |
|
1978 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 8 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
SnBr2 |
|
488 |
SnCl2 |
|
581 |
SnF4 |
|
978 |
SnI2 |
|
788 |
SnO |
|
1353 |
SnSO4 |
|
>635 |
SnS |
|
1153 |
SrB6 |
|
2508 |
SrBr2 |
|
916 |
SrC2 |
|
>1970 |
SrCl2 |
|
1148 |
SrF2 |
|
1736 |
SrI2 |
|
593 |
Sr(NO3)2 |
|
643 |
SrO |
|
2933 |
SrSO4 |
|
1878 |
SrS |
|
>2275 |
TaB |
|
>2270 |
TaBr5 |
|
538 |
TaC |
|
3813 |
TaCl5 |
|
489 |
TaF5 |
|
370 |
Ta2N |
|
3360 |
Ta2O5 |
|
2100 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 9 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
TaSi2 |
|
2670 |
TaS4 |
|
>1575 |
TeBr2 |
|
612 |
TeCl2 |
|
448 |
TeO2 |
|
1006 |
ThB4 |
|
>2270 |
ThBr4 |
|
883 |
ThC |
|
2898 |
ThCl4 |
|
1043 |
ThF4 |
|
1375 |
ThN |
|
2903 |
ThO2 |
|
3493 |
ThS2 |
|
2198 |
TiB2 |
|
3253 |
TiBr4 |
|
312 |
TiC |
|
3433 |
TiCl4 |
|
250 |
TiF3 |
|
1475 |
TiI2 |
|
873 |
TiN |
|
3200 |
TiO2 |
|
2113 |
TiSi2 |
|
1813 |
UB2 |
|
>1770 |
UBr4 |
|
789 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 10 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
UC |
|
2863 |
UCl4 |
|
843 |
UF4 |
|
1233 |
UI4 |
|
779 |
UN |
|
3123 |
UO2 |
|
3151 |
USi2 |
|
1970 |
US2 |
|
>1375 |
VB2 |
|
2373 |
VC |
|
3600 |
VCl4 |
|
245 |
VF3 |
|
>1075 |
FI2 |
|
1048 |
VN |
|
2593 |
V2O5 |
|
947 |
VSi2 |
|
2023 |
V2S3 |
|
>875 |
WB |
|
3133 |
WC |
|
2900 |
WCl6 |
|
548 |
WO3 |
|
1744 |
WSi2 |
|
2320 |
WS2 |
|
1523 |
ZnBr2 |
|
667 |
ZnCl2 |
|
548 |
ZnF2 |
|
1145 |
ZnI2 |
|
719 |
ZnO |
|
2248 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC

Table 72. MELTING POINTS OF CERAMICS
|
(SHEET 11 OF 11) |
|
|
|
|
Compound |
|
(K) |
|
|
|
|
|
|
ZnSO4 |
|
873 |
ZrB2 |
|
3313 |
ZrBr2 |
|
>625 |
ZrC |
|
3533 |
ZrCl2 |
|
623 |
ZrF4 |
|
873 |
ZrI4 |
|
772 |
ZrN |
|
3250 |
ZrO2 |
|
3123 |
Zr(SO4)2 |
|
683 |
ZrS2 |
|
1823 |
|
|
|
Source: data from: Lynch, Charles T., Ed., CRC Handbook of Materials Science, Vol. 1, CRC Press, Boca Raton, 1974, 348.
©2001 CRC Press LLC
Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 1 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
Actinium227 |
Ac |
1050±50 |
(11.0) |
(3400) |
Aluminum |
Al |
658.5 |
94.5 |
2550 |
Aluminum bromide |
Al2Br6 |
87.4 |
10.1 |
5420 |
Aluminum chloride |
Al2Cl6 |
192.4 |
63.6 |
19600 |
Aluminum iodide |
Al2I6 |
190.9 |
9.8 |
7960 |
Aluminum oxide |
Al2O3 |
2045.0 |
(256.0) |
(26000) |
Antimony |
Sb |
630 |
39.1 |
4770 |
Antimony pentachloride |
SbCl5 |
4.0 |
8.0 |
2400 |
Antimony tribromide |
SbBr3 |
96.8 |
9.7 |
3510 |
Antimony trichloride |
SbCl3 |
73.3 |
13.3 |
3030 |
Antimony trioxide |
Sb4O6 |
655.0 |
(46.3) |
(26990) |
Antimony trisulfide |
Sb4S6 |
546.0 |
33.0 |
11200 |
Argon |
Ar |
190.2 |
7.25 |
290 |
Arsenic |
As |
816.8 |
(22.0) |
(6620) |
Arsenic pentafluoride |
AsF5 |
80.8 |
16.5 |
2800 |
Arsenic tribromide |
AsBr3 |
30.0 |
8.9 |
2810 |
Arsenic trichloride |
AsCl3 |
–16.0 |
13.3 |
2420 |
Arsenic trifluoride |
AsF3 |
–6.0 |
18.9 |
2486 |
Arsenic trioxide |
As4O6 |
312.8 |
22.2 |
8000 |
Barium |
Ba |
725 |
13.3 |
1830 |
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 2 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Barium bromide |
BaBr2 |
846.8 |
21.9 |
6000 |
Barium chloride |
BaCl2 |
959.8 |
25.9 |
5370 |
Barium fluoride |
BaF2 |
1286.8 |
17.1 |
3000 |
Barium iodide |
BaI2 |
710.8 |
(17.3) |
(6800) |
Barium nitrate |
Ba(NO3)2 |
594.8 |
(22.6) |
(5900) |
Barium oxide |
BaO |
1922.8 |
93.2 |
13800 |
Barium phosphate |
Ba3(PO4)2 |
1727 |
30.9 |
18600 |
Barium sulfate |
BaSO4 |
1350 |
41.6 |
9700 |
Beryllium |
Be |
1278 |
260.0 |
– |
Beryllium bromide |
BeBr2 |
487.8 |
(26.6) |
(4500) |
Beryllium chloride |
BeCl2 |
404.8 |
(30) |
(3000) |
Beryllium oxide |
BeO |
2550.0 |
679.7 |
17000 |
Bismuth |
Bi |
271 |
12.0 |
2505 |
Bismuth trichloride |
BiCl3 |
223.8 |
8.2 |
2600 |
Bismuth trifluoride |
BiF3 |
726.0 |
(23.3) |
(6200) |
Bismuth trioxide |
Bi2O3 |
815.8 |
14.6 |
6800 |
Boron |
B |
2300 |
(490) |
(5300) |
Boron tribromide |
BBr3 |
–48.8 |
(2.9) |
(700) |
Boron trichloride |
BCl3 |
–107.8 |
(4.3) |
(500) |
Boron trifluoride |
BF3 |
–128.0 |
7.0 |
480 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 3 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Boron trioxide |
B2O3 |
448.8 |
78.9 |
5500 |
Bromine |
Br2 |
–7.2 |
16.1 |
2580 |
Bromine pentafluoride |
BrF5 |
–61.4 |
7.07 |
1355 |
Cadmium |
Cd |
320.8 |
12.9 |
1460 |
Cadmium bromide |
CdBr2 |
567.8 |
(18.4) |
(5000) |
Cadmium chloride |
CdCl2 |
567.8 |
28.8 |
5300 |
Cadmium fluoride |
CdF2 |
1110 |
(35.9) |
(5400) |
Cadmium iodide |
CdI2 |
386.8 |
10.0 |
3660 |
Cadmium sulfate |
CdSO4 |
1000 |
22.9 |
4790 |
Calcium |
Ca |
851 |
55.7 |
2230 |
Calcium bromide |
CaBr2 |
729.8 |
20.9 |
4180 |
Calcium carbonate |
CaCO3 |
1282 |
(126) |
(12700) |
Calcium chloride |
CaCl2 |
782 |
55 |
6100 |
Calcium fluoride |
CaF2 |
1382 |
52.5 |
4100 |
Calcium metasilicate |
CaSiO3 |
1512 |
115.4 |
13400 |
Calcium nitrate |
Ca(NO3)2 |
560.8 |
31.2 |
5120 |
Calcium oxide |
CaO |
2707 |
(218.1) |
(12240) |
Calcium sulfate |
CaSO4 |
1297 |
49.2 |
6700 |
Carbon dioxide |
CO2 |
–57.6 |
43.2 |
1900 |
Carbon monoxide |
CO |
–205 |
7.13 |
199.7 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC
Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 4 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
Cyanogen |
C2N2 |
–27.2 |
39.6 |
2060 |
Cyanogen chloride |
CNCl |
–5.2 |
36.4 |
2240 |
Cerium |
Ce |
775 |
27.2 |
2120 |
Cesium |
Cs |
28.3 |
3.7 |
500 |
Cesium chloride |
CsCl |
38.5 |
21.4 |
3600 |
Cesium nitrate |
CsNO3 |
406.8 |
16.6 |
3250 |
Chlorine |
Cl2 |
–103±5 |
22.8 |
1531 |
Chromium |
Cr |
1890 |
62.1 |
3660 |
Chromium (II) chloride |
CrCl2 |
814 |
65.9 |
7700 |
Chromium (III) sequioxide |
Cr2O3 |
2279 |
27.6 |
4200 |
Chromium trioxide |
CrO3 |
197 |
37.7 |
3770 |
Cobalt |
Co |
1490 |
62.1 |
3640 |
Cobalt (II) chloride |
CoCl2 |
727 |
56.9 |
7390 |
Copper |
Cu |
1083 |
49.0 |
3110 |
Copper (II) chloride |
CuCl2 |
430 |
24.7 |
4890 |
Copper (I) chloride |
CuCl |
429 |
26.4 |
2620 |
Copper(l) cyanide |
Cu2(CN)2 |
473 |
(30.1) |
(5400) |
Copper (I) iodide |
CuI |
587 |
(13.6) |
(2600) |
Copper (II) oxide |
CuO |
1446 |
35.4 |
2820 |
Copper (I) oxide |
Cu2O |
1230 |
(93.6) |
(l3400) |
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 5 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Copper (I) sulfide |
Cu2S |
1129 |
62.3 |
5500 |
Dysprosium |
Dy |
1407 |
25.2 |
4100 |
Erbium |
Er |
1496 |
24.5 |
4100 |
Europium |
Eu |
826 |
16.4 |
2500 |
Europium trichloride |
EuCl3 |
622 |
(20.9) |
(8000) |
Fluorine |
F2 |
–219.6 |
6.4 |
244.0 |
Gadolinium |
Gd |
1312 |
23.8 |
3700 |
Gallium |
Ga |
29 |
19.1 |
1336 |
Germanium |
Ge |
959 |
(114.3) |
(8300) |
Gold |
Au |
1063 |
(15.3) |
3030 |
Hafnium |
Hf |
2214 |
(34.1) |
(6000) |
Holmium |
Ho |
1461 |
24.8 |
4100 |
Hydrogen |
H2 |
–259.25 |
13.8 |
28 |
Hydrogen bromide |
HBr |
–86.96 |
7.1 |
575.1 |
Hydrogen chloride |
HCl |
–114.3 |
13.0 |
476.0 |
Hydrogen fluoride |
HF |
83.11 |
54.7 |
1094 |
Hydrogen iodide |
HI |
–50.91 |
5.4 |
686.3 |
Hydrogen nitrate |
HNO3 |
–47.2 |
9.5 |
601 |
Hydrogen oxide (water) |
H2O |
0 |
79.72 |
1436 |
Deuterium oxide |
D2O |
3.78 |
75.8 |
1516 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 6 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Hydrogen peroxide |
H2O2 |
–0.7 |
8.58 |
2920 |
Hydrogen selenate |
H2SeO4 |
57.8 |
23.8 |
3450 |
Hydrogen sulfate |
H2SO4 |
10.4 |
24.0 |
2360 |
Hydrogen sulfide |
H2S |
–85.6 |
16.8 |
5683 |
Hydrogen sulfide, di– |
H2S2 |
–89.7 |
27.3 |
1805 |
Hydrogen telluride |
H2Te |
–49.0 |
12.9 |
1670 |
Indium |
In |
156.3 |
6.8 |
781 |
lodine |
I2 |
112.9 |
14.3 |
3650 |
lodine chloride (α) |
ICl |
17.1 |
16.4 |
2660 |
lodine chloride (β) |
ICl |
13.8 |
13.3 |
2270 |
Iron |
Fe |
1530.0 |
63.7 |
3560 |
Iron carbide |
Fe3C |
1226.8 |
68.6 |
12330 |
Iron (III) chloride |
Fe2Cl6 |
303.8 |
63.2 |
20500 |
Iron (II) chloride |
FeCl2 |
677 |
61.5 |
7800 |
Iron (II) oxide |
FeO |
1380 |
(107.2) |
(7700) |
Iron oxide |
Fe3O4 |
1596 |
142.5 |
33000 |
Iron pentacarbonyl |
Fe(CO)5 |
–21.2 |
16.5 |
3250 |
Iron (II) sulfide |
FeS |
1195 |
56.9 |
5000 |
Lanthanum |
La |
920 |
17.4 |
2400 |
Lead |
Pb |
327.3 |
5.9 |
1224 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 7 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Leadbromide |
PbBr2 |
487.8 |
11 7 |
4290 |
Lead chloride |
PbCl2 |
497 8 |
20.3 |
5650 |
Lead fluoride |
PbF2 |
823 |
7.6 |
1860 |
Lead iodide |
PbI2 |
412 |
17.9 |
5970 |
Lead molybdate |
PbMoO4 |
1065 |
70.8 |
(25800) |
Lead oxide |
PbO |
890 |
12.6 |
2820 |
Lead sulfate |
PbSO4 |
1087 |
31.6 |
9600 |
Lead sulfide |
PbS |
1114 |
17.3 |
4150 |
Lithium |
Li |
178.8 |
158.5 |
1100 |
Lithium bromide |
LiBr |
552 |
33 4 |
2900 |
Lithium chloride |
LiCl |
614 |
75.5 |
3200 |
Lithium fluoride |
LiF |
896 |
(91.1) |
(2360) |
Lithium hydroxide |
LiOH |
462 |
103.3 |
2480 |
Lithium iodide |
LiI |
440 |
(10.6) |
(1420) |
Lithium metasilicate |
Li2SiO3 |
1177 |
80.2 |
7210 |
Lithium molybdate |
Li2MoO4 |
705 |
24.1 |
4200 |
Lithium nitrate |
LiNO3 |
250 |
87.8 |
6060 |
Lithium orthosilicate |
Li4SiO4 |
1249 |
60.5 |
7430 |
Lithium sulfate |
Li2SO4 |
857 |
27.6 |
3040 |
Lithium tungstate |
Li2WO4 |
742 |
(25.6) |
(6700) |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 8 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Lutetium |
Lu |
1651 |
26.3 |
4600 |
Magnesium |
Mg |
650 |
88.9 |
2160 |
Magnesium bromide |
MgBr2 |
711 |
45.0 |
8300 |
Magnesium chloride |
MgCl2 |
712 |
82.9 |
8100 |
Magnesium fluoride |
MgF2 |
1221 |
94.7 |
5900 |
Magnesium oxide |
MgO |
2642 |
459.0 |
18500 |
Magnesium silicate |
MgSiO3 |
1524 |
146.4 |
14700 |
Magnesium sulfate |
MgSO4 |
1327 |
28.9 |
3500 |
Manganese |
Mn |
1220 |
62.7 |
3450 |
Manganese dichloride |
MnCl2 |
650 |
58.4 |
7340 |
Manganese metasilicate |
MnSiO3 |
1274 |
(62.6) |
(8200) |
Manganese (II) oxide |
MnO |
1784 |
183.3 |
13000 |
Manganese oxide |
Mn3O4 |
1590 |
(170.4) |
(39000) |
Mercury |
Hg |
–39 |
2.7 |
557.2 |
Mercury bromide |
HgBr2 |
241 |
10.9 |
3960 |
Mercury chloride |
HgCl2 |
276.8 |
15.3 |
4150 |
Mercury iodide |
HgI2 |
250 |
9.9 |
4500 |
Mercury sulfate |
HgSO4 |
850 |
(4.8) |
(1440) |
Molybdenum |
Mo |
2622 |
(68.4) |
(6600) |
Molybdenum dichloride |
MoCl2 |
726.8 |
3.58 |
6000 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 9 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Molybdenum hexafluoride |
MoF6 |
17 |
11.9 |
2500 |
Molybdenum trioxide |
MoO3 |
795 |
(17.3) |
(2500) |
Neodymium |
Nd |
1020 |
11.8 |
1700 |
Neon |
Ne |
– 248.6 |
3.83 |
77.4 |
Nickel |
Ni |
1452 |
71.5 |
4200 |
Nickel chloride |
NiCl2 |
1030 |
142 5 |
18470 |
Nickel subsulfide |
Ni3S2 |
790 |
25.8 1 |
5800 |
Niobium |
Nb |
2496 |
(68.9) |
(6500) |
Niobium pentachloride |
NbCl5 |
21.1 |
30 8 |
8400 |
Niobium pentoxide |
Nb2O5 |
1511 |
91.0 |
24200 |
Nitric oxide |
NO |
–163.7 |
18.3 |
549.5 |
Nitrogen |
N2 |
–210 |
6.15 |
172.3 |
Nitrogen tetroxide |
N2O4 |
–13.2 |
60.2 |
5540 |
Nitrous oxide |
N2O |
–90.9 |
35.5 |
1563 |
Osmium |
Os |
2700 |
(36.7) |
(7000) |
Osmium tetroxide (white) |
OsO4 |
41.8 |
9.2 |
2340 |
Osmium tetroxide (yellow) |
OsO4 |
55.8 |
15.5 |
4060 |
Oxygen |
O2 |
–218.8 |
3.3 |
106.3 |
Palladium |
Pd |
1555 |
38.6 |
4120 |
Phosphoric acid |
H3PO4 |
42.3 |
25.8 |
2520 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 10 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Phosphoric acid. hypo– |
H4P2O6 |
54.8 |
51.2 |
8300 |
Phosphorus acid, hypo– |
H3PO2 |
17.3 |
35.0 |
2310 |
Phosphorus acid, ortho– |
H3PO3 |
73.8 |
37.4 |
3070 |
Phosphorus oxychloride |
POCl3 |
1.0 |
20.3 |
3110 |
Phosphorus pentoxide |
P4O10 |
569.0 |
60.1 |
17080 |
Phosphorus trioxide |
P4O6 |
23.7 |
15.3 |
3360 |
Phosphorus, yellow |
P4 |
44.1 |
4.8 |
600 |
Platinum |
Pt |
1770 |
24.1 |
4700 |
Potassium |
K |
63.4 |
14.6 |
574 |
Potassium borate, meta– |
KBO2 |
947 |
(69.1) |
(5660) |
Potassium bromide |
KBr |
742 |
42.0 |
5000 |
Potassium carbonate |
K2CO3 |
897 |
56.4 |
7800 |
Potassium chloride |
KCl |
770 |
85.9 |
6410 |
Potassium chromate |
K2CrO4 |
984 |
35.6 |
6920 |
Potassium cyanide |
KCN |
623 |
(53.7) |
(3500) |
Potassium dichromate |
K2Cr2O7 |
398 |
29.8 |
8770 |
Potassium fluoride |
KF |
875 |
111.9 |
6500 |
Potassium hydroxide |
KOH |
360 |
(35.3) |
(1980) |
Potassium iodide |
Kl |
682 |
24.7 |
4100 |
Potassium nitrate |
KNO3 |
338 |
78.1 |
2840 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 11 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Potassium peroxide |
K2O2 |
490 |
55.3 |
6100 |
Potassium phosphate |
K3PO4 |
1340 |
41.9 |
8900 |
Potassium pyro– phosphate |
K4P2O7 |
1092 |
42.4 |
14000 |
Potassium sulfate |
K2SO4 |
1074 |
46.4 |
8100 |
Potassium thiocyanate |
KSCN |
179 |
23.1 |
2250 |
Praseodymium |
Pr |
931 |
19.0 |
2700 |
Rhenium |
Re |
3167±60 |
(42.4) |
(7900) |
Rhenium heptoxide |
Re2O7 |
296 |
30.1 |
15340 |
Rhenium hexafluoride |
ReF6 |
19.0 |
16.6 |
5000 |
Rubidium |
Rb |
38 .9 |
6. 1 |
525 |
Rubidium bromide |
RbBr |
677 |
22.4 |
3700 |
Rubidium chloride |
RbCl |
717 |
36.4 |
4400 |
Rubidium fluoride |
RbF |
833 |
39.5 |
4130 |
Rubidium iodide |
Rbl |
638 |
14.0 |
2990 |
Rubidium nitrate |
RbNO3 |
305 |
9.1 |
1340 |
Samarium |
Sm |
1072 |
17.3 |
2600 |
Scandium |
Sc |
1538 |
84.4 |
3800 |
Selenium |
Se |
217 |
15.4 |
1220 |
Seleniumoxychloride |
SeOCl3 |
9.8 |
6.1 |
1010 |
Silane, hexaHuoro– |
Si2F6 |
–28.6 |
22.9 |
3900 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 12 OF 16)
|
|
Melting |
Heat of fusion |
||
|
|
|
|
||
|
|
point |
|
|
|
|
|
|
|
||
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
|
|
|
Silicon |
Si |
1427 |
337.0 |
9470 |
|
Silicon dioxide |
SiO2 |
1723 |
35.0 |
2100 |
|
(Cristobalite) |
|||||
|
|
|
|
||
Silicon tetrachloride |
SiCl4 |
–67.7 |
10.8 |
1845 |
|
Silver |
Ag |
961 |
25.0 |
2700 |
|
Silver bromide |
AgBr |
430 |
11.6 |
2180 |
|
Silver chloride |
AgCl |
455 |
22.0 |
3155 |
|
Silver cyanide |
AgCN |
350 |
20.5 |
2750 |
|
Silver iodide |
AgI |
557 |
9.5 |
2250 |
|
Silver nitrate |
AgNO3 |
209 |
16.2 |
2755 |
|
Silver sulfate |
Ag2SO4 |
657 |
(13.7) |
(4280) |
|
Silver sulfide |
Ag2S |
841 |
13.5 |
3360 |
|
Sodium |
Na |
97.8 |
27.4 |
630 |
|
Sodium borate, meta– |
NaBO2 |
966 |
134.6 |
8660 |
|
Sodium bromide |
NaBr |
747 |
59.7 |
6140 |
|
Sodium carbonate |
Na2CO3 |
854 |
66.0 |
7000 |
|
Sodium chlorate |
NaClO3 |
255 |
49.7 |
5290 |
|
Sodium chloride |
NaCl |
800 |
123.5 |
7220 |
|
Sodium cyanide |
NaCN |
562 |
(88.9) |
(4360) |
|
Sodium fluoride |
NaF |
992 |
166.7 |
7000 |
|
Sodium hydroxide |
NaOH |
322 |
50.0 |
2000 |
|
|
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 13 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Sodium iodide |
NaI |
662 |
35.1 |
5340 |
Sodium molybdate |
Na2MoO4 |
687 |
17.5 |
3600 |
Sodium nitrate |
NaNO3 |
310 |
44.2 |
3760 |
Sodium peroxide |
Na2O2 |
460 |
75.1 |
5860 |
Sodium phosphate, meta– |
NaPO3 |
988 |
(48.6) |
(4960) |
Sodium pyrophosphate |
Na4P2O7 |
970 |
(51.5) |
(13700) |
Sodiumsilicate,aluminum– |
NaAlSi3O8 |
1107 |
50.1 |
13150 |
Sodium silicate, di– |
Na2Si2O5 |
884 |
46.4 |
8460 |
Sodium silicate, meta– |
Na2SiO3 |
1087 |
84.4 |
10300 |
Sodium sulfate |
Na2SO4 |
884 |
41.0 |
5830 |
Sodium sulfide |
Na2S |
920 |
15.4 |
(1200) |
Sodium thiocyanate |
NaSCN |
323 |
54.8 |
4450 |
Sodium tungstate |
Na2WO4 |
702 |
19.6 |
5800 |
Strontium |
Sr |
757 |
25.0 |
2190 |
Strontium bromide |
SrBr2 |
643 |
19.3 |
4780 |
Strontium chloride |
SrCl2 |
872 |
26.5 |
4100 |
Strontium fluoride |
SrF2 |
1400 |
34.0 |
4260 |
Strontium oxide |
SrO |
2430 |
161.2 |
16700 |
Sulfur (monatomic) |
S |
119 |
9.2 |
295 |
Sulfur dioxide |
SO2 |
–73.2 |
32.2 |
2060 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 14 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Sulfur trioxide (α) |
SO3 |
16.8 |
25.8 |
2060 |
Sulfur trioxide (β) |
SO3 |
32.3 |
36.1 |
2890 |
Sulfur trioxide (γ) |
SO3 |
62.1 |
79.0 |
6310 |
Tantalum |
Ta |
2996 ± 50 |
34.6–41.5 |
(7500) |
Tantalum pentachloride |
TaCl5 |
206.8 |
25.1 |
9000 |
Tantalum pentoxide |
Ta2O5 |
1877 |
108.6 |
48000 |
Tellurium |
Te |
453 |
25.3 |
3230 |
Terbium |
Tb |
1356 |
24.6 |
3900 |
Thallium |
Tl |
302.4 |
5.0 |
1030 |
Thallium bromide, mono– |
TlBr |
460 |
21.0 |
5990 |
Thallium carbonate |
Tl2CO3 |
273 |
9.5 |
4400 |
Thallium chloride, mono– |
TICl |
427 |
17.7 |
4260 |
Thallium iodide, mono– |
TlI |
440 |
9.4 |
3125 |
Thallium nitrate |
TINO3 |
207 |
8.6 |
2290 |
Thallium sulfate |
Tl2SO4 |
632 |
10.9 |
5500 |
Thallium sulfide |
Tl2S |
449 |
6.8 |
3000 |
Thorium |
Th |
1845 |
(<19.8) |
(<4600) |
Thorium chloride |
ThCl4 |
765 |
61.6 |
22500 |
Thorium dioxide |
ThO2 |
2952 |
1102.0 |
291100 |
Thulium |
Tm |
1545 |
26.0 |
4400 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 15 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Tin |
Sn |
231.7 |
14.4 |
1720 |
Tin bromide, di– |
SnBr2 |
231.8 |
(6.1) |
(1720) |
Tin bromide, tetra– |
SnBr4 |
29.8 |
6.8 |
3000 |
Tin chloride, di– |
SnCl2 |
247 |
16.0 |
3050 |
Tin chloride,tetra– |
SnCl4 |
–33.3 |
8.4 |
2190 |
Tin iodide, tetra– |
SnI4 |
143.4 |
(6.9) |
(4330) |
Tin oxide |
SnO |
1042 |
(46.8) |
(6400) |
Titanium |
Ti |
1800 |
(104.4) |
(5000) |
Titanium bromide, tetra– |
TiBr4 |
38 |
(5.6) |
(2060) |
Titanium chloride, tetra– |
TiCl4 |
–23.2 |
11.9 |
2240 |
Titanium dioxide |
TiO2 |
1825 |
(142.7) |
(11400) |
Titanium oxide |
TiO |
991 |
219 |
14000 |
Tungsten |
W |
3387 |
(45.8) |
(8420) |
Tungsten dioxide |
WO2 |
1270 |
60 1 |
13940 |
Tungsten hexafluoride |
WF6 |
–0.5 |
6.0 |
1800 |
Tungsten tetrachloride |
WCl4 |
327 |
18.4 |
6000 |
Tungsten trioxide |
WO3 |
1470 |
60 1 |
13940 |
Uranium235 |
U |
~1133 |
20 |
3700 |
Uranium tetrachloride |
UCl4 |
590 |
27.1 |
10300 |
Vanadium |
V |
1917 |
(70) |
(4200) |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC

Table 73. HEAT OF FUSION FOR ELEMENTS AND
INORGANIC COMPOUNDS (SHEET 16 OF 16)
|
|
Melting |
Heat of fusion |
|
|
|
|
|
|
|
|
point |
|
|
|
|
|
|
|
Compound |
Formula |
˚C |
cal/g |
cal/g mole |
|
|
|
|
|
|
|
|
|
|
Vanadium dichloride |
VCl2 |
1027 |
65.6 |
8000 |
Vanadium oxide |
VO |
2077 |
224.0 |
15000 |
Vanadium pentoxide |
V2O5 |
670 |
85.5 |
15560 |
Xenon |
Xe |
–111.6 |
5.6 |
740 |
Ytterbium |
Yb |
823 |
12.7 |
2200 |
Yttrium |
Y |
1504 |
46.1 |
4100 |
Yttrium oxide |
Y2O3 |
2227 |
110.7 |
25000 |
Zinc |
Zn |
419.4 |
24.4 |
1595 |
Zinc chloride |
ZnCl2 |
283 |
(406) |
(5540) |
Zinc oxide |
ZnO |
1975 |
54.9 |
4470 |
Zinc sulfide |
ZnS |
1745 |
(93.3) |
(9100) |
Zirconium |
Zr |
1857 |
(60) |
(5500) |
Zirconium dichloride |
ZrCl2 |
727 |
45.0 |
7300 |
Zirconium oxide |
ZrO2 |
2715 |
168.8 |
20800 |
|
|
|
|
|
For heat of fusion in J/kg, multiply values in cal/g by 4184.
For heat of fusion in J/mol, multiply values in cal/g-mol (=cal/mol) by 4.184. For melting point in K, add 273.15 to values in ˚C.
Values in parentheses are of uncertain reliability.
Source: data from Weast, R C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, (1974); and Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, (1973)
©2001 CRC Press LLC
Table 74. HEATS OF SUBLIMATION OF METALS
AND THEIR OXIDES
|
kcal/mole |
kJ/mole |
Metal |
(25˚C) |
(25˚C) |
|
|
|
|
|
|
Al |
78 |
326 |
Cu |
81 |
338 |
Fe |
100 |
416 |
Mg |
113 |
473 |
Metal Oxide |
|
|
FeO |
122 |
509 |
MgO |
145 |
605 |
α-TiO |
143 |
597 |
TiO2 (rutile) |
153 |
639 |
|
|
|
Data from: JANAF Thermochemical Tables, 2nd ed., National Standard Reference Data Series, Natl. Bur. Std. (U.S.), 37 (1971) and Supplement in J. Phys. Chem. Ref. Data 4(1), 1- 175 (1975).
©2001 CRC Press LLC
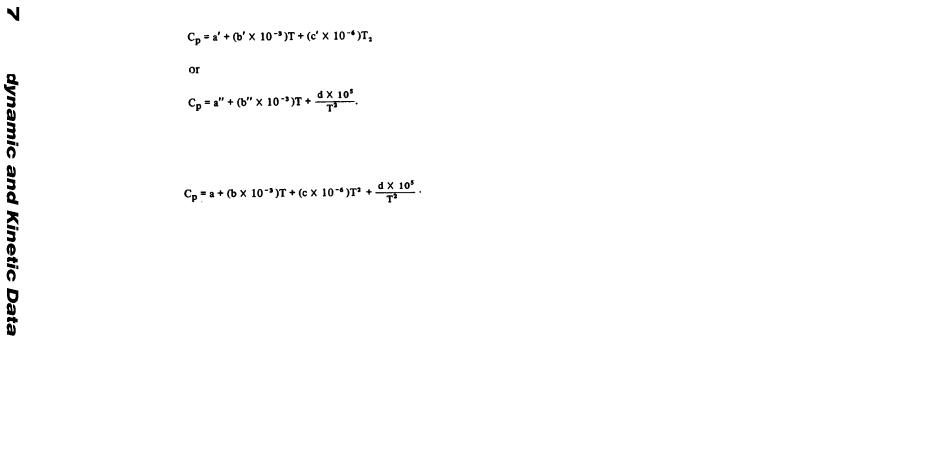
Table 75. KEY TO TABLES OF THERMODYNAMIC COEFFICIENTS
(SHEET 1 OF 4)
Thermodynamic calculations over a wide range of temperatures are generally made with the aid of algebraic equations representing the characteristic properties of the substances being considered. The necessary integrations and differentiations, or other mathematical manipulations, are then most easily effected. The most convenient starting point in making such calculations for a given substance is the heat capacity at constant pressure. From this quantity and a knowledge of the properties of any phase transitions, the other thermodynamic properties may be computed by the well-known equations given in standard texts on thermodynamics. Please note that the units for a, b, c, and d are cal/g mole, whereas those for A are kcal/g mole. The necessary adjustment must be made when the data are substituted into the equations. Empirical heat capacity equations are generated in the form of a power series, with the absolute temperature T as the independent variable:
Since both forms are used in the following, let
©2001 CRC Press LLC
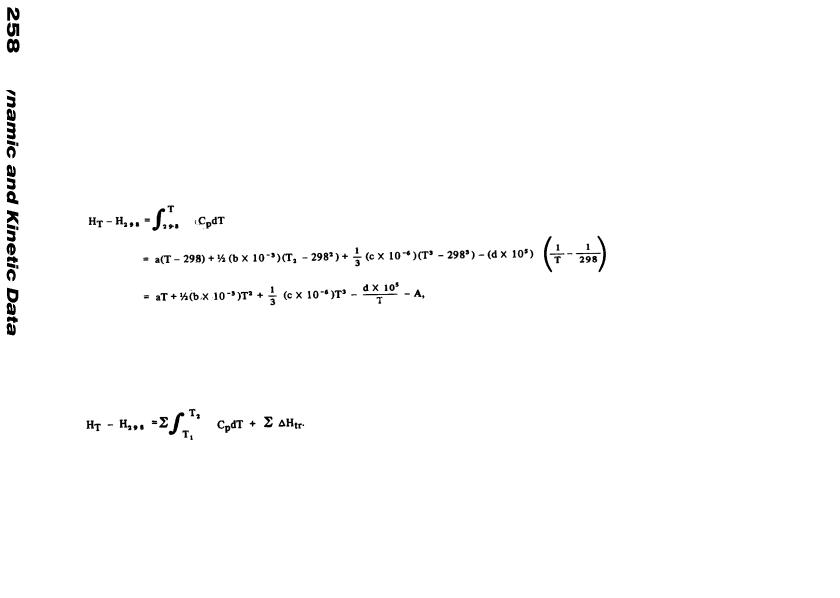
Table 75. KEY TO TABLES OF THERMODYNAMIC COEFFICIENTS
(SHEET 2 OF 4)
The constants a, b, c, and d are to be determined either experimentally or by some theoretical or semi-empirical approach. The heat content, or enthalpy (H), is determined from the heat capacity by a simple integration of the range of temperatures for which the formula for cp is valid. Thus, if 298K is taken as a reference temperature,
where all the constants on the right-hand side of the equation have been incorporated in the term –A.
In general, the enthalpy is given by a sum of terms for each phase of the substance involved in the temperature range considered plus terms that represent the heats of transitions:
In a similar manner, the entropy S is obtained by performing the integration
©2001 CRC Press LLC

Table 75. KEY TO TABLES OF THERMODYNAMIC COEFFICIENTS
(SHEET 3 OF 4)
where
From the definition of free energy (F):
the quantity
©2001 CRC Press LLC
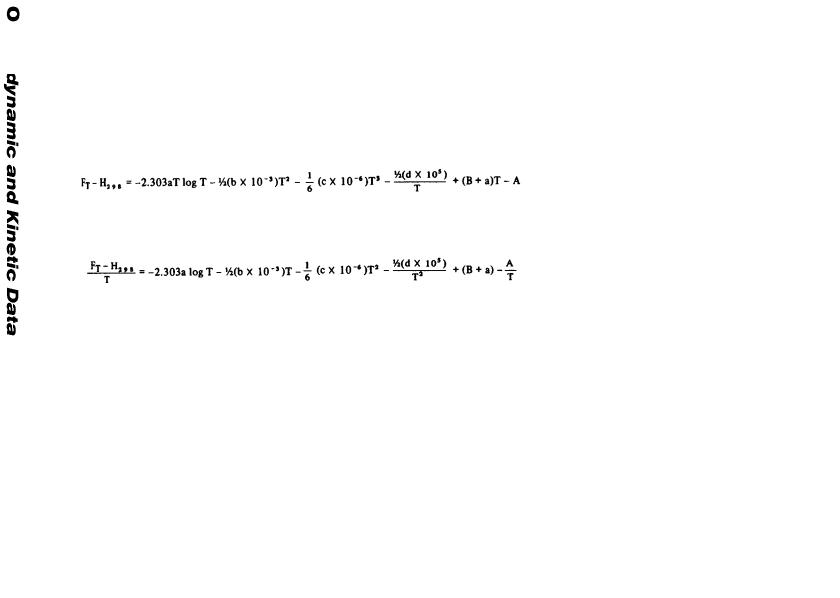
Table 75. KEY TO TABLES OF THERMODYNAMIC COEFFICIENTS
(SHEET 4 OF 4)
may be written as:
and also the free energy function
Values of these thermodynamic coefficients are given in the following tables. The first column in each table lists the material. The second column gives the phase to which the coefficients are applicable. The remaining columns list the values of the constants a, b, c, d, A, and B required in the thermodynamic equations. All values that represent estimates are enclosed in parentheses. The heat capacities at temperatures beyond the range of experimental determination were estimated by extrapolation. Where no experimental values were found, analogy with compounds of neighboring elements in the periodic table was used.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 1 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ac |
solid |
(5.4) |
(3.0) |
– |
– |
(1.743) |
(18.7) |
|
liquid |
(8) |
– |
– |
– |
(0.295) |
(31.3) |
Ag |
solid |
5.09 |
1.02 |
– |
0.36 |
1.488 |
19.21 |
|
liquid |
7.30 |
– |
– |
– |
0.164 |
30.12 |
|
gas |
(4.97) |
– |
– |
– |
(–66.34) |
(–12.52) |
Al |
solid |
4.94 |
2.96 |
– |
– |
1.604 |
22.26 |
|
liquid |
7.0 |
– |
– |
– |
0.33 |
30.83 |
Am |
solid |
(4.9) |
(4.4) |
– |
– |
(1.657) |
(16.2) |
|
liquid |
(8.5) |
– |
– |
– |
(0.409) |
(34.5) |
As |
solid |
5.17 |
2.34 |
– |
– |
1.646 |
21.8 |
Au |
solid |
6.14 |
–0.175 |
0.92 |
– |
1.831 |
23.65 |
|
liquid |
7.00 |
– |
– |
– |
–0.631 |
26.99 |
B |
solid |
1.54 |
4.40 |
– |
– |
0.655 |
8.67 |
|
liquid |
(6.0) |
– |
– |
– |
(–4.599) |
(31.4) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 2 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ba |
solid, α |
5.55 |
4.50 |
– |
– |
1.722 |
16.1 |
|
solid, β |
5.55 |
1.50 |
– |
– |
1.582 |
15.9 |
|
liquid |
(7.4) |
– |
– |
– |
(0.843) |
(25.3) |
|
gas |
(497) |
– |
– |
– |
(–39.65) |
(–11.7) |
Be |
solid |
5.07 |
1.21 |
– |
–1.15 |
1.951 |
27.62 |
|
liquid |
5.27 |
– |
– |
– |
–1.611 |
25.68 |
Bi |
solid |
5.38 |
2.60 |
– |
– |
1.720 |
17.8 |
|
liquid |
7.60 |
– |
– |
– |
–0.087 |
25.6 |
|
gas |
(4.97) |
– |
– |
– |
(–46.19) |
(–15.9) |
C |
solid |
4.10 |
1.02 |
– |
–2.10 |
1.972 |
23.484 |
Ca |
solid, α |
5.24 |
3.50 |
– |
– |
1.718 |
2095 |
|
solid, β |
6.29 |
1.40 |
– |
– |
1.689 |
26.01 |
|
liquid |
7.4 |
– |
– |
– |
–0.147 |
30.28 |
|
gas |
(4.97) |
– |
– |
– |
(–43.015) |
(–9.88) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 3 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cd |
solid |
5.31 |
2.94 |
– |
– |
1.714 |
18.8 |
|
liquid |
7.10 |
– |
– |
– |
0.798 |
26.1 |
|
gas |
(4.97) |
– |
– |
– |
(–25.28) |
(–11.7) |
Ce |
solid |
4.40 |
6.0 |
– |
– |
1.579 |
13.1 |
|
liquid |
(7.9) |
– |
– |
– |
(–0.148) |
(29.1) |
Cl2 |
gas |
8.76 |
0.27 |
– |
–0.65 |
2.845 |
–2.929 |
Co |
solid, α |
4.72 |
4.30 |
– |
– |
1.598 |
21.4 |
|
solid, β |
3.30 |
5.86 |
– |
– |
0.974 |
3.1 |
|
solid, γ |
9.60 |
– |
– |
– |
3.961 |
50.5 |
|
liquid |
8.30 |
– |
– |
– |
–2.034 |
38.7 |
Cr |
solid |
5.35 |
2.36 |
– |
–0.44 |
1.848 |
25.75 |
|
liquid |
9.40 |
– |
– |
– |
1.556 |
50.13 |
|
gas |
(4.97) |
– |
– |
– |
(–82.47) |
(–13.8) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 4 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cs |
solid |
7.42 |
– |
– |
– |
2.212 |
22.5 |
|
liquid |
8.00 |
– |
– |
– |
1.887 |
24.1 |
|
gas |
(4.97) |
– |
– |
– |
(–17.35) |
(–13.6) |
Cu |
solid |
5.41 |
1.50 |
– |
– |
1.680 |
23.30 |
|
liquid |
7.50 |
– |
– |
– |
0.024 |
34.05 |
F2 |
gas |
8.29 |
0.44 |
– |
–0.80 |
2.760 |
–0.76 |
Fe |
solid, α |
3.37 |
7.10 |
– |
0.43 |
1.176 |
14.59 |
|
solid, β |
10.40 |
– |
– |
– |
4.281 |
55.66 |
|
solid, γ |
4.85 |
3.00 |
– |
– |
0.396 |
19.76 |
|
solid, δ |
10.30 |
– |
– |
– |
4.382 |
55.11 |
|
liquid |
10.00 |
– |
– |
– |
–0.021 |
50.73 |
Ga |
solid |
5.237 |
3.33 |
– |
– |
1.710 |
21.01 |
|
liquid |
(6.645) |
– |
– |
– |
(0.648) |
(23.64) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 5 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ge |
solid |
5.90 |
1.13 |
– |
– |
1.764 |
23.8 |
|
liquid |
(7.3) |
– |
– |
– |
(–5.668) |
(25.7) |
H2 |
gas |
6.62 |
0.81 |
– |
– |
2.010 |
6.75 |
Hf |
solid |
(6.00) |
(0.52) |
– |
– |
(1.812) |
(21.2) |
Hg |
liquid |
6.61 |
– |
– |
– |
1.971 |
19.20 |
|
gas |
4.969 |
– |
– |
– |
–13.048 |
–13.54 |
In |
solid |
5.81 |
2.50 |
– |
– |
1.844 |
19.97 |
|
liquid |
7.50 |
– |
– |
– |
1.564 |
27.34 |
|
gas |
(4.97) |
– |
– |
– |
(–58.42) |
(–14.46) |
Ir |
solid |
5.56 |
1.42 |
– |
– |
1.721 |
23.4 |
K |
solid |
1.3264 |
19.405 |
– |
– |
1.258 |
–1.86 |
|
liquid |
8.8825 |
4.565 |
2.9369 |
– |
1.923 |
32.55 |
|
gas |
(4.97) |
– |
– |
– |
(–19.689) |
(–9.46) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 6 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
La |
solid |
6.17 |
1.60 |
– |
– |
1.911 |
21.9 |
|
liquid |
(7.3) |
– |
– |
– |
(–0.15) |
(26.0) |
Li |
solid |
3.05 |
8.60 |
– |
– |
1.292 |
12.92 |
|
liquid |
7.0 |
– |
– |
– |
1.509 |
32.00 |
|
gas |
(4.97) |
– |
– |
– |
(–34.30) |
(–2.84) |
Mg |
solid |
5.33 |
2.45 |
– |
–0.103 |
1.733 |
23.39 |
|
liquid |
(8.0) |
– |
– |
– |
0.942 |
36.967 |
|
gas |
(4.97) |
– |
– |
– |
(–34.78) |
(–7.60) |
Mn |
solid, α |
6.70 |
3.38 |
– |
–0.37 |
1.974 |
26.11 |
|
solid, β |
8.33 |
0.66 |
– |
– |
2.672 |
41.02 |
|
solid, γ |
10.70 |
– |
– |
– |
4.760 |
56.84 |
|
solid, δ |
11.30 |
– |
– |
– |
5.176 |
60.88 |
|
liquid |
11.00 |
– |
– |
– |
1.221 |
56.38 |
|
gas |
6.26 |
– |
|
|
–63.704 |
–3.13 |
Mo |
solid |
5.48 |
1.30 |
– |
– |
1.692 |
24.78 |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 7 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N2 |
gas |
6.76 |
0.606 |
0.13 |
– |
2.044 |
–7.064 |
Na |
solid |
5.657 |
3.252 |
0.5785 |
– |
1.836 |
20.92 |
|
liquid |
8.954 |
–4.577 |
2.540 |
– |
1.924 |
36.0 |
|
|
(4.97) |
– |
– |
– |
(–24.40) |
(–8.7) |
Nb |
solid |
5.66 |
0.96 |
– |
– |
1.730 |
24.24 |
Nd |
solid |
5.61 |
5.34 |
– |
– |
1.910 |
19.7 |
|
liquid |
(9.1) |
– |
– |
– |
(–0.606) |
35.8 |
Ni |
solid α |
4.06 |
7.04 |
– |
– |
1.523 |
18.095 |
|
solid β |
6.00 |
1.80 |
– |
– |
1.619 |
27.16 |
|
liquid |
9.20 |
– |
– |
– |
0.251 |
45.47 |
Np |
solid |
(5.3) |
(3.4) |
– |
– |
(1.731) |
(17.9) |
|
liquid |
(9.0) |
– |
– |
– |
(1.392) |
(37.5) |
O2 |
gas |
8.27 |
0.258 |
– |
–1.877 |
3.007 |
–0.750 |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 8 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Os |
solid |
5.69 |
0.88 |
– |
– |
1.736 |
24.9 |
P4 |
solid, white |
13.62 |
28.72 |
– |
– |
5.338 |
43.8 |
|
liquid |
19.23 |
0.51 |
– |
–2.98 |
6.035 |
66.7 |
|
gas |
(19.5) |
(–0.4) |
(1.3) |
– |
(–6.32) |
(46.1) |
Pa |
solid |
(5.2) |
(4.0) |
– |
– |
(1.728) |
(17.3) |
|
liquid |
(8.0) |
– |
– |
– |
(–3.823) |
(28.8) |
Pb |
solid |
5.64 |
2.30 |
– |
– |
1.784 |
17.33 |
|
liquid |
7.75 |
–0.73 |
– |
– |
1.362 |
27.11 |
|
gas |
(4.97) |
– |
– |
– |
(–45.25) |
(–13.6) |
Pd |
solid |
5.80 |
1.38 |
– |
– |
1.791 |
24.6 |
|
liquid |
(9.0) |
– |
– |
– |
(1.215) |
(43.8) |
Po |
solid |
(5.2) |
(3.2) |
– |
– |
(1.693) |
(17.6) |
|
liquid |
(9.0) |
– |
– |
– |
(0.847) |
(35.2) |
|
gas |
(4.97) |
– |
– |
– |
(–28.73) |
(–13.5) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 9 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pr |
solid |
(5.0) |
(4.6) |
– |
– |
(1.705) |
(16.4) |
|
liquid |
(8.0) |
– |
– |
– |
(–0.519) |
(30.0) |
Pt |
solid |
5.74 |
1.34 |
– |
0.10 |
1.737 |
23.0 |
|
liquid |
(9.0) |
– |
– |
– |
(0.406) |
(42.6) |
Pu |
solid |
(5.2) |
(3.6) |
– |
– |
(1.710) |
(17.7) |
|
liquid |
(8.0) |
– |
– |
– |
(0.506) |
(31.0) |
Ra |
solid |
(5.8) |
(1.2) |
– |
– |
(1.783) |
(16.4) |
|
liquid |
(8.0) |
– |
– |
– |
(1.284) |
(28.6) |
|
gas |
(4.97) |
– |
– |
– |
(–38.87) |
(–14.5) |
Rb |
solid |
3.27 |
13.1 |
– |
– |
1.557 |
5.9 |
|
liquid |
7.85 |
– |
– |
– |
1.814 |
26.5 |
|
gas |
(4.97) |
– |
– |
– |
(–19.04) |
(–12.3) |
Re |
solid |
(5.85) |
(0.8) |
– |
– |
(1.780) |
(24.7) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 10 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rh |
solid |
5.40 |
2.19 |
– |
– |
1.707 |
23.8 |
|
liquid |
(9.0) |
– |
– |
– |
(–0.923) |
(44.4) |
Ru |
solid, α |
5.25 |
1.50 |
– |
– |
1.632 |
23.5 |
|
solid, β |
7.20 |
– |
– |
– |
2.867 |
35.5 |
|
solid, γ |
7.20 |
– |
– |
– |
2.867 |
35.5 |
|
solid, δ |
7.50 |
– |
– |
– |
3.169 |
37.6 |
S |
solid, α |
3.58 |
6.24 |
– |
– |
1.345 |
14.64 |
|
solid, β |
3.56 |
6.95 |
– |
– |
1.298 |
14.54 |
|
liquid |
5.4 |
5.0 |
– |
– |
1.576 |
24.02 |
1/2 S2 |
gas |
(4.25) |
(0.15) |
– |
(–1.0) |
(–2.859) |
(9.57) |
Sb |
solid, α, β, γ |
5.51 |
1.74 |
– |
– |
1.720 |
21.4 |
|
liquid |
7.50 |
– |
– |
– |
1.992 |
28.1 |
1/2 Sb2 |
gas |
4.47 |
– |
– |
–0.11 |
–53.876 |
–21.7 |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 11 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sc |
solid |
(5.13) |
(3.0) |
– |
– |
1.663 |
21.1 |
|
liquid |
(7.50) |
– |
|
|
(–2.563) |
31.3 |
Se |
solid |
3.30 |
8.80 |
– |
– |
1.375 |
11.28 |
|
liquid |
7.0 |
– |
– |
– |
0.881 |
27.34 |
Si |
solid |
5.70 |
1.02 |
– |
–1.06 |
2.100 |
28.88 |
|
liquid |
7.4 |
– |
|
|
7.646 |
33.17 |
Sm |
solid |
(6.7) |
(3.4) |
– |
– |
(2.149) |
(24.2) |
|
liquid |
(9.0) |
– |
– |
– |
(–2.296) |
(33.4) |
Sn |
solid, α, β |
4.42 |
6.30 |
– |
– |
1.598 |
14.8 |
|
liquid |
7.30 |
– |
– |
– |
0.559 |
26.2 |
|
gas |
(4.97) |
– |
– |
– |
60.21) |
(–14.3) |
Sr |
solid |
(5.60) |
(1.37) |
– |
– |
(1.731) |
(19.3) |
|
liquid |
(7.7) |
– |
– |
– |
(0.976) |
(30.4) |
|
gas |
(4.97) |
– |
– |
– |
(37.16) |
(–10.2) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 12 OF 14)
|
|
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ta |
|
solid |
5.82 |
0.78 |
– |
– |
1.770 |
23.4 |
|
Tc |
|
solid |
(5.6) |
(2.0) |
– |
– |
(1.759) |
(24.5) |
|
|
|
|
liquid |
– |
– |
– |
– |
(3.459) |
(59.4) |
Te |
|
solid, α |
4.58 |
5.25 |
– |
– |
1.599 |
15.78 |
|
|
|
|
solid, β |
4.58 |
5.25 |
– |
– |
1.469 |
15.57 |
|
|
|
liquid |
9.0 |
– |
– |
– |
–0.988 |
34.96 |
1/ |
Te |
2 |
gas |
4.47 |
– |
– |
–0.10 |
–19.048 |
–6.47 |
2 |
|
|
|
|
|
|
|
|
|
Th |
|
solid |
8.2 |
–0.77 |
2.04 |
– |
2.591 |
33.64 |
|
|
|
|
liquid |
(8.0) |
– |
– |
– |
(–7.602) |
(26.84) |
Ti |
|
solid, α |
5.25 |
2.52 |
– |
– |
1.677 |
23.33 |
|
|
|
|
solid, β |
7.50 |
– |
– |
– |
1.645 |
35.46 |
|
|
|
liquid |
(7.8) |
– |
– |
– |
(–2.355) |
(35.45) |
|
|
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 13 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tl |
solid, α |
5.26 |
3.46 |
– |
– |
1.722 |
15.6 |
|
solid, β |
7.30 |
– |
– |
– |
2.230 |
26.4 |
|
liquid |
7.50 |
– |
– |
– |
1.315 |
25.9 |
|
gas |
(4.97) |
– |
– |
– |
(–41.88) |
(–15.4) |
U |
solid, α |
3.25 |
8.15 |
– |
0.80 |
1.063 |
8.47 |
|
solid, β |
10.28 |
– |
– |
– |
3.493 |
48.27 |
|
solid, γ |
9.12 |
– |
– |
– |
1.110 |
39.09 |
|
liquid |
(8.99) |
– |
– |
– |
(–2.073) |
36.01 |
V |
solid |
5.57 |
0.97 |
– |
– |
1.704 |
24.97 |
|
liquid |
(8.6) |
– |
– |
– |
1.827 |
44.06 |
W |
solid |
5.74 |
0.76 |
– |
– |
1.745 |
24.9 |
Y |
solid |
(5.6) |
(2.2) |
– |
– |
(1.767) |
(21.6) |
|
liquid |
(7.5) |
– |
– |
– |
2.277) |
(29.6) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC
Table 76. THERMODYNAMIC COEFFICIENTS FOR SELECTED ELEMENTS *
(SHEET 14 OF 14)
|
|
a |
b |
c |
d |
A |
B |
Element |
Phase |
– |
– |
(cal • g mole-1 ) |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Zn |
solid |
5.35 |
2.40 |
– |
– |
1.702 |
21.25 |
|
liquid |
7.50 |
– |
– |
– |
1.020 |
31.35 |
|
|
(4.97) |
– |
– |
– |
(–29.407) |
(–9.81) |
Zr |
solid, α |
6.83 |
1.12 |
– |
–0.87 |
2.378 |
30.45 |
|
solid, β |
7.27 |
– |
– |
– |
1.159 |
31.43 |
|
liquid |
(8.0) |
– |
– |
– |
(–2.190) |
(34.7) |
|
|
|
|
|
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
*Please refer to Table 75, ”Key to Tables of Thermodynamic Coefficients” on page 257 for an explanation of the coefficients.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 1 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ac2O3 |
Solid |
(20.0) |
(20.4) |
– |
– |
(6.870) |
(80.9) |
|
Liquid |
(40) |
– |
– |
– |
(–19.767) |
(180.5) |
Ag2O |
Solid |
13.26 |
7.04 |
– |
– |
4.266 |
48.56 |
Ag2O2 |
Solid |
(16.4) |
(12.2) |
– |
– |
(5.432) |
(76.7) |
Al2O3 |
Solid |
26.12 |
4.388 |
– |
–7.269 |
10.422 |
142.03 |
|
Liquid |
(33) |
– |
– |
– |
(– 11.655) |
(174.1) |
Am2O3 |
Solid |
(20.0) |
(15.6) |
– |
– |
(6.657) |
(81.6) |
|
Liquid |
(38.5) |
– |
– |
– |
(–7.796) |
(181.8) |
AmO2 |
Solid |
(14.0) |
(6.8) |
– |
– |
(4.477) |
(61.8) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 2 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As2O3 |
Solid, α |
8.37 |
48.6 |
– |
– |
4.656 |
36.6 |
|
Solid, β |
8.37 |
48.6 |
– |
– |
0.556 |
28.4 |
|
Liquid |
(39) |
– |
– |
– |
(5.760) |
(187.6) |
|
Gas |
(21.5) |
– |
– |
– |
(–14.164) |
(62.5) |
AsO2 |
Solid |
(8.5) |
(9.4) |
– |
– |
(2.952) |
(38.2) |
|
Liquid |
(21) |
– |
– |
– |
(2.184) |
(108.0) |
As2O5 |
Solid |
(31.1) |
(16.4) |
– |
(–5.4) |
(11.813) |
(159.9) |
Au2O3 |
Solid |
(23.5) |
(4.8) |
– |
– |
(7.220) |
(105.3) |
B2O3 |
Solid |
8.73 |
25.40 |
– |
–1.31 |
4.171 |
45.04 |
|
Liquid |
30.50 |
– |
– |
– |
7.822 |
161.59 |
Ba2O |
Solid |
(20.0) |
(2.2) |
– |
– |
(6.061) |
(91.1) |
|
Liquid |
(22) |
– |
– |
– |
(1.769) |
(96.8) |
|
Gas |
(15) |
– |
– |
– |
(–25.51) |
(29.0) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 3 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BaO |
Solid |
12.74 |
1.040 |
– |
–1.984 |
4.510 |
57.2 |
|
Liquid |
(13.9) |
– |
– |
– |
(–9.341) |
(57.5) |
BaO2 |
Solid |
(13.6) |
(2.0) |
– |
– |
(4.144) |
(59.6) |
|
Liquid |
(21) |
– |
– |
– |
(3.241) |
(99.0) |
BeO |
Solid |
8.69 |
3.65 |
– |
–3.13 |
3.803 |
48.99 |
BiO |
Solid |
(9.7) |
(3.0) |
– |
– |
(3.025) |
(41.2) |
|
Liquid |
(14) |
– |
– |
– |
(2.306) |
(64.9) |
|
Gas |
(8.9) |
– |
– |
– |
(–61.49) |
(–1.8) |
Bi2O3 |
Solid |
23.27 |
11.05 |
– |
– |
7.429 |
99.7 |
|
Liquid |
(35.7) |
– |
– |
– |
(7.614) |
(168.3) |
CO |
Gas |
6.60 |
1.2 |
– |
– |
2.021 |
–9.34 |
CO2 |
Gas |
7.70 |
5.3 |
–0.83 |
– |
2.490 |
–5.64 |
CaO |
Solid |
10.00 |
4.84 |
– |
–1.08 |
3.559 |
49.5 |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 4 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CdO |
Solid |
9.65 |
2.08 |
– |
– |
2.970 |
42.5 |
Ce2O3 |
Solid |
(–23.0) |
(9.0) |
– |
– |
(7.258) |
(100.2) |
|
Liquid |
(37) |
– |
– |
– |
(–2.591) |
(178.5) |
CeO2 |
Solid |
15.0 |
2.5 |
– |
– |
4.579 |
68.5 |
CoO |
Solid |
(9.8) |
(2.2) |
– |
– |
(3.020) |
(46.0) |
|
Liquid |
(15.5) |
– |
– |
– |
(–1.886) |
(79.2) |
Co3O4 |
Solid |
(29.5) |
(17.0) |
– |
– |
(9.551) |
(137.6) |
Cr2O3 |
Solid |
28.53 |
2.20 |
– |
–3.736 |
9.857 |
145.9 |
CrO2 |
Solid |
(16.1) |
(3.0) |
– |
(–3.0) |
(5.946) |
(82.8) |
CrO3 |
Solid |
(18.1) |
(4.0) |
– |
(–2.0) |
(6.245) |
(87.9) |
|
Liquid |
(27) |
– |
– |
– |
(3.381) |
(127.0) |
|
Gas |
(20) |
– |
– |
– |
(–28.62) |
(53.6) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 5 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cs2O |
Solid |
(16.51) |
(5.4) |
– |
– |
(5.160) |
(72.6) |
|
Liquid |
(22) |
– |
– |
– |
(3.205) |
(99.0) |
Cs2O2 |
Solid |
(21.4) |
(11.4) |
– |
– |
(6.887) |
(85.3) |
|
Liquid |
(29.5) |
– |
– |
– |
(4.125) |
(123.8) |
Cs2O3 |
Solid |
(24.0) |
(22.6) |
– |
– |
(8.160) |
(96.5) |
|
Liquid |
(35) |
– |
– |
– |
(2.148) |
(142.2) |
Cu2O |
Solid |
(13.4) |
(8.6) |
– |
– |
(4.378) |
(96.0) |
|
Liquid |
(21.5) |
– |
– |
– |
(3.721) |
(54.9) |
CuO |
Solid |
14.34 |
6.2 |
– |
– |
4.551 |
61.11 |
|
Liquid |
(22) |
– |
– |
– |
(–4.339) |
(98.91) |
FeO |
Solid |
9.27 |
4.80 |
– |
– |
(2.977) |
(43.8) |
|
Liquid |
(14.5) |
– |
– |
– |
(–3.721) |
(69.2) |
Fe3O4 |
Solid, α |
12.38 |
1.62 |
– |
–0.38 |
3.826 |
58.3 |
|
Solid, β |
(14.5) |
– |
– |
– |
(–2.399) |
(66.7) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 6 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fe2O3 |
Solid, α |
|
|
|
|
|
|
|
Solid, β |
21.88 |
48.20 |
– |
– |
8.666 |
104.0 |
|
Solid, γ |
48.00 |
18.6 |
– |
– |
12.652 |
238.3 |
Ga2O |
Solid |
23.49 |
18.6 |
– |
–3.55 |
9.021 |
119.9 |
|
Liquid |
36.00 |
– |
– |
– |
11.979 |
187.6 |
|
Gas |
31.71 |
1.8 |
– |
– |
8.467 |
159.7 |
Ga2O3 |
Solid |
(13.8) |
– |
– |
– |
(4.497) |
(58.7) |
|
Liquid |
(21.5) |
– |
– |
– |
(–0.559) |
(94.1) |
GeO |
Solid |
(14) |
– |
– |
– |
(–28.06) |
(22.3) |
|
Gas |
11.77 |
25.2 |
– |
– |
(4.630) |
(54.35) |
GeO2 |
Solid (α,β) |
(35.5) |
– |
– |
– |
(–20.66) |
(173.2) |
|
Liquid |
(10.4) |
(2.6) |
– |
(–0.5) |
(3.3) |
(47.8) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 7 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In2O |
Solid |
(14.7) |
(7.8) |
– |
– |
(4.730) |
(58.1) |
|
Liquid |
(22) |
– |
– |
– |
(3.206) |
(92.6) |
|
Gas |
(15) |
– |
– |
– |
(–18.39) |
(25.8) |
InO |
Solid |
(10.0) |
(3.2) |
– |
– |
(3.124) |
(43.4) |
|
Liquid |
(14) |
– |
– |
– |
(1.615) |
(64.9) |
|
Gas |
(9.0) |
– |
– |
– |
(–68.38) |
(–3.1) |
In2O3 |
Solid |
(22.6) |
(6.0) |
– |
– |
(7.005) |
(100.5) |
|
Liquid |
(35) |
– |
– |
– |
(–0.195) |
(172.8) |
Ir2O3 |
Solid |
(21.8) |
(14.4) |
– |
– |
(7.140) |
(102.0) |
|
Liquid |
(35) |
– |
– |
– |
(0.706) |
(170.3) |
|
Gas |
(20) |
(10) |
– |
– |
(–57.73) |
(54.8) |
IrO2 |
Solid |
9.17 |
15.20 |
– |
– |
3.410 |
40.9 |
K2O |
Solid |
(15.9) |
(6.4) |
– |
– |
(5.025) |
(69.5) |
|
Liquid |
(22) |
– |
– |
– |
(1.130) |
(98.3) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 8 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
K2O2 |
Solid |
(20.8) |
(5.4) |
– |
– |
(6.442) |
(93.1) |
|
Liquid |
(29) |
– |
– |
– |
(4.127) |
(134.2) |
|
Gas |
(20) |
– |
– |
– |
(–57.07) |
(41.7) |
K2O3 |
Solid |
(19.1) |
(23.2) |
– |
– |
(6.750) |
(82.2) |
|
Liquid |
(35.5) |
– |
– |
– |
(6.447) |
(164.7) |
|
Gas |
(20) |
(5.0) |
– |
– |
(–31.29) |
(37.3) |
KO2 |
Solid |
(15.0) |
(12.0) |
– |
– |
(5.006) |
(61.1) |
|
Liquid |
(24) |
– |
– |
– |
(3.424) |
(105.5) |
La2O3 |
Solid |
28.86 |
3.076 |
– |
–3.275 |
9.840 |
(130.7) |
Li2O |
Solid |
(11.4) |
(5.4) |
– |
– |
(3.639) |
(57.5) |
|
Liquid |
(21) |
– |
– |
– |
(–1.961) |
(112.7) |
Li2O2 |
Solid |
(17.0) |
(5.4) |
– |
– |
(5.309) |
(82.0) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 9 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MgO |
Solid |
10.86 |
1.197 |
– |
–2.087 |
3.991 |
57.0 |
MgO2 |
Solid |
(12.1) |
(2A) |
– |
– |
(3.714) |
(49.2) |
MnO |
Solid |
11.11 |
1.94 |
– |
–0.88 |
3.689 |
50.10 |
|
Liquid |
(13.5) |
– |
– |
– |
(–8.543) |
(58.02) |
Mn3O4 |
Solid, α |
34.64 |
10.82 |
– |
–2.20 |
11.312 |
166.3 |
|
Solid, β |
50.20 |
– |
– |
– |
17.376 |
260.4 |
|
Liquid |
(49) |
– |
– |
– |
(–17.86) |
(233.4) |
Mn2O3 |
Solid |
24.73 |
8.38 |
– |
–3.23 |
8.829 |
118.8 |
MnO2 |
Solid |
16.60 |
2.44 |
– |
–3.88 |
6.359 |
84.8 |
MoO2 |
Solid |
(16.2) |
(3.0) |
– |
(–3.0) |
(5.973) |
(80.4) |
|
Liquid |
(23) |
– |
– |
– |
(–2.463) |
(118.4) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 10 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MoO3 |
Solid |
13.6 |
13.5 |
– |
– |
4.655 |
62.83 |
|
Liquid |
(28.4) |
– |
– |
– |
(0.222) |
(139.88) |
|
Gas |
(18.1) |
– |
– |
– |
(–48.54) |
(42.8) |
N2O |
Gas |
(10.92) |
2.06 |
– |
–2.04 |
4.032 |
11.40 |
Na2O |
Solid |
15.70 |
5.40 |
– |
– |
4.921 |
73.7 |
|
Liquid |
(22) |
– |
– |
– |
(1.494) |
(105.9) |
Na2O2 |
Solid |
(20.2) |
(3.8) |
– |
– |
(6.192) |
(93.6) |
NaO2 |
Solid |
(16.2) |
(3.6) |
– |
– |
(4.990) |
(65.7) |
|
Liquid |
(23) |
– |
– |
– |
(3.175) |
(100.9) |
|
Gas |
(15) |
– |
– |
– |
(–35.22) |
(22.0) |
NbO |
Solid |
(9.6) |
(4.4) |
– |
– |
(3.058) |
(44.0) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 11 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NbO2 |
Solid |
(17.1) |
(1.6) |
– |
(–2.8) |
(6.109) |
(84.6) |
|
Liquid |
(24) |
– |
– |
– |
(1.033) |
(127.2) |
Nb2O5 |
Solid |
21.88 |
28.2 |
– |
– |
7.776 |
100.3 |
|
Liquid |
(44.2) |
– |
– |
– |
(–24.09) |
(201.6) |
Nd2O3 |
Solid |
28.99 |
5.760 |
– |
(–4.159) |
10.295 |
(133.9) |
NiO |
Solid |
13.69 |
0.83 |
– |
–2.915 |
5.097 |
70.67 |
|
Liquid |
(14.3) |
– |
– |
– |
(–7.861) |
(67.91) |
NpO2 |
Solid |
(17.7) |
(3.2) |
– |
(–2.6) |
(6.292) |
(84.08) |
Np2O5 |
Solid |
(32.4) |
(12.6) |
– |
– |
(10.22) |
(145.4) |
OsO2 |
Solid |
(11.5) |
(6.0) |
– |
– |
(3.696) |
(52.8) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 12 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OsO4 |
Solid |
(16.4) |
(23.1) |
– |
(–2.4) |
(6.726) |
(67.0) |
|
Liquid |
(33) |
– |
– |
– |
(6.612) |
(143.0) |
|
Gas |
16.46 |
8.60 |
– |
–4.6 |
(–7.644) |
(25.3) |
P2O3 |
Liquid |
(34.5) |
– |
– |
– |
(10.287) |
(162.6) |
|
Gas |
(153) |
(10) |
– |
– |
(–1.953) |
(38.0) |
PO2 |
Solid |
(11.3) |
(5.0) |
– |
– |
(3.591) |
(54.4) |
|
Liquid |
(20) |
– |
– |
– |
(3.640) |
(95.9) |
P2O5 |
Solid |
8.375 |
5.40 |
– |
– |
4.897 |
30.3 |
|
Gas |
36.80 |
– |
– |
– |
3.284 |
165.6 |
PaO2 |
Solid |
(14.4) |
(2.6) |
– |
– |
(4.409) |
(65.0) |
Pa2O5 |
Solid |
(28.4) |
(11.4) |
– |
– |
(8.975) |
(127.7) |
|
Liquid |
(48) |
– |
– |
– |
(–0.800) |
(241.1) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 13 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PbO |
Solid, red |
10.60 |
4.00 |
– |
– |
3.338 |
45.4 |
|
Solid, yellow |
9.05 |
6.40 |
– |
– |
2.454 |
36.4 |
|
Liquid |
(14.6) |
– |
– |
– |
1.788 |
65.7 |
|
Gas |
(8.1) |
(0.4) |
– |
– |
(–59.94) |
(–11.0) |
Pb2O4 |
Solid |
(31.1) |
(17.6) |
– |
– |
(10.055) |
(132.0) |
PbO2 |
Solid |
12.7 |
7.80 |
– |
– |
4.133 |
56.4 |
PdO |
Solid |
3.30 |
14.2 |
– |
– |
1.615 |
(13.9) |
PoO2 |
Solid |
(14.3) |
(5.6) |
– |
– |
(4.513) |
(66.1) |
|
Liquid |
(22) |
– |
– |
– |
(3.460) |
(106.5) |
Pr2O3 |
Solid |
(29.0) |
(4.0) |
– |
(–4.0) |
(10.166) |
(133.2) |
|
Liquid |
(36) |
– |
– |
– |
(–6.298) |
(168.3) |
PrO2 |
Solid |
(17.6) |
(3.4) |
– |
(–2.8) |
(6.338) |
(85.9) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 14 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PtO |
Solid |
(9.0) |
(6.4) |
– |
– |
(2.968) |
(39.7) |
Pt3O4 |
Solid |
(30.8) |
(17.4) |
– |
– |
(9.957) |
(139.7) |
PtO2 |
Solid |
(11.1) |
(9.6) |
– |
– |
(3.736) |
(49.6) |
|
Liquid |
(21) |
– |
– |
– |
(3.785) |
(101.5) |
PuO |
Solid |
(12.0) |
(2.4) |
– |
– |
(3.685) |
(49.1) |
|
Liquid |
(14.5) |
– |
– |
– |
(–2.287) |
(58.3) |
|
Gas |
(8.9) |
– |
– |
– |
(–62.307) |
(–5.3) |
Pu2O3 |
Solid |
(21.2) |
(18.2) |
– |
– |
(7.130) |
(88.2) |
|
Liquid |
(40) |
– |
– |
– |
(–5.691) |
(187.2) |
PuO2 |
Solid |
(17.1) |
(3.4) |
– |
(–2.6) |
(6.122) |
(80.2) |
|
Liquid |
(20.5) |
– |
– |
– |
(–10.62) |
(92.2) |
RaO |
Solid |
(10.5) |
(2.0) |
– |
– |
(3.220) |
(43.4) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 15 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rb2O |
Solid |
(15.4) |
(5.8) |
– |
– |
(4.850) |
(62.5) |
|
Liquid |
(22) |
– |
– |
– |
(2.754) |
(95.9) |
Rb2O2 |
Solid |
(20.9) |
(8.0) |
– |
– |
(6.587) |
(94.0) |
|
Liquid |
(29) |
– |
– |
– |
(3.273) |
(133.2) |
Rb2O3 |
Solid |
(20.5) |
(13.0) |
– |
– |
(6.690) |
(88.2) |
|
Liquid |
(34) |
– |
– |
– |
(5.603) |
(157.8) |
RbO2 |
Solid |
(13.8) |
(6.4) |
– |
– |
(4.399) |
(59.0) |
|
Liquid |
(21) |
– |
– |
– |
(3.720) |
(95.7) |
ReO2 |
Solid |
(10.8) |
(9.8) |
– |
– |
(3.656) |
(49.5) |
|
Liquid |
(24.5) |
– |
– |
– |
(1.204) |
(127.0) |
ReO3 |
Solid |
(18.0) |
(5.8) |
– |
– |
(5.625) |
(84.5) |
|
Liquid |
29 |
– |
– |
– |
(4.644) |
(136.8) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 16 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Re2O7 |
Solid |
(41.8) |
(14.8) |
– |
(–3.0) |
(14.127) |
(200.3) |
|
Liquid |
(65.7) |
– |
– |
– |
(9.203) |
(314.7) |
|
Gas |
(38.2) |
– |
– |
– |
(–25.97) |
(109.3) |
ReO4 |
Solid |
(21.4) |
(10.8) |
– |
(–2.0) |
(7.531) |
(91.8) |
|
Liquid |
(33) |
– |
– |
– |
(6.775) |
(146.7) |
|
Gas |
(16.5) |
(8.6) |
– |
(–5.0) |
(–8.118) |
(30.6) |
Rh2O |
Solid |
15.59 |
6.47 |
– |
– |
4.936 |
(65.3) |
RhO |
Solid |
(9.84) |
(553) |
– |
– |
(3.179) |
(45.7) |
Rh2O3 |
Solid |
20.73 |
13.80 |
– |
– |
6.794 |
(99.2) |
RuO2 |
Solid |
(11.4) |
(6.0) |
– |
– |
3.666 |
(54.2) |
RuO4 |
Solid |
(20) |
– |
– |
– |
(5.963) |
(81.5) |
|
Liquid |
(33) |
– |
– |
– |
(6.663) |
(144.9) |
SO2 |
Gas |
11.4 |
1.414 |
– |
–2.045 |
4.148 |
7.12 |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 17 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sb2O3 |
Solid |
19.10 |
17.1 |
– |
– |
6.455 |
84.5 |
|
Liquid |
(36) |
– |
– |
– |
(0.035) |
(168.2) |
|
Gas |
(20.8) |
– |
– |
– |
(–34.70) |
(49.9) |
SbO2 |
Solid |
11.30 |
8.1 |
– |
– |
3.725 |
51.6 |
Sb2O5 |
Solid |
(22.4) |
(23.6) |
– |
– |
(7.723) |
(104.8) |
Sc2O3 |
Solid |
23.17 |
5.64 |
– |
– |
7.159 |
1089 |
SeO |
Solid |
(9.1) |
(3.8) |
– |
– |
(2.882) |
(42.0) |
|
Liquid |
(15.5) |
– |
– |
– |
(0.490) |
(77.5) |
|
Gas |
8.20 |
0.50 |
– |
–0.80 |
(–58.54) |
(0.7) |
SeO2 |
Solid |
(12.8) |
(6.1) |
– |
(–0.2) |
(4.150) |
(59.9) |
|
Gas |
(14.5) |
– |
– |
– |
(–20.45) |
(26.4) |
SiO |
Solid |
(7.3) |
(2.4) |
– |
– |
(2.283) |
(35.8) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 18 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SiO2 |
Solid, β |
11.22 |
8.20 |
– |
–2.70 |
4.615 |
57.83 |
|
Solid, α |
14.41 |
1.94 |
– |
– |
4.602 |
73.67 |
|
Liquid |
(20) |
– |
– |
– |
(9.649) |
(111.08) |
Sm2O3 |
Solid |
(25.9) |
(7.0) |
– |
– |
(8.033) |
(113.2) |
|
Liquid |
(36) |
– |
– |
– |
(–6.431) |
(166.3) |
SnO |
Solid |
9.40 |
3.62 |
– |
– |
2.964 |
41.1 |
|
Liquid |
(14.5) |
– |
– |
– |
(0.141) |
(68.1) |
|
Gas |
(9.0) |
– |
– |
– |
(–69.76) |
(–6.4) |
SnO2 |
Solid |
17.66 |
2.40 |
– |
–5.16 |
7.103 |
91.7 |
|
Liquid |
(22.5) |
– |
– |
– |
(0.304) |
(117.7) |
SrO |
Solid |
12.34 |
1.120 |
– |
–1.806 |
4.335 |
58.7 |
SrO2 |
Solid |
(16.8) |
(2.2) |
– |
(–3.0) |
(6.113) |
(83.3) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 19 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ta2O5 |
Solid |
29.2 |
10.0 |
– |
– |
9.151 |
135.2 |
|
Liquid |
(46) |
– |
– |
– |
(6.158) |
(235.1) |
TcO2 |
Solid |
(10.4) |
(9.2) |
– |
– |
(3.510) |
(48.6) |
|
Liquid |
(25) |
– |
– |
– |
(–5.946) |
(132.7) |
TcO3 |
Solid |
(19.4) |
(5.2) |
– |
(–2.0) |
(6.686) |
(93.7) |
Tc2O7 |
Solid |
(39.1) |
(18.6) |
– |
(–2.4) |
(13.29) |
(187.2) |
|
Liquid |
(64) |
– |
– |
– |
(10.02) |
(299.8) |
|
Gas |
(25) |
(28) |
– |
– |
(–21.98) |
(43.8) |
TeO |
Solid |
(8.6) |
(6.2) |
– |
– |
(2.840) |
(37.8) |
|
Liquid |
(15.5) |
– |
– |
– |
(–0.448) |
(72.3) |
|
Gas |
(8.9) |
– |
– |
– |
(–62.16) |
(–5.2) |
TeO3 |
Solid |
13.85 |
6.87 |
– |
– |
4.435 |
63.97 |
|
Liquid |
(20) |
– |
– |
– |
(3.940) |
(96.4) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 20 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TeO2 |
Solid |
(11.0) |
(2.4) |
– |
– |
(3.386) |
(47.4) |
|
Liquid |
(15) |
– |
– |
– |
(–6.561) |
(66.9) |
ThO2 |
Solid |
16.45 |
2.346 |
– |
–2.124 |
5.721 |
80.03 |
TiO |
Solid, α |
10.57 |
3.60 |
– |
–1.86 |
3.935 |
54.03 |
|
Solid, β |
11.85 |
3.00 |
– |
– |
4.108 |
61.71 |
Ti2O3 |
Solid, α |
7.31 |
53.52 |
– |
– |
4.559 |
38.78 |
|
Solid, β |
34.68 |
1.30 |
– |
–10.20 |
13.605 |
184.48 |
|
Liquid |
(37.5) |
– |
– |
– |
(–7.796) |
(193.2) |
Ti3O5 |
Solid, α |
35.47 |
29.50 |
– |
– |
11.887 |
179.98 |
|
Solid, β |
41.60 |
8.00 |
– |
– |
10.230 |
202.80 |
|
Liquid |
(60) |
– |
– |
– |
(–18.701) |
(306.4) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 21 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TiO2 |
Solid |
17.97 |
0.28 |
– |
–4.35 |
6.829 |
92.92 |
|
Liquid |
(21.4) |
– |
– |
– |
(–2.610) |
(111.08 |
Ti2O |
Solid |
(15.8) |
(6.0) |
– |
(–0.3) |
(5.078) |
(68.2) |
|
Liquid |
(22.1) |
– |
– |
– |
(2.651) |
(96.0) |
|
Gas |
(13.7) |
– |
– |
– |
(–20.94) |
(18.0) |
Tl2O3 |
Solid |
(23.0) |
(5.0) |
– |
– |
(7.080) |
(99.0) |
|
Liquid |
(35.5) |
– |
– |
– |
(4.604) |
(167.8) |
UO |
Solid |
(10.6) |
(2.0) |
– |
– |
(3.249) |
(45.0) |
UO2 |
Solid |
19.20 |
1.62 |
– |
–3.957 |
7.124 |
93.37 |
U3O8 |
Solid |
(65) |
(7.5) |
– |
(–10.9) |
(23.37) |
(312.7) |
UO3 |
Solid |
22.09 |
2.54 |
– |
–2.973 |
7.969 |
104.72 |
VO |
Solid |
11.32 |
1.61 |
– |
–1.26 |
3.869 |
56.4 |
|
Liquid |
(14.5) |
– |
– |
– |
(–8.157) |
(70.9) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 22 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
V2O3 |
Solid |
29.35 |
4.76 |
– |
–5.42 |
10.780 |
148.12 |
|
Liquid |
(38) |
– |
– |
– |
(–6.028) |
(193.4) |
V3O4 |
Solid |
(36) |
(30) |
– |
– |
(12.07) |
(182.1) |
|
Liquid |
(55.6) |
– |
– |
– |
(–54.72) |
(249.1) |
VO2 |
Solid, α |
14.96 |
– |
– |
– |
4.460 |
72.92 |
|
Solid, β |
17.85 |
1.70 |
– |
–3.94 |
5.680 |
89.09 |
|
Liquid |
25.50 |
– |
– |
– |
2.962 |
135.87 |
V2O5 |
Solid |
46.54 |
–390 |
– |
–13.22 |
18.136 |
240.2 |
|
Liquid |
45.60 |
– |
– |
– |
2.122 |
220.1 |
|
Gas |
(40) |
– |
– |
– |
(–73.90) |
(149.6) |
WO2 |
Solid |
(17.6) |
(4.2) |
– |
(–4.0) |
(6.772) |
(88.8) |
|
Liquid |
(24) |
– |
– |
– |
(–0.112) |
(121.8) |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 77. THERMODYNAMIC COEFFICIENTS FOR OXIDES
(SHEET 23 OF 23)
|
|
a |
b |
c |
d |
A |
B |
Oxide |
Phase |
– |
(cal • g mole-1 ) |
– |
– |
(kcal • g mole-1) |
(e.u.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WO3 |
Solid |
17.33 |
7.74 |
– |
– |
5.511 |
81.15 |
|
Liquid |
(30) |
– |
– |
– |
(–1.162) |
(152.5) |
|
Gas |
(18) |
– |
– |
– |
(–69.36) |
(40.2) |
Y2O3 |
Solid |
(26.0) |
(8.2) |
– |
(–2.2) |
(8.846) |
(122.3) |
ZnO |
Solid |
11.71 |
1.22 |
– |
–2.18 |
4.277 |
57.88 |
ZrO2 |
Solid, α |
16.64 |
1.80 |
– |
–3.36 |
6.168 |
85.21 |
|
Solid, β |
17.80 |
– |
– |
– |
4.270 |
89.96 |
|
|
|
|
|
|
|
|
For discussion of these coefficients, please see Table 75, Key to Tables of Thermodynamic Coefficients on page 257
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-58.
©2001 CRC Press LLC

Table 78. ENTROPY OF THE ELEMENTS
(SHEET 1 OF 3)
|
|
Entropy at 298K |
Element |
Phase |
(e.u.) |
|
|
|
|
|
|
Ac |
solid |
(13) |
Ag |
solid |
10.20 |
Al |
solid |
6.769 |
Am |
solid |
(13) |
As |
solid |
8.4 |
Au |
solid |
11.32 |
B |
solid |
1.42 |
Ba |
solid, α |
16 |
Be |
solid |
2.28 |
Bi |
solid |
13.6 |
C |
solid |
1.3609 |
Ca |
solid, α |
9.95 |
Cd |
solid |
12.3 |
Ce |
solid |
13.8 |
Cl2 |
gas |
53.286 |
Co |
solid, α |
6.8 |
Cr |
solid |
5.68 |
Cs |
solid |
19.8 |
Cu |
solid |
7.97 |
F2 |
gas |
48.58 |
Fe |
solid, α |
6.491 |
Ga |
solid |
9.82 |
Ge |
solid |
10.1 |
H2 |
gas |
31.211 |
Hf |
solid |
13.1 |
Hg |
liquid |
18.46 |
In |
solid |
13.88 |
Ir |
solid |
8.7 |
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 78. ENTROPY OF THE ELEMENTS
(SHEET 2 OF 3)
|
|
Entropy at 298K |
Element |
Phase |
(e.u.) |
|
|
|
|
|
|
K |
solid |
15.2 |
La |
solid |
13.7 |
Li |
solid |
6.70 |
Mg |
solid |
7.77 |
Mn |
solid, α |
7.59 |
Mo |
solid |
6.83 |
N2 |
gas |
45.767 |
Na |
solid |
12.31 |
Nb |
solid |
8.3 |
Nd |
solid |
13.9 |
Ni |
solid, α |
7.137 |
Np |
solid |
(14) |
O2 |
gas |
49.003 |
Os |
solid |
7.8 |
P4 |
solid, white |
42.4 |
Pa |
solid |
(13.5) |
Pb |
solid |
15.49 |
Pd |
solid |
8.9 |
Po |
solid |
13 |
Pr |
solid |
(13.5) |
Pt |
solid |
10.0 |
Pu |
solid |
(13.0) |
Ra |
solid |
(17) |
Rb |
solid |
16.6 |
Re |
solid |
(8.89) |
Rh |
solid |
7.6 |
Ru |
solid, α |
6.9 |
S |
solid, α |
7.62 |
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 78. ENTROPY OF THE ELEMENTS
(SHEET 3 OF 3)
|
|
Entropy at 298K |
Element |
Phase |
(e.u.) |
|
|
|
|
|
|
Sb |
solid (α, β, γ) |
10.5 |
Sc |
solid |
(9.0) |
Se |
solid |
10.144 |
Si |
solid |
4.50 |
Sm |
solid |
(15) |
Sn |
solid (α, β) |
12.3 |
Sr |
solid |
13.0 |
Ta |
solid |
9.9 |
Tc |
solid |
(8.0) |
Te |
solid, α |
11.88 |
Th |
solid |
12.76 |
Ti |
solid, α |
7.334 |
Tl |
solid, α |
15.4 |
U |
solid, α |
12.03 |
V |
solid |
7.05 |
W |
solid |
8.0 |
Y |
solid |
(11) |
Zn |
solid |
9.95 |
Zr |
solid, α |
9.29 |
|
|
|
Source: data from Weast, R. C. Ed., Handbook of Chemistry and Physics, 69th ed., CRC Press, Boca Raton, Fla., 1988, D44.
©2001 CRC Press LLC

Table 79. VAPOR PRESSURE OF THE ELEMENTS
AT VERY LOW PRESSURES (SHEET 1 OF 2)
|
|
|
|
Pressure (mm Hg) |
|
|
|
|
Melting point |
|
|
|
|
|
|
|
10 -5 |
10-4 |
10-3 |
10-2 |
10-1 |
1 |
|
Element |
(˚C) |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ag |
961 |
767 |
848 |
936 |
1047 |
1184 |
1353 |
Al |
660 |
724 |
808 |
889 |
996 |
1123 |
1279 |
Au |
1063 |
1083 |
1190 |
1316 |
1465 |
1646 |
1867 |
Ba |
717 |
418 |
476 |
546 |
629 |
730 |
858 |
Be |
1284 |
942 |
1029 |
1130 |
1246 |
1395 |
1582 |
Bi |
271 |
474 |
536 |
609 |
698 |
802 |
934 |
C |
|
2129 |
2288 |
2471 |
2681 |
2926 |
3214 |
Cd |
321 |
148 |
180 |
220 |
264 |
321 |
|
Co |
1478 |
1249 |
1362 |
1494 |
1649 |
1833 |
2056 |
Cr |
1900 |
907 |
992 |
1090 |
1205 |
1342 |
1504 |
Cu |
1083 |
946 |
1035 |
1141 |
1273 |
1432 |
1628 |
Fe |
1535 |
1094 |
1195 |
1310 |
1447 |
1602 |
1783 |
Hg |
–38.9 |
–23.9 |
–5.5 |
18.0 |
48.0 |
82.0 |
126 |
In |
157 |
667 |
746 |
840 |
952 |
1088 |
1260 |
Ir |
2454 |
1993 |
2154 |
2340 |
2556 |
2811 |
3118 |
Mg |
651 |
287 |
331 |
383 |
443 |
515 |
605 |
Mn |
1244 |
717 |
791 |
878 |
980 |
1103 |
1251 |
Mo |
2622 |
1923 |
2095 |
2295 |
2533 |
|
|
Ni |
1455 |
1157 |
1257 |
1371 |
1510 |
1679 |
1884 |
Os |
2697 |
2101 |
2264 |
2451 |
2667 |
2920 |
3221 |
|
|
|
|
|
|
|
|
To convert mm Hg (torr) to N/m2, divide by 133.3
To convert atm to MN/m2, divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
The values given in this table are from a variety of sources that are not always in agreement; for that reason, the table should be used only as a general guide.
Source: from Dushman, S., Scientific Foundations of Vacuum Technique, John Wiley & Sons, New York, (1949)
©2001 CRC Press LLC

Table 79. VAPOR PRESSURE OF THE ELEMENTS
AT VERY LOW PRESSURES (SHEET 2 OF 2)
|
|
|
|
Pressure (mm Hg) |
|
|
|
|
Melting point |
|
|
|
|
|
|
|
10 -5 |
10-4 |
10-3 |
10-2 |
10-1 |
1 |
|
Element |
(˚C) |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pb |
328 |
483 |
548 |
625 |
718 |
832 |
975 |
Pd |
1555 |
1156 |
1271 |
1405 |
1566 |
1759 |
2000 |
Pt |
1774 |
1606 |
1744 |
1904 |
2090 |
2313 |
2582 |
Sb |
630 |
466 |
525 |
595 |
678 |
779 |
904 |
Si |
1410 |
1024 |
1116 |
1223 |
1343 |
1485 |
1670 |
Sn |
232 |
823 |
922 |
1042 |
1189 |
1373 |
1609 |
Ta |
2996 |
2407 |
2599 |
2820 |
|
|
|
W |
3382 |
2554 |
2767 |
3016 |
3309 |
|
|
Zn |
419 |
211 |
248 |
292 |
343 |
405 |
|
Zr |
2127 |
1527 |
1660 |
1816 |
2001 |
2212 |
2459 |
|
|
|
|
|
|
|
|
To convert mm Hg (torr) to N/m2, divide by 133.3
To convert atm to MN/m2, divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
The values given in this table are from a variety of sources that are not always in agreement; for that reason, the table should be used only as a general guide.
Source: from Dushman, S., Scientific Foundations of Vacuum Technique, John Wiley & Sons, New York, (1949)
©2001 CRC Press LLC

Table 80. VAPOR PRESSURE OF THE ELEMENTS
AT MODERATE PRESSURES (SHEET 1 OF 3)
|
|
|
Pressure (mm Hg) |
|
||
|
|
|
|
|
|
|
Element |
Symbol |
1 |
10 |
100 |
400 |
760 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminum |
Al |
1540 |
1780 |
2080 |
2320 |
2467 |
Antimony |
Sb |
|
960 |
1280 |
1570 |
1750 |
Arsenic |
As |
380 |
440 |
510 |
580 |
610 |
Barium |
Ba |
860 |
1050 |
1300 |
1520 |
1640 |
Beryllium |
Be |
1520 |
1860 |
2300 |
2770 |
2970 |
Bismuth |
Bi |
|
1060 |
1280 |
1450 |
1560 |
Boron |
B |
2660 |
3030 |
3460 |
3810 |
4000 |
Bromine |
Br |
–60 |
–30 |
+9 |
39 |
59 |
Cadmium |
Cd |
393 |
486 |
610 |
710 |
765 |
Calcium |
Ca |
800 |
970 |
1200 |
1390 |
1490 |
Cesium |
Cl |
|
373 |
513 |
624 |
690 |
Chlorine |
Cl |
–123 |
–101 |
–71 |
–46 |
–34 |
Chromium |
Cr |
1610 |
1840 |
2140 |
2360 |
2480 |
Cobalt |
Co |
1910 |
2170 |
2500 |
2760 |
2870 |
Copper |
Cu |
|
1870 |
2190 |
2440 |
2600 |
Fluorine |
F |
|
|
–203 |
–193 |
–188 |
Gallium |
Ca |
1350 |
1570 |
1850 |
2060 |
2180 |
Germanium |
Ge |
|
2080 |
2440 |
2710 |
2830 |
Gold |
Au |
1880 |
2160 |
2520 |
2800 |
2940 |
Indium |
In |
|
|
|
1960 |
2080 |
|
|
|
|
|
|
|
To convert mm Hg (torr) to N/m2, divide by 133.3
To convert atm to MN/m2, divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
The values given in this table are from a variety of sources that are not always in agreement; for that reason, the table should be used only as a general guide.
Source: from Dushman, S., Scientific Foundations of Vacuum Technique, John Wiley & Sons, New York, (1949)
©2001 CRC Press LLC

Table 80. VAPOR PRESSURE OF THE ELEMENTS
AT MODERATE PRESSURES (SHEET 2 OF 3)
|
|
|
Pressure (mm Hg) |
|
||
|
|
|
|
|
|
|
Element |
Symbol |
1 |
10 |
100 |
400 |
760 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iodine |
I |
40 |
72 |
115 |
160 |
185 |
Iridium |
Ir |
2830 |
3170 |
3630 |
3960 |
4130 |
Iron |
Fe |
1780 |
2040 |
2370 |
2620 |
2750 |
Lanthanum |
La |
|
|
|
3230 |
3420 |
Lead |
Pb |
970 |
1160 |
1420 |
1630 |
1740 |
Lithium |
Li |
750 |
890 |
1080 |
1240 |
1310 |
Magnesium |
Mg |
620 |
740 |
900 |
1040 |
1110 |
Manganese |
Mn |
|
1510 |
1810 |
2050 |
2100 |
Mercury |
Hg |
|
|
260 |
330 |
356.9 |
Molybdenum |
Mo |
3300 |
3770 |
4200 |
4580 |
4830 |
Neodymium |
Nd |
|
|
|
2870 |
3100 |
Nickel |
Ni |
1800 |
2090 |
2370 |
2620 |
2730 |
Palladium |
Pd |
1470 |
2290 |
2670 |
2950 |
3140 |
Phosphorus |
P |
|
127 |
199 |
253 |
283 |
Platinum |
Pt |
2600 |
2940 |
3360 |
3650 |
3830 |
Polonium |
Po |
472 |
587 |
752 |
890 |
960 |
Potassium |
K |
|
|
590 |
710 |
770 |
Rhodium |
Rh |
2530 |
2850 |
3260 |
3590 |
3760 |
Rubidium |
Rb |
|
390 |
527 |
640 |
700 |
Selenium |
Se |
|
429 |
547 |
640 |
685 |
|
|
|
|
|
|
|
To convert mm Hg (torr) to N/m2, divide by 133.3
To convert atm to MN/m2, divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
The values given in this table are from a variety of sources that are not always in agreement; for that reason, the table should be used only as a general guide.
Source: from Dushman, S., Scientific Foundations of Vacuum Technique, John Wiley & Sons, New York, (1949)
©2001 CRC Press LLC

Table 80. VAPOR PRESSURE OF THE ELEMENTS
AT MODERATE PRESSURES (SHEET 3 OF 3)
|
|
|
Pressure (mm Hg) |
|
||
|
|
|
|
|
|
|
Element |
Symbol |
1 |
10 |
100 |
400 |
760 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silver |
Ag |
1310 |
1540 |
1850 |
2060 |
2210 |
Sodium |
Na |
440 |
546 |
700 |
830 |
890 |
Strontium |
Sr |
740 |
900 |
1100 |
1280 |
1380 |
Sulfur |
S |
|
246 |
333 |
407 |
445 |
Tellurium |
Te |
520 |
633 |
792 |
900 |
962 |
Thallium |
Tl |
|
1000 |
1210 |
1370 |
1470 |
Tin |
Sn |
1610 |
1890 |
2270 |
2580 |
2750 |
Titanium |
Ti |
2180 |
2480 |
2860 |
3100 |
3260 |
Tungsten |
W |
3980 |
4490 |
5160 |
5470 |
5940 |
Uranium |
U |
2450 |
2800 |
3270 |
3620 |
3800 |
Vanadium |
V |
2290 |
2570 |
2950 |
3220 |
3380 |
Zinc |
Zn |
|
590 |
730 |
840 |
907 |
|
|
|
|
|
|
|
To convert mm Hg (torr) to N/m2, divide by 133.3
To convert atm to MN/m2, divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
The values given in this table are from a variety of sources that are not always in agreement; for that reason, the table should be used only as a general guide.
Source: from Dushman, S., Scientific Foundations of Vacuum Technique, John Wiley & Sons, New York, (1949)
©2001 CRC Press LLC

Table 81. VAPOR PRESSURE OF THE ELEMENTS
AT HIGH PRESSURES (SHEET 1 OF 3)
|
|
|
Pressure (atm) |
|
|
|
|
|
|
|
|
|
|
Element |
Symbol |
2 |
5 |
10 |
20 |
40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminum |
Al |
2610 |
2850 |
3050 |
3270 |
3530 |
Antimony |
Sb |
1960 |
2490 |
|
|
|
Arsenic |
As |
|
|
|
|
|
Barium |
Ba |
1790 |
2030 |
2230 |
|
|
Beryllium |
Be |
3240 |
3730 |
4110 |
4720 |
5610 |
Bismuth |
Bi |
1660 |
1850 |
2000 |
2180 |
|
Boron |
B |
|
|
|
|
|
Bromine |
Br |
78 |
110 |
|
|
|
Cadmium |
Cd |
830 |
930 |
1030 |
1120 |
1240 |
Calcium |
Ca |
1630 |
1850 |
2020 |
2290 |
|
Cesium |
Cl |
|
|
|
|
|
Chlorine |
Cl |
–17 |
+9 |
30 |
55 |
97 |
Chromium |
Cr |
2630 |
2850 |
3010 |
3180 |
|
Cobalt |
Co |
3040 |
3270 |
|
|
|
Copper |
Cu |
2760 |
3010 |
3500 |
3460 |
3740 |
Fluorine |
F |
–180.7 |
–169.1 |
–159.6 |
|
|
Gallium |
Ca |
2320 |
2560 |
2730 |
|
|
Germanium |
Ge |
2970 |
3200 |
3430 |
|
|
Gold |
Au |
3120 |
3490 |
3630 |
3890 |
|
Indium |
In |
2230 |
2440 |
2600 |
|
|
Iodine |
I |
216 |
265 |
|
|
|
Iridium |
Ir |
4310 |
4650 |
|
|
|
Iron |
Fe |
2900 |
3150 |
3360 |
3570 |
|
Lanthanum |
La |
3620 |
3960 |
4270 |
|
|
|
|
|
|
|
|
|
To convert atm to MN/m2 divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
Source: from Loebel, R., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, (1974)
©2001 CRC Press LLC

Table 81. VAPOR PRESSURE OF THE ELEMENTS
AT HIGH PRESSURES (SHEET 2 OF 3)
|
|
|
|
Pressure (atm) |
|
|
|
|
|
|
|
|
|
|
|
Element |
Symbol |
2 |
5 |
|
10 |
20 |
40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lead |
Pb |
1880 |
2140 |
|
2320 |
2620 |
|
Lithium |
Li |
1420 |
1518 |
|
|
|
|
Magnesium |
Mg |
1190 |
1330 |
|
1430 |
1560 |
|
Manganese |
Mn |
2360 |
2580 |
|
2850 |
|
|
Mercury |
Hg |
398 |
465 |
|
517 |
581 |
657 |
Molybdenum |
Mo |
5050 |
5340 |
|
5680 |
5980 |
|
Neodymium |
Nd |
3300 |
3680 |
|
3990 |
|
|
Nickel |
Ni |
2880 |
3120 |
|
3300 |
3310 |
|
Palladium |
Pd |
3270 |
3560 |
|
3840 |
|
|
Phosphorus |
P |
319 |
|
|
|
|
|
Platinum |
Pt |
4000 |
4310 |
|
4570 |
4860 |
|
Polonium |
Po |
1060 |
1200 |
|
1340 |
|
|
Potassium |
K |
850 |
950 |
|
1110 |
1240 |
1420 |
Rhodium |
Rh |
3930 |
4230 |
|
4440 |
|
|
Rubidium |
Rb |
|
|
|
|
|
|
Selenium |
Se |
750 |
850 |
|
920 |
1010 |
1120 |
Silver |
Ag |
2360 |
2600 |
|
2850 |
3050 |
3300 |
Sodium |
Na |
980 |
1120 |
|
1230 |
1370 |
|
Strontium |
Sr |
1480 |
1670 |
|
1850 |
2030 |
|
Sulfur |
S |
493 |
574 |
|
640 |
720 |
|
Tellurium |
Te |
1030 |
1160 |
|
1250 |
|
|
Thallium |
Tl |
1560 |
1750 |
|
1900 |
2050 |
2260 |
Tin |
Sn |
2950 |
3270 |
|
3540 |
3890 |
|
Titanium |
Ti |
3400 |
3650 |
|
3800 |
|
|
|
|
|
|
|
|
|
|
To convert atm to MN/m2 divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
Source: from Loebel, R., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, (1974)
©2001 CRC Press LLC

Table 81. VAPOR PRESSURE OF THE ELEMENTS
AT HIGH PRESSURES (SHEET 3 OF 3)
|
|
|
|
Pressure (atm) |
|
|
|
|
|
|
|
|
|
|
|
Element |
Symbol |
2 |
5 |
|
10 |
20 |
40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tungsten |
W |
6260 |
6670 |
|
7250 |
7670 |
|
Uranium |
U |
4040 |
4420 |
|
|
|
|
Vanadium |
V |
3540 |
3800 |
|
|
|
|
Zinc |
Zn |
970 |
1090 |
|
1180 |
1290 |
|
|
|
|
|
|
|
|
|
To convert atm to MN/m2 divide by 0.1013
This table lists the temperature in degrees Celsius (Centigrade) at which an element has a vapor pressure indicated by the headings of the columns.
Source: from Loebel, R., in Handbook of Chemistry and Physics, 55th ed., Weast, R. C., Ed., CRC Press, Cleveland, (1974)
©2001 CRC Press LLC

Table 82. VAPOR PRESSURE OF ELEMENTS AND
INORGANIC COMPOUNDS* (SHEET 1 OF 5)
|
|
|
|
Temperature. Range |
|
|
|
|
of Validity |
Compound |
Formula |
a |
b |
˚C |
|
|
|
|
|
|
|
|
|
|
Aluminum Oxide |
Al2O3 |
540000 |
14.22 |
1840 to 2200 liq. |
Ammonia |
NH3 |
31211 |
9.9974 |
–127 to –78 sol. |
Ammonium Bromide |
NH4Br |
90208 |
9.9404 |
250 to 400 sol. |
Ammonium Chloride |
NH4Cl |
83486 |
10.0164 |
100 to 400 sol. |
Ammonium Cyanide |
NH4CN |
41484 |
9.978 |
7 to 17 sol. |
Ammonium Iodide |
NH4I |
95730 |
10.2700 |
300 to 400 sol. |
Ammonium Sulfhydrate |
NH4HS |
46025 |
10.7500 |
6 to 40 sol. |
Antimony |
Sb |
189000 |
9.051 |
1070 to 1325 liq. |
Argon |
Ar |
7814.5 |
7.5741 |
–208 to –189 sol. |
|
|
6826 |
6.9605 |
–189 to –183 liq. |
Arsenic |
As |
47100 |
6.692 |
800 to 860 liq. |
|
|
133000 |
10.800 |
440 to 815 sol. |
Arsenous Oxide |
As2O3 |
111350 |
12.127 |
100 to 315 sol. |
|
|
52120 |
6.513 |
315 to 490 liq. |
Barium |
Ba |
350000 |
15.765 |
930 to 1130 liq. |
Bismuth |
Bi |
200000 |
8.876 |
1210 to 1420 liq. |
Bismuth Trichloride |
BiCl3 |
13125 |
2.681 |
91 to 213 sol. |
Cadimium |
Cd |
10900 |
8.564 |
150 to 320.9 sol.. |
|
|
99900 |
7.897 |
500 to 840 liq |
Cadimium Iodide |
CdI2 |
122200 |
9.269 |
385 to 450 liq. |
Cesium |
Cs |
73400 |
6.949 |
200 to 350 liq. |
Cesium Chloride |
CsCl |
163200 |
8.340 |
986 to 1295 liq. |
Calcium |
Ca |
370000 |
16.240 |
960 to 1110 liq. |
Carbon |
C |
540000 |
9.596 |
3880 to 4430 liq. |
Carbon Dioxide |
CO2 |
26179.3 |
9.9082 |
–135 to –56.7 liq. |
Carbon Monooxide |
CO |
6354 |
6.976 |
–220 to –206 liq. |
Chlorine |
Cl |
29293 |
9.950 |
–154 to –103 liq. |
Cobalt |
Co |
309000 |
7.571 |
2375 liq. |
|
|
|
|
|
Source: data compiled by J.S. Park from CRC Handbook of Chemistry and Physics, David R. Lide, Ed., CRC Press, Boca Raton, (1990).
©2001 CRC Press LLC

Table 82. VAPOR PRESSURE OF ELEMENTS AND
INORGANIC COMPOUNDS* (SHEET 2 OF 5)
|
|
|
|
Temperature. Range |
|
|
|
|
of Validity |
Compound |
Formula |
a |
b |
˚C |
|
|
|
|
|
|
|
|
|
|
Copper |
Cu |
468000 |
12.344 |
2100 to 2310 liq. |
Cuprous Chloride |
Cu2Cl2 |
80700 |
5.454 |
878 to 1369 liq. |
Cyanogen |
(CN)2 |
32437 |
9.6539 |
–72 to –28 sol. |
|
|
23750 |
7.808 |
–32 to –6 liq. |
Ferrous Chloride |
FeCl2 |
135200 |
8.33 |
700 to 390 sol. |
Gold |
Au |
385000 |
9.853 |
2315 to 2500 liq. |
Hydriodic Acid |
HI |
24160 |
8.259 |
–97 to –51 sol. |
|
|
21580 |
7.630 |
–50 to –34 liq. |
Hydrobromic Acid |
HBr |
22420 |
8.734 |
–114 to –86 sol. |
|
|
17960 |
7.427 |
–86 ot –66 liq. |
Hydrochloric Acid |
HCl |
19588 |
8.4430 |
–158 to –110 sol. |
Hydrocyanic Acid |
HCN |
27830 |
7.7446 |
–8 to 27 liq. |
Hydrofluoric Acid |
HF |
25180 |
7.370 |
–83 to 48 liq. |
Hydrogen Peroxide |
H2O2 |
48530 |
8.853 |
10 to 90 liq. |
Hydrogen Sulfide |
H2S |
20690 |
7.880 |
–110 to –83 sol. |
Iron |
Fe |
309000 |
7.482 |
2220 to 2450 liq. |
Krypton |
Kr |
10065 |
7.1770 |
–189 to –169 sol. |
|
|
9377 |
6.92387 |
–169 to –150 liq. |
Lead |
Pb |
188500 |
7.827 |
525 to 1325 liq. |
Lead Bromide |
PbBr2 |
118000 |
7.827 |
735 to 918 liq. |
Lead Chloride |
PbCl2 |
141900 |
8.961 |
500 to 950 liq. |
Lithium Bromide |
LiBr |
152700 |
8.068 |
1010 to 1265 liq. |
Lithium Chloride |
LiCl |
155900 |
7.939 |
1045 to 1325 liq. |
Lithium Fluoride |
LiF |
218400 |
8.753 |
1398 to 1666 liq. |
Lithium Iodide |
LiI |
143600 |
8.011 |
940 to 1140 liq. |
|
|
|
|
|
Source: data compiled by J.S. Park from CRC Handbook of Chemistry and Physics, David R. Lide, Ed., CRC Press, Boca Raton, (1990).
©2001 CRC Press LLC

Table 82. VAPOR PRESSURE OF ELEMENTS AND
INORGANIC COMPOUNDS* (SHEET 3 OF 5)
|
|
|
|
Temperature. Range |
|
|
|
|
of Validity |
Compound |
Formula |
a |
b |
˚C |
|
|
|
|
|
|
|
|
|
|
Magnesium |
Mg |
260000 |
12.993 |
900 to 1070 liq. |
Magnase |
Mn |
267000 |
9.300 |
1510 to 1900 liq. |
Mercuric Bromide |
HgBr2 |
79800 |
10.181 |
111 to 235 sol. |
|
|
61250 |
8.284 |
238 to 331 lig. |
Mercuric Chloride |
HgCl2 |
85030 |
10.888 |
60 to 130 sol. |
|
|
78850 |
10.094 |
130 to 270 sol. |
|
|
61020 |
8.409 |
275 to 309 liq. |
Mercuric Iodide |
HgI2 |
82340 |
10.057 |
100 to 250 sol. |
|
|
62770 |
8.115 |
266 to 360 liq. |
Mercury |
Hg |
73000 |
10.383 |
–80 to –38.87 sol. |
|
|
58700 |
7.752 |
400 to 1300 liq. |
Molybdenum |
Mo |
680000 |
10.844 |
1800 to 2240 sol. |
Nitrogen |
N2 |
6881.3 |
7.66558 |
–215 to –210 sol. |
Nitrogen Dioxide |
NO |
16423 |
10.048 |
–200 to –161 sol. |
|
|
13040 |
8.440 |
–163.7 to –148 liq. |
Nitrogen Monoxide |
N2O |
23590 |
9.579 |
–144 to –90 sol. |
|
|
16440 |
7.535 |
–90.1 to –88.7 liq. |
Nitrogen Pentoxide |
N2O5 |
57180 |
12.647 |
–30 to 30 sol. |
Nitrogen Tetroxide |
N2O4 |
55160 |
13.400 |
–100 to –40 sol. |
|
|
45440 |
11.214 |
–40 to –10 sol. |
|
|
33430 |
8.814 |
–8 to 43.2 liq. |
Nitrogen Trioxide |
N2O3 |
39400 |
10.30 |
–25 to 0 liq. |
Phosphorus (White) |
P |
63123 |
9.6511 |
20 to 44.1 sol. |
Phosphorus (Violet) |
P |
108510 |
11.0842 |
380 to 590 sol. |
Platinum |
Pt |
486000 |
7.786 |
1425 to 1765 sol. |
Potassium |
K |
84900 |
7.183 |
260 to 760 liq. |
|
|
|
|
|
Source: data compiled by J.S. Park from CRC Handbook of Chemistry and Physics, David R. Lide, Ed., CRC Press, Boca Raton, (1990).
©2001 CRC Press LLC

Table 82. VAPOR PRESSURE OF ELEMENTS AND
INORGANIC COMPOUNDS* (SHEET 4 OF 5)
|
|
|
|
Temperature. Range |
|
|
|
|
of Validity |
Compound |
Formula |
a |
b |
˚C |
|
|
|
|
|
|
|
|
|
|
Potassium Bromide |
KBr |
168100 |
8.2470 |
906 to 1063 liq. |
|
|
163800 |
7.936 |
1095 to 1375 liq. |
Potassium Chloride |
KCl |
174500 |
8.3526 |
906 to 1105 liq. |
|
|
169700 |
8.130 |
1116 to 1428 liq. |
Potassium Flouride |
KF |
207500 |
9.000 |
1278 to 1500 liq. |
Potassium Hydroxide |
KOH |
136000 |
7.330 |
1170 to 1327 liq. |
Potassium Iodide |
KI |
157600 |
8.0957 |
843 to 1028 liq. |
|
|
155700 |
7.949 |
1063 to 1333 liq. |
Rubidium |
Rb |
76000 |
6.976 |
250 to 370 liq. |
Rubidium Chloride |
RbCl |
198600 |
9.111 |
1142 to 1395 liq. |
Silicon |
Si |
170000 |
5.950 |
1200 to 1320 sol. |
Silicon Dioxide |
SiO2 |
506000 |
13.43 |
1860 to 2230 liq. |
Silver |
Ag |
250000 |
8.762 |
1650 to 1950 liq. |
Silver Chloride |
AgCl |
185500 |
8.179 |
1255 to 1442 liq. |
Sodium |
Na |
103300 |
7.553 |
180 to 883 liq. |
Sodium Bromide |
NaBr |
161600 |
7.948 |
1138 to 1394 liq. |
Sodium Chloride |
NaCl |
180300 |
8.3297 |
976 to 1155 liq. |
|
|
185800 |
8.548 |
1156 to 1430 liq. |
Sodium Cyanide |
NaCN |
155520 |
7.472 |
800 to 1360 liq. |
Sodium Fluoride |
NaF |
218200 |
8.640 |
1562 to 1701 liq. |
Sodium Hydroxide |
NaOH |
132000 |
7.030 |
1010 to 1042 liq. |
Sodium Iodide |
NaI |
165100 |
8.371 |
1063 to 1307 liq. |
Stannic Chloride |
SnCl4 |
46740 |
9.824 |
–52 to –38 liq. |
Stronium |
Sr |
360000 |
16.056 |
940 to 1140 liq. |
|
|
|
|
|
Source: data compiled by J.S. Park from CRC Handbook of Chemistry and Physics, David R. Lide, Ed., CRC Press, Boca Raton, (1990).
©2001 CRC Press LLC
Table 82. VAPOR PRESSURE OF ELEMENTS AND
INORGANIC COMPOUNDS* (SHEET 5 OF 5)
|
|
|
|
Temperature. Range |
|
|
|
|
of Validity |
Compound |
Formula |
a |
b |
˚C |
|
|
|
|
|
|
|
|
|
|
Sulfur Dioxide |
SO2 |
35827 |
10.5916 |
–95 to –75 liq. |
Sulfur Trioxide |
SO3 |
43450 |
10.022 |
24 to 48 liq. |
Thallium |
Tl |
120000 |
6.140 |
950 to 1200 liq. |
Thallium Chloride |
TlCl |
105200 |
7.947 |
665 to 807 liq. |
Tin |
Sn |
328000 |
9.643 |
1950 to 2270 liq. |
Tungsten |
W |
897000 |
9.920 |
2230 to 2770 liq. |
Zinc |
Zn |
133000 |
9.200 |
250 to 491.4 sol. |
|
|
118000 |
8.108 |
600 to 985 liq. |
|
|
|
|
|
Source: data compiled by J.S. Park from CRC Handbook of Chemistry and Physics, David R. Lide, Ed., CRC Press, Boca Raton, (1990).
*The vapor pressure with respect to temperature may be represented by the following equation:
log10 p = –0.05223a/T + b
where p is the pressure in mm of mercury of the saturated vapor at the absolute temperature T. (T = t˚C + 273.1) The values obtained by the use of the equation given above are valid within the temperature ranges indicated for each of the compounds.
©2001 CRC Press LLC

Table 83. VALUES OF THE ERROR FUNCTION
|
(SHEET 1 OF 2) |
|
|
z |
erf (z) |
|
|
|
|
0.00 |
0.0000 |
0.01 |
0.0113 |
0.02 |
0.0226 |
0.03 |
0.0338 |
0.04 |
0.0451 |
0.05 |
0.0564 |
0.10 |
0.1125 |
0.15 |
0.1680 |
0.20 |
0.2227 |
0.25 |
0.2763 |
0.30 |
0.3286 |
0.35 |
0.3794 |
0.40 |
0.4284 |
0.45 |
0.4755 |
0.50 |
0.5205 |
0.55 |
0.5633 |
0.60 |
0.6039 |
0.65 |
0.6420 |
0.70 |
0.6778 |
0.75 |
0.7112 |
0.80 |
0.7421 |
0.85 |
0.7707 |
0.90 |
0.7969 |
0.95 |
0.8209 |
1.00 |
0.8427 |
1.10 |
0.8802 |
1.20 |
0.9103 |
1.30 |
0.9340 |
|
|
Source: from: Handbook of Mathematical Functions, M. Abramowitz and I. A. Stegun, eds., National Bureau of Standards, Applied Mathematics Series 55, Washington, D.C., 1972.
©2001 CRC Press LLC
Table 83. VALUES OF THE ERROR FUNCTION
|
(SHEET 2 OF 2) |
|
|
z |
erf (z) |
|
|
|
|
1.40 |
0.9523 |
1.50 |
0.9661 |
1.60 |
0.9763 |
1.70 |
0.9838 |
1.80 |
0.9891 |
1.90 |
0.9928 |
2.00 |
0.9953 |
|
|
Source: from: Handbook of Mathematical Functions, M. Abramowitz and I. A. Stegun, eds., National Bureau of Standards, Applied Mathematics Series 55, Washington, D.C., 1972.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 1 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminum |
Ag110 |
S |
99.999 |
371–655 |
27.83 |
0.118 |
|
Al27 |
S |
|
450–650 |
34.0 |
1.71 |
|
Au198 |
S |
99.999 |
423–609 |
27.0 |
0.077 |
|
Cd115 |
S |
99.999 |
441–631 |
29.7 |
1.04 |
|
Ce141 |
P |
99.995 |
450–630 |
26.60 |
1.9 x 10–6 |
|
Co60 |
S |
99.999 |
369–655 |
27.79 |
0.131 |
|
Cr51 |
S |
99.999 |
422–654 |
41.74 |
464 |
|
Cu64 |
S |
99.999 |
433–652 |
32.27 |
0.647 |
|
Fe59 |
S |
99.99 |
550–636 |
46.0 |
135 |
|
Ga72 |
S |
99.999 |
406–652 |
29.24 |
0.49 |
|
Ge71 |
S |
99.999 |
401–653 |
28.98 |
0.481 |
|
In114 |
P |
99.99 |
400–600 |
27.6 |
0.123 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 2 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminum (con’t) |
La140 |
P |
99.995 |
500–630 |
27.0 |
1.4 x 10–6 |
|
Mn54 |
P |
99.99 |
450–650 |
28.8 |
0.22 |
|
Mo99 |
P |
99.995 |
400–630 |
13.1 |
1.04 x 10–9 |
|
Nb95 |
P |
99.95 |
350–480 |
19.65 |
1.66 x 10–7 |
|
Nd147 |
P |
99.995 |
450–630 |
25.0 |
4.8 x 10–7 |
|
Ni63 |
P |
99.99 |
360–630 |
15.7 |
2.9 x 10–8 |
|
Pd103 |
P |
99.995 |
400–630 |
20.2 |
1.92 x 10–7 |
|
Pr142 |
P |
99.995 |
520–630 |
23.87 |
3.58 x 10–7 |
|
Sb124 |
P |
|
448–620 |
29.1 |
0.09 |
|
Sm153 |
P |
99.995 |
450–630 |
22.88 |
3.45 x 10–7 |
|
Sn113 |
P |
|
400–600 |
28.5 |
0.245 |
|
V48 |
P |
99.995 |
400–630 |
19.6 |
6.05 x 10–8 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 3 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminum (con’t) |
Zn65 |
S |
99.999 |
357–653 |
28.86 |
0.259 |
Beryllium |
Ag110 |
S c |
99.75 |
650–900 |
43.2 |
1.76 |
|
||||||
|
Ag110 |
S||c |
99.75 |
650–900 |
39.3 |
0.43 |
|
Be7 |
S c |
99.75 |
565–1065 |
37.6 |
0.52 |
|
Be7 |
S||c |
99.75 |
565–1065 |
39.4 |
0.62 |
|
Fe59 |
S |
99.75 |
700–1076 |
51.6 |
0.67 |
|
Ni63 |
P |
|
800–1250 |
58.0 |
0.2 |
Cadmium |
Ag110 |
S |
99.99 |
180–300 |
25.4 |
2.21 |
|
Cd115 |
S |
99.95 |
110–283 |
19.3 |
0.14 |
|
Zn65 |
S |
99.99 |
180–300 |
19.0 |
0.0016 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 4 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calcium |
C14 |
|
99.95 |
550–800 |
29.8 |
3.2 x 10–5 |
|
Ca45 |
|
99.95 |
500–800 |
38.5 |
8.3 |
|
Fe59 |
|
99.95 |
500–800 |
23.3 |
2.7 x 10–3 |
|
Ni63 |
|
99.95 |
550–800 |
28.9 |
1.0 x 10–6 |
|
U235 |
|
99.95 |
500–700 |
34.8 |
l.l x 10–5 |
Carbon |
Ag110 |
c |
|
750–1050 |
64.3 |
9280 |
|
C14 |
c |
|
2000–2200 |
163 |
5 |
|
Ni63 |
|
540–920 |
47.2 |
102 |
|
|
Ni63 |
||c |
|
750–1060 |
53.3 |
2.2 |
|
Th228 |
c |
|
1400–2200 |
145.4 |
1.33 x 10–5 |
|
Th228 |
||c |
|
1800–2200 |
114.7 |
2.48 |
|
U232 |
c |
|
140~2200 |
115.0 |
6760 |
|
U232 |
||c |
|
1400 1820 |
129.5 |
385 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 5 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chromium |
C14 |
P |
|
120–1500 |
26.5 |
9.0 x 10–3 |
|
Cr51 |
P |
99.98 |
1030–1545 |
73.7 |
0.2 |
|
Fe59 |
P |
99.8 |
980–1420 |
79.3 |
0.47 |
|
Mo99 |
P |
|
1100–1420 |
58.0 |
2.7 x 10–3 |
Cobalt |
C14 |
P |
99.82 |
600–1400 |
34.0 |
0.21 |
|
Co60 |
P |
99.9 |
1100–1405 |
67.7 |
0.83 |
|
Fe59 |
P |
99.9 |
1104–1303 |
62.7 |
0.21 |
|
Ni63 |
P |
|
1192–1297 |
60.2 |
0.10 |
|
S35 |
P |
99.99 |
1150–1250 |
5.4 |
1.3 |
Copper |
Ag110 |
S, P |
|
580–980 |
46.5 |
0.61 |
|
As76 |
P |
|
810–1075 |
42.13 |
0.20 |
|
Au193 |
S, P |
|
400–1050 |
42.6 |
0.03 |
|
Cd115 |
S |
99.98 |
725–950 |
45.7 |
0.935 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 6 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Copper (Con’t) |
Ce141 |
P |
99.999 |
766–947 |
27.6 |
2.17 x 10–3 |
|
Cr51 |
S, P |
|
800–1070 |
53.5 |
1.02 |
|
Co60 |
S |
99.998 |
701–1077 |
54.1 |
1.93 |
|
Cu67 |
S |
99.999 |
698–1061 |
50.5 |
0.78 |
|
Eu152 |
P |
99.999 |
750–970 |
26.85 |
1.17 x 10–7 |
|
Fe59 |
S. P |
|
460–1070 |
52.0 |
1.36 |
|
Ga72 |
|
|
_ |
45.90 |
0.55 |
|
Ge68 |
S |
99.998 |
653–1015 |
44.76 |
0.397 |
|
Hg203 |
P |
|
_ |
44.0 |
0.35 |
|
Lu177 |
P |
99.999 |
857–1010 |
26.15 |
4.3 x 10–9 |
|
Mn54 |
S |
99.99 |
754–950 |
91.4 |
107 |
|
Nb95 |
P |
99.999 |
807–906 |
60.06 |
2.04 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 7 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Copper (Con’t) |
Ni63 |
P |
|
620–1080 |
53.8 |
1.1 |
|
Pd102 |
S |
99.999 |
807–1056 |
54.37 |
1.71 |
|
Pm147 |
P |
99.999 |
720–955 |
27.5 |
3.62 x 10–8 |
|
Pt195 |
P |
|
843–997 |
37.5 |
4.8 x 10–4 |
|
S35 |
S |
99.999 |
800–1000 |
49.2 |
23 |
|
Sb124 |
S |
99.999 |
600–1000 |
42.0 |
0.34 |
|
Sn113 |
P |
|
680–910 |
45.0 |
0.11 |
|
Tb160 |
P |
99.999 |
770–980 |
27.45 |
8.96 x 10–9 |
|
Tl204 |
S |
99.999 |
785–996 |
43.3 |
0.71 |
|
Tm170 |
P |
99.999 |
705–950 |
24.15 |
7.28 x 10–9 |
|
Zn65 |
P |
99.999 |
890–1000 |
47.50 |
0.73 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 8 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Germanium |
Cd115 |
S |
|
750–950 |
102.0 |
1.75 x 109 |
|
Fe59 |
S |
|
775–930 |
24.8 |
0.13 |
|
Ge71 |
S |
|
766–928 |
68.5 |
7.8 |
|
In114 |
S |
|
600–920 |
39.9 |
2.9 x 10–4 |
|
Sb124 |
S |
|
720–900 |
50.2 |
0.22 |
|
Te125 |
S |
|
770–900 |
56.0 |
2.0 |
|
Tl204 |
S |
|
800–930 |
78.4 |
1700 |
Gold |
Ag110 |
S |
99.99 |
699–1007 |
40.2 |
0.072 |
|
Au198 |
S |
99.97 |
850–1050 |
42.26 |
0.107 |
|
Co60 |
P |
99.93 |
702–948 |
41.6 |
0.068 |
|
Fe59 |
P |
99.93 |
701–948 |
41.6 |
0.082 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 9 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gold (Con’t) |
Hg203 |
S |
99.994 |
600–1027 |
37.38 |
0.116 |
|
Ni63 |
P |
99.96 |
880–940 |
46.0 |
0.30 |
|
Pt195 |
P, S |
99.98 |
800–1060 |
60.9 |
7.6 |
β–Hafnium |
Hf181 |
P |
97.9 |
1795–1995 |
38.7 |
1.2 x10–3 |
Indium |
Ag110 |
S c |
99.99 |
25–140 |
12.8 |
0.52 |
|
Ag110 |
S||c |
99.99 |
25–140 |
11.5 |
0.11 |
|
Au198 |
S |
99.99 |
25–140 |
6.7 |
9 x 10–3 |
|
In114 |
S c |
99.99 |
44–144 |
18.7 |
3.7 |
|
In114 |
S||c |
99.99 |
44–144 |
18.7 |
2.7 |
|
Tl204 |
S |
99.99 |
49–157 |
15.5 |
0.049 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 10 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α-Iron |
Ag110 |
P |
|
748–888 |
69.0 |
1950 |
|
Au198 |
P |
99.999 |
800–900 |
62.4 |
31 |
|
C14 |
P |
99.98 |
616–844 |
29.3 |
2.2 |
|
Co60 |
P |
99.995 |
638–768 |
62.2 |
7.19 |
|
Cr51 |
P |
99.95 |
775–875 |
57.5 |
2.53 |
|
Cu64 |
P |
99.9 |
800 1050 |
57.0 |
0.57 |
|
Fe55 |
P |
99.92 |
809–889 |
60.3 |
5.4 |
|
K42 |
P |
99.92 |
500–800 |
42.3 |
0.036 |
|
Mn54 |
P |
99.97 |
800–900 |
52.5 |
0.35 |
|
Mo99 |
P |
|
750–875 |
73.0 |
7800 |
|
Ni63 |
P |
99.97 |
680–800 |
56.0 |
1.3 |
|
P32 |
P |
|
860–900 |
55.0 |
2.9 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 11 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α-Iron (Con’t) |
Sb124 |
P |
|
800–900 |
66.6 |
1100 |
|
V48 |
P |
|
755–875 |
55.4 |
1.43 |
|
W185 |
P |
|
755–875 |
55.1 |
0.29 |
γ-Iron |
Be7 |
P |
99.9 |
1100–1350 |
57.6 |
0.1 |
|
C14 |
P |
99.34 |
800–1400 |
34.0 |
0.15 |
|
Co60 |
P |
99.98 |
1138–1340 |
72.9 |
1.25 |
|
Cr51 |
P |
99.99 |
950–1400 |
69.7 |
10.8 |
|
Fe59 |
P |
99.98 |
1171–1361 |
67.86 |
0.49 |
|
Hf181 |
P |
99.99 |
1110–1360 |
97.3 |
3600 |
|
Mn54 |
P |
99.97 |
920–1280 |
62.5 |
0.16 |
|
Ni63 |
P |
99.97 |
930–2050 |
67.0 |
0.77 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 12 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
γ-Iron (Con’t) |
P32 |
P |
99.99 |
950–1200 |
43.7 |
0.01 |
|
||||||
|
S35 |
P |
|
900–1250 |
53.0 |
1.7 |
|
V48 |
P |
99.99 |
1120–1380 |
69.3 |
0.28 |
|
W185 |
P |
99.5 |
1050–1250 |
90.0 |
1000 |
δ-Iron |
Co60 |
P |
99.995 |
1428–1521 |
61.4 |
6.38 |
|
Fe59 |
P |
99.95 |
1428–1492 |
57.5 |
2.01 |
|
P32 |
P |
99.99 |
1370–1460 |
55.0 |
2.9 |
Lanthanum |
Au198 |
P |
99.97 |
600–800 |
45.1 |
1.5 |
|
La140 |
P |
99.97 |
690–850 |
18.1 |
2.2 x 10–2 |
Lead |
Ag110 |
P |
99.9 |
200–310 |
14.4 |
0.064 |
|
Au198 |
S |
99.999 |
190–320 |
10.0 |
8.7 x 10–3 |
|
Cd115 |
S |
99.999 |
150–320 |
21.23 |
0.409 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 13 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lead (Con’t) |
Cu64 |
S |
|
150–320 |
14.44 |
0.046 |
|
Pb204 |
S |
99.999 |
150–320 |
25.52 |
0.887 |
|
Tl205 |
P |
99.999 |
207–322 |
24.33 |
0.511 |
Lithium |
Ag110 |
P |
92.5 |
65–161 |
12.83 |
0.37 |
|
Au195 |
P |
92.5 |
47–153 |
10.49 |
0.21 |
|
Bi |
P |
99.95 |
141–177 |
47.3 |
5.3 x 1013 |
|
Cd115 |
P |
92.5 |
80–174 |
16.05 |
2.35 |
|
Cu64 |
P |
99.98 |
51–120 |
9.22 |
0.47 |
|
Ga72 |
P |
99.98 |
58–173 |
12.9 |
0.21 |
|
Hg203 |
P |
99.98 |
58–173 |
14.18 |
1.04 |
|
In114 |
P |
92.5 |
80–175 |
15.87 |
0.39 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 14 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lithium (Con’t) |
Li6 |
P |
99.98 |
35–178 |
12.60 |
0.14 |
|
Na22 |
P |
92.5 |
52–176 |
12.61 |
0.41 |
|
Pb204 |
P |
99.95 |
129–169 |
25.2 |
160 |
|
Sb124 |
P |
99.95 |
141–176 |
41.5 |
1.6 x 1010 |
|
Sn113 |
P |
99.95 |
108–174 |
15.0 |
0.62 |
|
Zn65 |
P |
92.5 |
60–175 |
12.98 |
0.57 |
Magnesium |
Ag110 |
P |
99.9 |
476–621 |
28.50 |
0.34 |
|
Fe59 |
P |
99.95 |
400–600 |
21.2 |
4 x 10–6 |
|
In114 |
P |
99.9 |
472–610 |
28.4 |
5.2 x 10–2 |
|
Mg28 |
S c |
|
467–635 |
32.5 |
1.5 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 15 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Magnesium (Con’t) |
Mg28 |
S||c |
|
467–635 |
32.2 |
1.0 |
|
Ni63 |
P |
99.95 |
400 600 |
22.9 |
1.2 x 10–5 |
|
U235 |
P |
99.95 |
500–620 |
27.4 |
1.6 x 10–5 |
|
Zn65 |
P |
99.9 |
467–620 |
28.6 |
0.41 |
Molybdenum |
C14 |
P |
99.98 |
1200–1600 |
41.0 |
2.04 x 10–2 |
|
Co60 |
P |
99.98 |
1850–2350 |
106.7 |
18 |
|
Cr51 |
P |
|
1000–1500 |
54.0 |
2.5 x 10–4 |
|
Cs134 |
S |
99.99 |
1000–1470 |
28.0 |
8.7 x 10–11 |
|
K42 |
S |
|
800–1100 |
25.04 |
5.5 x 10–9 |
|
Mo99 |
P |
|
1850–2350 |
96.9 |
0.5 |
|
Na24 |
S |
|
800–1100 |
21.25 |
2.95 x 10–9 |
|
Nb95 |
P |
99.98 |
1850–2350 |
108.1 |
14 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 16 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molybdenum (Con’t) |
P32 |
P |
99.97 |
2000–2200 |
80.5 |
0.19 |
|
Re186 |
P |
|
1700–2100 |
94.7 |
0.097 |
|
S35 |
S |
99.97 |
2220–2470 |
101.0 |
320 |
|
Ta182 |
P |
|
1700–2150 |
83.0 |
3.5 x 10–4 |
|
U235 |
P |
99.98 |
1500–2000 |
76.4 |
7.6 x 10–3 |
|
Wl85 |
P |
99.98 |
1700–2260 |
110 |
1.7 |
Nickel |
Au198 |
S,P |
99.999 |
700–1075 |
55.0 |
0.02 |
|
Be7 |
P |
99.9 |
1020–1400 |
46.2 |
0.019 |
|
Cl4 |
P |
99.86 |
600–1400 |
34.0 |
0.012 |
|
Co60 |
P |
99.97 |
1149–1390 |
65.9 |
1.39 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 17 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nickel (Con’t) |
Cr51 |
P |
99.95 |
1100–1270 |
65.1 |
1.1 |
|
Cu64 |
P |
99.95 |
1050–1360 |
61.7 |
0.57 |
|
Fe59 |
P |
|
1020–1263 |
58.6 |
0.074 |
|
Mo99 |
P |
|
900–1200 |
51.0 |
1.6 x 10–3 |
|
Ni63 |
P |
99.95 |
1042–1404 |
68.0 |
1.9 |
|
Pu238 |
P |
|
1025–1125 |
51.0 |
0.5 |
|
Sb124 |
P |
99.97 |
1020–1220 |
27.0 |
1.8 x 10–5 |
|
Sn113 |
P |
99.8 |
700–1350 |
58.0 |
0.83 |
|
V48 |
P |
99.99 |
800–1300 |
66.5 |
0.87 |
|
W185 |
P |
99.95 |
1100–1300 |
71.5 |
2.0 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 18 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Niobium |
C14 |
P |
|
800–1250 |
32.0 |
1.09 x 10–5 |
|
Co60 |
P |
99.85 |
1500–2100 |
70.5 |
0.74 |
|
Cr51 |
S |
|
943–1435 |
83.5 |
0.30 |
|
Fe51 |
P |
99.85 |
1400–2100 |
77.7 |
1.5 |
|
K42 |
S |
|
900–1100 |
22.10 |
2.38 x 10–7 |
|
Nb95 |
P, S |
99.99 |
878–2395 |
96.0 |
1.1 |
|
P32 |
P |
99.0 |
1300–1800 |
51.5 |
5.1 x 10–2 |
|
S35 |
S |
99.9 |
1100–1500 |
73.1 |
2600 |
|
Sn113 |
P |
99.85 |
1850–2400 |
78.9 |
0.14 |
|
Ta182 |
P, S |
99.997 |
878–2395 |
99.3 |
1.0 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 19 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Niobium (Cont) |
Ti44 |
S |
|
994–1492 |
86.9 |
0.099 |
|
|
|||||
|
U235 |
P |
99.55 |
1500–2000 |
76.8 |
8.9 x10–3 |
|
V48 |
S |
99.99 |
1000–1400 |
85.0 |
2.21 |
|
W185 |
P |
99.8 |
1800–2200 |
91.7 |
5 x 10–4 |
Palladium |
Pd103 |
S |
99.999 |
1060–1500 |
63.6 |
0.205 |
Phosphorus |
P32 |
P |
|
0–44 |
9.4 |
1.07 x 10–3 |
Platinum |
Co60 |
P |
99.99 |
900–1050 |
74.2 |
19.6 |
|
Cu64 |
P |
|
1098–1375 |
59.5 |
0.074 |
|
Pt195 |
P |
99.99 |
1325–1600 |
68.2 |
0.33 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 20 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Potassium |
Au198 |
P |
99.95 |
5.6–52.5 |
3.23 |
1.29 x10–3 |
|
K42 |
S |
99.7 |
–52–61 |
9.36 |
0.16 |
|
Na22 |
P |
99.7 |
0–62 |
7.45 |
0.058 |
|
Rb86 |
P |
99.95 |
0.1–59.9 |
8.78 |
0.090 |
γ–Plutonium |
Pu238 |
P |
|
190–310 |
16.7 |
2.1 x 10–5 |
δ–Plutonium |
Pu238 |
P |
|
350–440 |
23.8 |
4.5 x 10–3 |
ε-Plutonium |
Pu238 |
P |
|
500–612 |
18.5 |
2.0 x 10–2 |
α-Praseodymium |
Ag110 |
P |
99.93 |
610–730 |
25.4 |
0.14 |
|
Au195 |
P |
99.93 |
650–780 |
19.7 |
4.3 x 10–2 |
|
Co60 |
P |
99.93 |
660–780 |
16.4 |
4.7 x 10–2 |
|
Zn65 |
P |
99.96 |
766–603 |
24.8 |
0.18 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 21 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
β-Praseodymium |
Ag110 |
P |
99.93 |
800–900 |
21.5 |
3.2 x 10–2 |
|
Au195 |
P |
99.93 |
800–910 |
20.1 |
3.3 x 10–2 |
|
Ho166 |
P |
99.96 |
800–930 |
26.3 |
9.5 |
|
In114 |
P |
99.96 |
800–930 |
28.9 |
9.6 |
|
La140 |
P |
99.96 |
800–930 |
25.7 |
1.8 |
|
Pr142 |
P |
99.93 |
800–900 |
29.4 |
8.7 |
|
Zn65 |
P |
99.96 |
822–921 |
27.0 |
0.63 |
Selenium |
Fe59 |
P |
|
40–100 |
8.88 |
— |
|
Hg203 |
P |
99.996 |
25–100 |
1.2 |
— |
|
S35 |
S c |
|
60–90 |
29.9 |
1700 |
|
S35 |
S||c |
|
60–90 |
15.6 |
1100 |
|
Se75 |
P |
|
35–140 |
11.7 |
1.4 x 10–4 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 22 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silicon |
Au198 |
S |
|
700–1300 |
47.0 |
2.75 x 10–3 |
|
C14 |
P |
|
1070–1400 |
67.2 |
0.33 |
|
Cu64 |
P |
|
800–1100 |
23.0 |
4 x 10–2 |
|
Fe59 |
S |
|
1000–1200 |
20.0 |
6.2 x 10–3 |
|
Ni63 |
P |
|
450–800 |
97.5 |
1000 |
|
P32 |
S |
|
1100–1250 |
41.5 |
– |
|
Sb124 |
S |
|
1190–1398 |
91.7 |
12.9 |
|
Si31 |
S |
99.99999 |
1225–1400 |
110.0 |
1800 |
Silver |
Au198 |
P |
99.99 |
718–942 |
48.28 |
0.85 |
|
Ag110 |
S |
99.999 |
640–955 |
45.2 |
0.67 |
|
Cd115 |
S |
99.99 |
592–937 |
41.69 |
0.44 |
|
Co60 |
S |
99.999 |
700–940 |
48.75 |
1.9 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 23 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silver (Con’t) |
Cu64 |
P |
99.99 |
717–945 |
46.1 |
1.23 |
|
Fe59 |
S |
99.99 |
720–930 |
49.04 |
2.42 |
|
Ge77 |
P |
|
640–870 |
36.5 |
0.084 |
|
Hg203 |
P |
99.99 |
653–948 |
38.1 |
0.079 |
|
In114 |
S |
99.99 |
592–937 |
40.80 |
0.41 |
|
Ni63 |
S |
99.99 |
749–950 |
54.8 |
21.9 |
|
Pb210 |
P |
|
700–865 |
38.1 |
0.22 |
|
Pd102 |
S |
99.999 |
736–939 |
56.75 |
9.56 |
|
Ru103 |
S |
99.99 |
793–945 |
65.8 |
180 |
|
S35 |
S |
99.999 |
600–900 |
40.0 |
1.65 |
|
Sb124 |
P |
99.999 |
780–950 |
39.07 |
0.234 |
|
Sn113 |
S |
99.99 |
592–937 |
39.30 |
0.255 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 24 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silver (Con’t) |
Te125 |
P |
|
770–940 |
38.90 |
0.47 |
|
Tl204 |
P |
|
640–870 |
37.9 |
0.15 |
|
Zn65 |
S |
99.99 |
640–925 |
41.7 |
0.54 |
Sodium |
Au198 |
P |
99.99 |
1.0–77 |
2.21 |
3.34 x l0–4 |
|
K42 |
P |
99.99 |
0–91 |
8.43 |
0.08 |
|
Na22 |
P |
99.99 |
0–98 |
10.09 |
0.145 |
|
Rb86 |
P |
99.99 |
0–85 |
8.49 |
0.15 |
Tantalum |
C14 |
P |
|
1450–2200 |
40.3 |
1.2 x 10–2 |
|
Fe59 |
P |
|
930–1240 |
71.4 |
0.505 |
|
Mo99 |
P |
|
1750–2220 |
81.0 |
1.8 x 10–3 |
|
Nb95 |
P, S |
99.996 |
921–2484 |
98.7 |
0.23 |
|
S35 |
P |
99.0 |
1970–2110 |
70.0 |
100 |
|
Ta182 |
P, S |
99.996 |
1250–2200 |
98.7 |
1.24 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 25 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tellurium |
Hg203 |
P |
|
270–440 |
18.7 |
3.14 x 10–5 |
|
Se75 |
P |
|
320–440 |
28.6 |
2.6 x 10–2 |
|
Tl204 |
P |
|
360–430 |
41.0 |
320 |
|
Te127 |
S c |
99.9999 |
300–400 |
46.7 |
3.91 x 104 |
|
Te127 |
S||c |
99.9999 |
300–400 |
35.5 |
130 |
α-Thallium |
Ag110 |
P c |
99.999 |
80–250 |
11.8 |
3.8 x 10–2 |
|
Ag110 |
P||c |
99.999 |
80–250 |
11.2 |
2.7 x 10–2 |
|
Au198 |
P c |
99.999 |
110–260 |
2.8 |
2.0 x 10–5 |
|
Au198 |
P||c |
99.999 |
110–260 |
5.2 |
5.3 x 10–4 |
|
Tl204 |
S c |
99.9 |
135–230 |
22.6 |
0.4 |
|
Tl204 |
S||c |
99.9 |
135–230 |
22.9 |
0.4 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 26 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
β-Thallium |
Ag110 |
P |
99.999 |
230–310 |
11.9 |
4.2 x 10–2 |
|
Au198 |
P |
99.999 |
230–310 |
6.0 |
5.2 x 10–4 |
|
Tl204 |
S |
99.9 |
230–280 |
20.7 |
0.7 |
α-Thorium |
Pa231 |
P |
99.85 |
770–910 |
74.7 |
126 |
|
Th228 |
P |
99.85 |
720–880 |
716 |
395 |
|
U233 |
P |
99.85 |
700–880 |
79.3 |
2210 |
Tin |
Ag110 |
S c |
|
135–225 |
18.4 |
0.18 |
|
Ag110 |
S||c |
|
135–225 |
12.3 |
7.1 x 10–3 |
|
Au198 |
S c |
|
135–225 |
17.7 |
0.16 |
|
Au198 |
S||c |
|
135–225 |
11.0 |
5.8 x 10–3 |
|
Co60 |
S,P |
|
140–217 |
22.0 |
5.5 |
|
In114 |
S c |
99.998 |
181–221 |
25.8 |
34.1 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 27 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tin (Con’t) |
In114 |
S||c |
99.998 |
181–221 |
25.6 |
12.2 |
|
Sn113 |
S c |
99.999 |
160–226 |
25.1 |
10.7 |
|
Sn113 |
S||c |
99.999 |
160–226 |
25.6 |
7.7 |
|
Tl204 |
P |
99.999 |
137–216 |
14.7 |
1.2 x 10–3 |
α-Titanium |
Ti44 |
P |
99.99 |
700–850 |
35.9 |
8.6 x 10–6 |
β-Titanium |
Ag110 |
P |
99.95 |
940 1570 |
43.2 |
3 x 10–3 |
|
Be7 |
P |
99.96 |
915–1300 |
40.2 |
0.8 |
|
C14 |
P |
99.62 |
1100–1600 |
20.0 |
3.02 x 10–3 |
|
Cr51 |
P |
99.7 |
950–1600 |
35.1 |
5 x 10–3 |
|
Co60 |
P |
99.7 |
900–1600 |
30.6 |
1.2 x 10–2 |
|
Fe59 |
P |
99.7 |
900–1600 |
31.6 |
7.8 x 10–3 |
|
Mo99 |
P |
99.7 |
900–1600 |
43.0 |
8.0 x 10–3 |
|
Mn54 |
P |
99.7 |
900–1600 |
33.7 |
6.1 x 10–3 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 28 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
β-Titanium (Con’t) |
Nb95 |
P |
99.7 |
1000–1600 |
39.3 |
5.0 x 10–3 |
|
Ni63 |
P |
99.7 |
925–1600 |
29.6 |
9.2 x 10–3 |
|
P32 |
P |
99.7 |
950–1600 |
24.1 |
3.62x10–3 |
|
Sc46 |
P |
99.95 |
940–1590 |
32.4 |
4.0 x 10–3 |
|
Sn113 |
P |
99.7 |
950–1600 |
31.6 |
3.8 x 10–4 |
|
Ti44 |
P |
99.95 |
900–1540 |
31.2 |
3.58 x 10–4 |
|
U235 |
P |
99.9 |
900–400 |
29.3 |
5.1 x 10–4 |
|
V48 |
P |
99.95 |
900–1545 |
32.2 |
3.1 x 10–4 |
|
W185 |
P |
99.94 |
900–1250 |
43.9 |
3.6 x 10–3 |
|
Zr95 |
P |
98.94 |
920–1500 |
35.4 |
4.7 x 10–3 |
Tungsten |
C14 |
P |
99.51 |
1200–1600 |
53.5 |
8.91 x 10–3 |
|
Fe59 |
P |
|
940–1240 |
66.0 |
1.4 x 10–2 |
|
Mo99 |
P |
|
1700–2100 |
101.0 |
0.3 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 29 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tungsten (Con’t) |
Nb95 |
P |
99.99 |
1305–2367 |
137.6 |
3.01 |
|
Re186 |
S |
|
2100–2400 |
141.0 |
19.5 |
|
Ta182 |
P |
99.99 |
1305–2375 |
139.9 |
3.05 |
|
W185 |
P |
99.99 |
1800–2403 |
140.3 |
1.88 |
α–Uranium |
U234 |
P |
|
580–650 |
40.0 |
2 x 10–3 |
β–Uranium |
Co60 |
P |
99.999 |
692–763 |
27.45 |
1.5 x 10–2 |
|
U235 |
P |
|
690–750 |
44.2 |
2.8 x10–3 |
γ-Uranium |
Au195 |
P |
99.99 |
785–1007 |
30.4 |
4.86 x 10–3 |
|
Co60 |
P |
99.99 |
783–989 |
12.57 |
3.51 x 10–4 |
|
Cr51 |
P |
99.99 |
797–1037 |
24.46 |
5.37 X 10–3 |
|
Cu64 |
P |
99.99 |
787–1039 |
24.06 |
1.96 x 10–3 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 30 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
γ-Uranium (Con’t) |
Fe55 |
P |
99.99 |
787–990 |
12.0 |
2.69 x 10–4 |
|
Mn54 |
P |
99.99 |
787–939 |
13.88 |
1.81 x 10–4 |
|
Nb95 |
P |
99.99 |
791–1102 |
39.65 |
4.87 x 10–2 |
|
Ni63 |
P |
99.99 |
787–1039 |
15.66 |
5.36 x10–4 |
|
U233 |
P |
99.99 |
800–1070 |
28.5 |
2.33 x 10–3 |
|
Zr95 |
P |
|
800–1000 |
16.5 |
3.9 x 10–4 |
Vanadium |
C14 |
P |
99.7 |
845–1130 |
27.3 |
4.9 x 10–3 |
|
Cr51 |
P |
99.8 |
960–1200 |
64.6 |
9.54 x10–3 |
|
Fe59 |
P |
|
960–1350 |
71.0 |
0.373 |
|
P32 |
P |
99.8 |
1200–1450 |
49.8 |
2.45 x l0–2 |
|
S35 |
P |
99.8 |
1320–1520 |
34.0 |
3.1 x l0–2 |
|
V48 |
S,P |
99.99 |
880–1360 |
73.65 |
0.36 |
|
V48 |
S,P |
99.99 |
1360–1830 |
94.14 |
214.0 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 31 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yttrium |
Y90 |
S c |
|
900–1300 |
67.1 |
5.2 |
|
Y90 |
S||c |
|
900–1300 |
60.3 |
0.82 |
Zinc |
Ag110 |
S c |
99.999 |
271–413 |
27.6 |
0.45 |
|
Ag110 |
S||c |
99.999 |
271–413 |
26.0 |
0.32 |
|
Au198 |
S c |
99.999 |
315–415 |
29.72 |
0.29 |
|
Au198 |
S||c |
99.999 |
315–415 |
29.73 |
0.97 |
|
Cd115 |
S c |
99.999 |
225–416 |
20.12 |
0.117 |
|
Cd115 |
S||c |
99.999 |
225–416 |
20.54 |
0.114 |
|
Cu64 |
S c |
99.999 |
338–415 |
~20 |
~2 |
|
Cu64 |
S||c |
99.999 |
338–415 |
29.53 |
2.22 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 32 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Zinc (Con’t) |
Ga72 |
S c |
|
240–403 |
18.15 |
0.018 |
|
Ga72 |
S||c |
|
240 403 |
18.4 |
0.016 |
|
Hg203 |
S c |
|
260–413 |
20.18 |
0.073 |
|
Hg203 |
S||c |
|
260–413 |
19.70 |
0.056 |
|
In114 |
S c |
|
271–413 |
19.60 |
0.14 |
|
In114 |
S||c |
|
271–413 |
19.10 |
0.062 |
|
Sn113 |
S c |
|
298–400 |
18.4 |
0.13 |
|
Sn113 |
S||c |
|
298–400 |
19.4 |
0.15 |
|
Zn65 |
S c |
99.999 |
240–418 |
23.0 |
0.18 |
|
Zn65 |
S||c |
99.999 |
240–418 |
21.9 |
0.13 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 33 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α-Zirconium |
Cr51 |
P |
99.9 |
700–850 |
18.0 |
1.19 x 10–8 |
|
Fe55 |
P |
|
750–840 |
48.0 |
2.5 x 10–2 |
|
Mo99 |
P |
|
600–850 |
24.76 |
6.22 x 10–8 |
|
Nb95 |
P |
99.99 |
740–857 |
31.5 |
6.6 x 10–6 |
|
Sn113 |
P |
|
300–700 |
22.0 |
1.0 x 10–8 |
|
Ta182 |
P |
99.6 |
700–800 |
70.0 |
100 |
|
V48 |
P |
99.99 |
600–850 |
22.9 |
1.12 x 10–8 |
|
Zr95 |
P |
99.95 |
750–850 |
45.5 |
5.6 x 10–4 |
β–Zirconium |
Be7 |
P |
99.7 |
915–1300 |
31.1 |
8.33 x 10–2 |
|
C14 |
P |
96.6 |
1100–1600 |
34.2 |
3.57 x 10–2 |
|
Ce141 |
P |
|
880–1600 |
41.4 |
3.16 |
|
Co60 |
P |
99.99 |
920–1600 |
21.82 |
3.26 x 10–3 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

Table 84. DIFFUSION IN METALLIC SYSTEMS*
(SHEET 34 OF 34)
|
|
|
|
Temperature |
Activation |
Frequency |
|
|
|
|
energy, Q |
factor, Do |
|
|
|
Crystalline |
Purity |
Range |
||
Metal |
Tracer |
Form |
(%) |
(˚C) |
(kcal • mol–1) |
(cm2 • s–1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
β–Zirconium (Con’t) |
Cr51 |
P |
99.9 |
700–850 |
18.0 |
1.19 x 10–8 |
|
Fe55 |
P |
|
750–840 |
48.0 |
2.5 x 10–2 |
|
Mo99 |
P |
|
900–1635 |
35.2 |
1.99 x 10–6 |
|
Nb95 |
P |
|
1230–1635 |
36.6 |
7.8 x 10–4 |
|
P32 |
P |
99.94 |
950–1200 |
33.3 |
0.33 |
|
Sn113 |
P |
|
300–700 |
22.0 |
1 x 10–8 |
|
Ta182 |
P |
99.6 |
900–1200 |
27.0 |
5.5 x 10–5 |
|
U235 |
P |
|
900–1065 |
30.5 |
5.7 x 10–4 |
|
V48 |
P |
99.99 |
870–1200 |
45.8 |
7.59 x 10–3 |
|
V48 |
P |
99.99 |
1200–1400 |
57.7 |
0.32 |
|
W185 |
P |
99.7 |
900–1250 |
55.8 |
0.41 |
|
Zr95 |
P |
|
1100–1500 |
30.1 |
2.4 x 10–4 |
|
|
|
|
|
|
|
Source: data from Askill, J.,in Handbook of Chemistry and Physics, 55th ed.,Weast, R.C., Ed., CRC Press, Cleveland,1974, F61.
©2001 CRC Press LLC

*The diffusion coefficient DT at a temperature T(K) is given by the following: DT =Do e–Q/RT
For activation energy in KJ/mol, multiply values in Kcal/mol by 4.184. For frequency factor in m2/s, multiply values in cm2/s by 10–4.
Abbreviations:
P= polycrystalline
S = single crystal
c = perpendicular to c direction || c = parallel to c direction
©2001 CRC Press LLC

Table 85. DIFFUSION |
OF METALS INTO METALS |
|||
|
(SHEET 1 OF 11) |
|
||
|
|
|
|
|
|
|
|
Diffusion |
Coefficient |
Diffusing |
Matrix |
|
Temperature |
|
|
(cm2 • hr–1) |
|||
Metal |
Metal |
|
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
Ag |
Al |
|
466 |
6.84–8.1 x10–7 |
|
|
|
500 |
7.2–3.96 x 10–8 |
|
|
|
573 |
1.26 x 10–5 |
|
Pb |
|
220 |
5.40 x 10–5 |
|
|
|
250 |
1.08 x 10–4 |
|
|
|
285 |
3.29 x 10–4 |
|
Sn |
|
500 |
1.73 x 10–1 |
Al |
Cu |
|
500 |
6.12 x 10–9 |
|
|
|
850 |
7.92 x 10–6 |
As |
Si |
|
|
0.32 e–82,000⁄RT |
Au |
Ag |
|
456 |
1.76 x 10–9 |
|
|
|
491 |
0.92–2.38 x 10–13 |
|
|
|
585 |
3.6 x 10–8 |
|
|
|
601 |
3.96 x 10–8 |
|
|
|
624 |
2.5–5 x 10–11 |
|
|
|
717 |
1.04–2.25 x 10–9 |
|
|
|
729 |
1.76 x 10–9 |
|
|
|
767 |
1.15 x 10–6 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 2 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Au (Con’t) |
Ag (Con’t) |
847 |
2.30 x 10–6 |
|
|
|
858 |
3.63 x 10–8 |
|
|
|
861 |
3.92 x 10–8 |
|
|
|
874 |
3.92 x 10–8 |
|
|
|
916 |
5.40 x 10–6 |
|
|
|
1040 |
1.17 x 10–6 |
|
|
|
1120 |
2.29 x 10–5 |
|
|
|
1189 |
5.42 x 10–6 |
|
|
Au |
800 |
1.17 x 10–8 |
|
|
|
900 |
9 x 10–8 |
|
|
|
1020 |
5.4 x 10–7 |
|
|
Bi |
500 |
1.88 x 10–1 |
|
|
Cu |
970 |
5.04 x 10–6 |
|
|
Hg |
11 |
3 x10–2 |
|
|
Pb |
100 |
8.28 x 10–8 |
|
|
|
150 |
1.80 x 10–4 |
|
|
|
200 |
3.10 x 10–4 |
|
|
|
240 |
1.58 x 10–3 |
|
|
|
300 |
5.40 x 10–3 |
|
|
|
500 |
1.33 x 10–1 |
|
|
|
|
0.001e–25,000⁄RT |
|
|
Sn |
500 |
1.94 x 10–1 |
|
B |
Si |
|
10.5 e–85,000⁄RT |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 3 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Ba |
Hg |
7.8 |
2.17 x 10–2 |
|
Bi |
Si |
|
1030e–107,000⁄RT |
|
|
Pb |
220 |
1.73 x 10–7 |
|
|
|
250 |
1.33 x 10–6 |
|
|
|
285 |
1.58 x 10–6 |
|
C |
W |
1700 |
1.87 x 10–3 |
|
|
Fe |
930 |
7.51–9.18 x 10–9 |
|
Ca |
Hg |
10.2 |
2.25 x 10–2 |
|
Cd |
Ag |
650 |
9.36 x 10–7 |
|
|
|
800 |
4.68 x 10–6 |
|
|
|
900 |
2.23 x 10–5 |
|
|
Hg |
8.7 |
6.05 x 10–2 |
|
|
|
15 |
6.51 x 10–2 |
|
|
|
20 |
5.47 x 10–2 |
|
|
|
99.1 |
1.23 x 10–1 |
|
|
Pb |
200 |
4.59 x 10–7 |
|
|
|
252 |
3.10 x 10–6 |
|
Cd, 1 atom% |
Pb |
167 |
1.66 x 10–7 |
|
Ce |
W |
1727 |
3.42 x 10–6 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 4 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Cs |
Hg |
7.3 |
1.88 x 10–2 |
|
|
W |
27 |
4.32 x 10–3 |
|
|
|
227 |
5.40 x 10–4 |
|
|
|
427 |
2.88 x 10–2 |
|
|
|
540 |
1.44 x 10–1 |
|
Cu |
Al |
440 |
1.8 x 10–7 |
|
|
|
457 |
2.88 x 10–7 |
|
|
|
540 |
5.04 x 10–6 |
|
|
|
565 |
4.68–5.00 x 10–4 |
|
|
Ag |
650 |
1.04 x 10–6 |
|
|
|
760 |
1.30 x 10–6 |
|
|
|
895 |
3.38 x 10–6 |
|
|
Au |
301 |
5.40 x 10–10 |
|
|
|
443 |
8.64 x 10–9 |
|
|
|
560 |
3.38 x 10–7 |
|
|
|
604 |
5.10 x 10–7 |
|
|
|
616 |
7.92 x 10–7 |
|
|
|
740 |
3.35 x 10–6 |
|
|
Cu |
650 |
1.15 x 10–5 |
|
|
|
750 |
2.34 x 10–8 |
|
|
|
830 |
1 .44 x 10–7 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION |
OF METALS INTO METALS |
|||
|
(SHEET 5 OF 11) |
|
||
|
|
|
|
|
|
|
|
Diffusion |
Coefficient |
Diffusing |
Matrix |
|
Temperature |
|
|
(cm2 • hr–1) |
|||
Metal |
Metal |
|
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
Cu (Con’t) |
Cu (Con’t) |
|
850 |
9.36 x 10–7 |
|
|
|
950 |
2.30 x 10–6 |
|
|
|
1030 |
1.01 x 10–5 |
|
Ge |
|
700–900 |
1.01± 0.1 x 10–1 |
|
Pt |
|
1041 |
7.83–9 x 10–8 |
|
|
|
1213 |
5.04 x 10–7 |
|
|
|
1401 |
6.12 x 10–6 |
Fe |
Au |
|
753 |
1.94 x 10–6 |
|
|
|
1003 |
2.70 x 10–5 |
|
|
|
|
0.0062 e–20,000⁄RT |
Ga |
Si |
|
|
3.6 e–81,000⁄RT |
Ge |
Al |
|
630 |
3.31 x 10–1 |
|
Au |
|
529 |
1.84 x 10–1 |
|
|
|
563 |
2.80 x 10–1 |
|
Ge |
|
766–928 |
7.8 e–68,509⁄RT |
|
|
|
1060–1200•K |
87 e–73,000⁄RT |
Hg |
Cd |
|
156 |
9.36 x 10–7 |
|
|
|
176 |
2.55 x 10–6 |
|
|
|
202 |
9 x 10–6 |
|
Pb |
|
177 |
8.34 x 10–8 |
|
|
|
197 |
2.09 x 10–5 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 6 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
In |
Ag |
650 |
1.04 x 10–6 |
|
|
|
800 |
6.84 x 10–6 |
|
|
|
895 |
4.68 x 10–5 |
|
|
|
|
16.5 e–90,000⁄RT |
|
K |
Hg |
10.5 |
2.21 x 10–2 |
|
|
W |
207 |
2.05 x 10–2 |
|
|
|
317 |
3.6 x 10–1 |
|
|
|
507 |
1.1 x 10+1 |
|
Li |
Hg |
8.2 |
2.75 x 10–2 |
|
Mg |
Al |
365 |
3.96 x 10–8 |
|
|
|
395 |
1.98–2.41 x 10–7 |
|
|
|
420 |
2.38–2.74 x 10–7 |
|
|
|
440 |
1.19 x 10–7 |
|
|
|
447 |
9.36 x 10–7 |
|
|
|
450 |
6.84 x 10–6 |
|
|
|
500 |
3.96–7.56 x 10–6 |
|
|
|
577 |
1.58 x 10–5 |
|
|
Pb |
220 |
4.32 x 10–7 |
|
Mn |
Cu |
400 |
7.2 x 10–10 |
|
|
|
850 |
4.68 x 10–7 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 7 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Mo |
W |
1533 |
9.36 x 10–10 |
|
|
|
1770 |
4.32 x 10–9 |
|
|
|
2010 |
7.92 x 10–8 |
|
|
|
2260 |
2.81 x 10–7 |
|
Na |
W |
20 |
2.88 x 10–2 |
|
|
|
227 |
1.80 |
|
|
|
417 |
9.72 |
|
|
|
527 |
1.19 x 10–1 |
|
Ni |
Au |
800 |
2.77 x 10–6 |
|
|
|
1003 |
2.48 x 10–5 |
|
|
Cu |
550 |
2.56 x 10–9 |
|
|
|
950 |
7.56 x 10–7 |
|
|
|
320 |
1.26 x 10–6 |
|
|
Pt |
1043 |
1.81 x 10–8 |
|
|
|
1241 |
1.73 x 10–6 |
|
|
|
1401 |
5.40 x 10–6 |
|
Ni, 1 atom % |
Pb |
285 |
8.34 x 10–7 |
|
Ni, 3 atom% |
Pb |
252 |
1.25 x 10–7 |
|
Pb |
Cd |
252 |
2.88 x 10–8 |
|
|
Pb |
250 |
5.42 x 10–8 |
|
|
|
285 |
2.92 x 10–7 |
|
|
Sn |
500 |
1.33 x 10–1 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION |
OF METALS INTO METALS |
|||
|
(SHEET 8 OF 11) |
|
||
|
|
|
|
|
|
|
|
Diffusion |
Coefficient |
Diffusing |
Matrix |
|
Temperature |
|
|
(cm2 • hr–1) |
|||
Metal |
Metal |
|
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
Pb, 2 atom % |
Hg |
|
9.4 |
6.46 x 10–9 |
|
|
|
15.6 |
5.71 x 10–2 |
|
|
|
99.2 |
8 x 10–2 |
Pd |
Ag |
|
444 |
4.68 x 10–9 |
|
|
|
571 |
1.33 x 10–7 |
|
|
|
642 |
4.32 x 10–7 |
|
|
|
917 |
4.32 x 10–6 |
|
Au |
|
727 |
2.09 x 10–8 |
|
|
|
970 |
1.15 x 10–6 |
|
Cu |
|
490 |
3.24 x 10–9 |
|
|
|
950 |
9.0–10.44 x 10–7 |
Po |
Au |
|
470 |
4.59 x 10–11 |
|
Al |
|
20 |
1.08 x 10–9 |
|
|
|
500 |
1.80 x 10–7 |
|
Bi |
|
150 |
1.80 x 10–7 |
|
|
|
200 |
1.80 x 10–6 |
|
Pb |
|
150 |
4.59 x 10–11 |
|
|
|
200 |
4.59 x 10–9 |
|
|
|
310 |
5.41 x 10–7 |
Pt |
Au |
|
740 |
1.69 x 10–8 |
|
|
|
986 |
6.12–10.08 x 10–7 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 9 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Pt (Con’t) |
Cu |
490 |
2.01 x 10–9 |
|
|
|
960 |
3.96–8.28 x 10–7 |
|
|
Pb |
490 |
7.04 x 10–2 |
|
Ra |
Au |
470 |
1.42 x 10–8 |
|
|
Pt |
470 |
3.42 x 10–8 |
|
Ra(β+γ) |
Ag |
470 |
1.57 x 10–8 |
|
Rb |
Hg |
7.3 |
1.92 x 10–9 |
|
Rh |
Pb |
500 |
1.27 x 10–1 |
|
Sb |
Ag |
650 |
1.37 x 10–6 |
|
|
|
760 |
5.40 x 10–6 |
|
|
|
895 |
1.55 x 10–5 |
|
|
|
|
5.6 e–91,000⁄RT |
|
Si |
Al |
465 |
1.22 x 10–6 |
|
|
|
510 |
7.2 x 10–6 |
|
|
|
600 |
3.35 x 10–5 |
|
|
|
667 |
1.44 x 10–1 |
|
|
|
697 |
3.13 x 10–1 |
|
|
Fe+C* |
1400–1600 |
3.24–5.4 x 10–2 |
|
Sn |
Ag |
650 |
2.23 x 10–6 |
|
|
|
895 |
2.63 x 10–6 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 10 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Sn (Con’t) |
Cu |
400 |
1.69 x 10–9 |
|
|
|
650 |
2.48 x 10–7 |
|
|
|
850 |
1.40 x 10–5 |
|
|
Hg |
10.7 |
6.38 x 10–2 |
|
|
Pb |
245 |
1.12 x 10–7 |
|
|
|
250 |
1.83 x 10–7 |
|
|
|
285 |
5.76 x 10–7 |
|
Sr |
Hg |
9.4 |
1.96 x 10–2 |
|
Th |
Mo |
1615 |
1.30 x 10–6 |
|
|
|
2000 |
3.60 x 10–3 |
|
|
Tl |
285 |
8.76 x 10–7 |
|
|
W |
1782 |
3.96 x 10–7 |
|
|
|
2027 |
4.03 x 10–6 |
|
|
|
2127 |
1 .29 x 10–5 |
|
|
|
2227 |
2.45 x 10–5 |
|
Th (β) |
Pb |
165 |
2.54 x 10–12 |
|
|
|
260 |
2.54 x 10–8 |
|
|
|
324 |
5.84 x 10–6 |
|
Tl |
Hg |
11.5 |
3.63 x 10–2 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
©2001 CRC Press LLC

Table 85. DIFFUSION OF METALS INTO METALS
(SHEET 11 OF 11)
|
|
Diffusion |
Coefficient |
|
Diffusing |
Matrix |
Temperature |
||
(cm2 • hr–1) |
||||
Metal |
Metal |
(˚C) |
||
|
|
|
|
|
|
|
|
|
|
Tl (Con’t) |
Pb |
220 |
1.01 x 10–7 |
|
|
|
250 |
7.92 x 10–7 |
|
|
|
270 |
3.96 x 10–7 |
|
|
|
285 |
1.12 x 10–6 |
|
|
|
315 |
2.09 x 10–6 |
|
|
|
|
16.5 e–85,000⁄RT |
|
U |
W |
1727 |
4.68 x 10–8 |
|
Y |
W |
1727 |
6.55 x 10–5 |
|
Zn |
Ag |
750 |
1.66 x 10–5 |
|
|
|
850 |
4.37 x 10–5 |
|
|
Al |
415 |
9 x 10–7 |
|
|
|
473 |
1.91 x 10–6 |
|
|
|
500 |
7.2–13.68 x 10–6 |
|
|
|
555 |
1.8 x 10–5 |
|
|
Hg |
11.5 |
9.09 x 10–2 |
|
|
|
15 |
8.72 x 10–2 |
|
|
|
99.2 |
1.20 x 10–1 |
|
|
Pb |
285 |
5.84 |
|
Zr |
W |
1727 |
1.17 x 10–5 |
|
|
|
|
|
For diffusion coefficients in m2/s, multiply values in cm2/hr by 2.778 x 10–8.
Source: data from Loebel, R., in Handbook of Chemistry and Physics, 51st ed., Weast, R. C., Ed., Chemical Rubber, Cleveland, 1970, F-55.
*Saturated FeC Alloy.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 1 OF 8)
|
|
|
|
Temperature |
|
|
|
Do |
|
Range |
|
|
Diffusing |
E |
of Validity |
||
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminum antimonide |
Al |
|
~1.8 |
|
|
(AlSb) |
|
|
|||
|
|
|
|
||
|
Cu |
3.5x10–3 |
0.36 |
150–500 |
|
|
Sb |
|
~1.5 |
|
|
|
Zn |
0.33±.15 |
1.93±0.04 |
660–860 |
|
Cadmium selenide |
Se |
2.6x10–3 |
1.55 |
700–1800 |
|
(CdSe) |
|||||
|
|
|
|
||
Cadmium sulfide (CdS) |
Ag |
2.5x10+1 |
1.2 |
250–500 |
|
|
Cd |
3.4 |
2.0 |
750–1000 |
|
|
Cu |
1.5x10–3 |
0.76 |
450–750 |
|
Cadmium telluride |
Au |
6.7x10+1 |
2.0 |
600–1000 |
|
(CdTe) |
|||||
|
|
|
|
||
|
In |
4.1x10–1 |
1.6 |
450–1000 |
|
Calcium ferrate (III) |
Ca |
30 |
3.7 |
|
|
(CaFe2O4) |
|
||||
|
|
|
|
||
|
Fe |
0.4 |
3.1 |
|
|
α-Calcium metasilicate |
Ca |
7.4x10+4 |
4.8 |
|
|
(CaSiO3) |
|
||||
|
|
|
|
||
Gallium antimonide |
Ga |
3.2x10+3 |
3.15 |
650–700 |
|
(GaSb) |
|||||
|
|
|
|
||
|
In |
1.2x10–7 |
0.53 |
400–650 |
|
|
Sb |
3.4x10+4 |
3.44 |
650–700 |
|
|
|
8.7x10+2 |
1.13 |
470–570 |
|
|
Sn |
2.4x10–5 |
0.80 |
320–570 |
|
|
Te |
3.8x10–4 |
1.2 |
400–650 |
|
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 2 OF 8)
|
|
|
|
Temperature |
|
|
Do |
|
Range |
|
Diffusing |
E |
of Validity |
|
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
Gallium arsenide |
Ag |
2.5x10–3 |
1.5 |
|
(GaAs) |
|
|||
|
|
|
|
|
|
|
4x10–4 |
0.8±0.05 |
500–1160 |
|
As |
4x1021 |
10.2±1.2 |
1200–1250 |
|
Au |
10–3 |
1.0±0.2 |
740–1024 |
|
Cd |
0.05±0.04 |
2.43±0.06 |
868–1149 |
|
|
b50x10–2 |
2.8a |
|
|
Cu |
0.03 |
0.52 |
100–600 |
|
Ga |
1x10+7 |
5.60±0.32 |
1125–1250 |
|
Li |
0.53 |
1.0 |
250–400 |
|
Mg |
1.4x10–4 |
1.89 |
|
|
|
2.3x10–2 |
2.6 |
740–1024 |
|
|
b2.6x10–2 |
2.7a |
|
|
|
b6.5x10–1 |
2.49a |
|
|
|
8.5x10–3 |
1.7 |
740–1024 |
|
S |
1.2x10–4 |
1.8 |
|
|
|
b1.6x10–5 |
1.63a |
|
|
|
2.6x10–5 |
1.86 |
|
|
|
4x103 |
4.04±0.15 |
1000–1200 |
|
Se |
3x103 |
4.16±0.16 |
1000–1200 |
|
Sn |
b3.8x10–2 |
2.7 |
|
|
|
6x10–4 |
2.5 |
1069–1215 |
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 3 OF 8)
|
|
|
|
Temperature |
|
|
|
Do |
|
Range |
|
|
Diffusing |
E |
of Validity |
||
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Gallium arsenide |
Zn |
b2.5x10–1 |
3.0a |
|
|
(GaAs) (Con’t) |
|
||||
|
|
|
|
||
|
|
3.0x10–7 |
1.0 |
|
|
|
|
6.0x10–7 |
0.6 |
|
|
|
|
15±7 |
2.49±0.05 |
800 |
|
Gallium phosphide |
Zn |
1.0 |
2.1 |
700–1300 |
|
(GaP) |
|||||
|
|
|
|
||
Germanium (Ge) |
Ag |
4.4x10–2 |
1.0 |
700–900 |
|
|
As |
6.3 |
2.4 |
600–850 |
|
|
Au |
2.2x10–2 |
2.5 |
|
|
|
B |
1.6x10–9 |
4.6 |
600–850 |
|
|
Cu |
1.9x10–4 |
0.18 |
600–850 |
|
|
Fe |
1.3x10–1 |
1.1 |
750–850 |
|
|
Ga |
4.0x10+1 |
3.1 |
600–850 |
|
|
Ge |
8.7x10+1 |
3.2 |
750–920 |
|
|
He |
6.1x10–3 |
0.69 |
750–850 |
|
|
In |
3x10–2 |
2.4 |
600–850 |
|
|
Li |
1.3x10-4 |
0.47 |
200–600 |
|
|
Ni |
8x10–1 |
0.9 |
700–875 |
|
|
P |
2.5 |
2.5 |
600–850 |
|
|
Pb |
– |
3.6 |
600–850 |
|
|
Sb |
4.0 |
2.4 |
600–850 |
|
|
Sn |
1.7x10–2 |
1.9 |
600–850 |
|
|
Zn |
1.0x10+1 |
2.5 |
600–850 |
|
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 4 OF 8)
|
|
|
|
Temperature |
|
|
Do |
|
Range |
|
Diffusing |
E |
of Validity |
|
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
Indium antimonide |
Ag |
1.0x10–7 |
0.25 |
|
(InSb) |
|
|||
|
|
|
|
|
|
Au |
b7x10–4 |
0.32a |
140–510 |
|
Cd |
b1.0x10–5 |
1.1a |
250–500 |
|
|
1.23x10–9 |
0.52 |
442–519 |
|
|
1.26 |
1.75 |
|
|
|
1.3x10–4 |
1.2 |
360–500 |
|
Co |
2.7x10–11 |
0.39 |
|
|
|
10–7 |
0.25 |
440–510 |
|
Cu |
3.0x10–5 |
0.37 |
|
|
|
b9.0x10–4 |
1.08a |
|
|
Fe |
10–7 |
0.25 |
440–510 |
|
Hb |
b4.0x10–6 |
1.17a |
|
|
In |
0.05 |
1.81 |
450–500 |
|
|
1.8x10–9 |
0.28 |
|
|
Ni |
10–7 |
0.25 |
440–510 |
|
Sb |
0.05 |
1.94 |
450–500 |
|
|
1.4x10–6 |
0.75 |
|
|
Sn |
5.5x10–8 |
0.75 |
390–512 |
|
Te |
1.7x10–7 |
0.57 |
300–500 |
|
Zn |
0.5 |
1.35 |
360–500 |
|
|
1.6x10–6 |
2.3±0.3 |
360–500 |
|
|
5.5 |
1.6 |
360–500 |
|
(Polycrystal) |
1.7x10–7 |
0.85 |
390–512 |
|
|
b5.3x10+7 |
2.61 |
|
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 5 OF 8)
|
|
|
|
Temperature |
|
|
|
Do |
|
Range |
|
|
Diffusing |
E |
of Validity |
||
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Indium antimonide |
Zinc (con’t) |
6.3x10+8 |
|
|
|
(High |
2.61 |
|
|||
(InSb) (Con’t) |
|
||||
concentration) |
|
|
|
||
|
|
|
|
||
|
|
b3.7x10–10 |
0.7a |
|
|
|
(Conc. = 2.2 x |
9.0x10–10 |
~0 |
|
|
|
1020 cm–3) |
|
|||
|
(Single crystal) |
1.4x10–7 |
0.86 |
390–512 |
|
Indium arsenide (InAs) |
Cd |
4.35x10–4 |
1.17 |
600–900 |
|
|
Cu |
|
0.52a |
|
|
|
Ge |
3.74x10–6 |
1.17 |
600–900 |
|
|
Mg |
1.98x10–6 |
1.17 |
600–900 |
|
|
S |
6.78 |
2.20 |
600–900 |
|
|
Se |
12.55 |
2.20 |
600–900 |
|
|
Sn |
1.49x10–6 |
1.17 |
600–900 |
|
|
Te |
3.43x10–5 |
1.28 |
600–900 |
|
|
Zn |
3.11x10–3 |
1.17 |
600–900 |
|
Indium phosphide |
In |
1x10+5 |
3.85 |
850–1000 |
|
(InP) |
|||||
|
|
|
|
||
|
p |
7x10+10 |
5.65 |
850–1000 |
|
Iron oxide (Fe3O4) |
Fe |
5.2 |
2.4 |
|
|
Lead metasilicate |
Pb |
85 |
2.6 |
|
|
(PbSiO3) |
|
||||
|
|
|
|
||
Lead orthosilicate |
Pb |
8.2 |
2.0 |
|
|
(PbSiO4) |
|
||||
|
|
|
|
||
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 6 OF 8)
|
|
|
|
Temperature |
|
|
|
Do |
|
Range |
|
|
Diffusing |
E |
of Validity |
||
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Mercury selenide |
Sb |
6.3x10–5 |
0.85 |
540–630 |
|
(HgSe) |
|||||
|
|
|
|
||
Nickel aluminate |
Cr |
1.17x10–3 |
2.2 |
|
|
(NiAl2O4) |
|
||||
|
|
|
|
||
|
Fe |
1.33 |
3.5 |
|
|
Nickel chromate (III) |
Cr |
0.74 |
3.1 |
|
|
(NiCr2O4) |
|
||||
|
|
|
|
||
|
Cr |
2.03x10–5 |
1.9 |
|
|
|
Fe |
1.35x10–3 |
2.6 |
|
|
|
Ni |
0.85 |
3.2 |
|
|
Selenium (Se) |
Fe |
1.1x10–5 |
0.38 |
300–400 |
|
(amorphous) |
|||||
|
|
|
|
||
|
Ge |
9.4x10–6 |
0.39 |
300–400 |
|
|
In |
5.2x10–6 |
0.32 |
300–400 |
|
|
Sb |
2.8x10–8 |
0.29 |
300–400 |
|
|
Se |
7.6x10–10 |
0.14 |
300–400 |
|
|
Sn |
4.8x10–8 |
0.39 |
300–400 |
|
|
Te |
5.4x10–6 |
0.53 |
300–400 |
|
|
Tl |
1.4x10–6 |
0.35 |
300–400 |
|
|
Zn |
3.8x10–7 |
0.29 |
300–400 |
|
Silicon (Si) |
Al |
8.0 |
3.5 |
1100–1400 |
|
|
Ag |
2x10–3 |
1.6 |
1100–1350 |
|
|
As |
3.2x10–1 |
3.5 |
1100–1350 |
|
|
Au |
1.1x10–3 |
1.1 |
800–1200 |
|
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 7 OF 8)
|
|
|
|
Temperature |
|
|
Do |
|
Range |
|
Diffusing |
E |
of Validity |
|
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
Silicon (Si) (Con’t) |
B |
1.0x10+1 |
3.7 |
950–1200 |
|
Bi |
1.04x10+3 |
4.6 |
1100–1350 |
|
Cu |
4x10–1 |
1.0 |
800–1100 |
|
Fe |
6.2x10–3 |
0.86 |
1000–1200 |
|
Ga |
3.6 |
3.5 |
1150–1350 |
|
Hl |
9.4x10–3 |
0.47 |
1000–1200 |
|
He |
1.1x10–1 |
0.86 |
1000–1200 |
|
In |
1.65x10+1 |
3.9 |
1100–1350 |
|
Li |
9.4x10–3 |
0.78 |
100–800 |
|
P |
1.0x10+1 |
3.7 |
1100–1350 |
|
Sb |
5.6 |
3.9 |
1100–1350 |
|
Tl |
1.65x10+1 |
3.9 |
1100–1350 |
Silicon carbide (SiC) |
Al |
2.0x10–1 |
4.9 |
1800–2250 |
|
B |
1.6x10+1 |
5.6 |
1850–2250 |
|
Cr |
2.3x10–1 |
4.8 |
1700–1900 |
Sulfur (S) |
S |
2.8x10+13 |
2.0 |
>100 |
Tin zinc oxide |
Sn |
2x10+5 |
4.7 |
|
(SnZn2O4) |
|
|||
|
|
|
|
|
|
Zn |
37 |
3.3 |
|
Zinc aluminate |
Zn |
2.5x10+2 |
3.4 |
|
(ZnAl2O4) |
|
|||
|
|
|
|
|
Zinc chromate (III) |
Cr |
8.5 |
3.5 |
|
(ZnCr2O4) |
|
|||
|
|
|
|
|
|
Zn |
60 |
3.7 |
|
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
©2001 CRC Press LLC

Table 86. DIFFUSION IN SEMICONDUCTORS*
(SHEET 8 OF 8)
|
|
|
|
Temperature |
|
|
Do |
|
Range |
|
Diffusing |
E |
of Validity |
|
Semiconductor |
Element |
(cm2 • s–1) |
(eV) |
(˚C) |
|
|
|
|
|
|
|
|
|
|
Zinc ferrate (III) |
Fe |
8.5x10+2 |
3.5 |
|
(ZnFe2O4) |
|
|||
|
|
|
|
|
|
Zn |
8.8x10+2 |
3.7 |
|
Zinc selenide (ZnSe) |
Cu |
1.7x10–5 |
0.56 |
200–570 |
Zinc sulfide (ZnS) |
Zn |
1.0x10+16 |
6.50 |
>1030 |
|
|
1.5x10+4 |
3.25 |
940–1030 |
|
|
3.0x10–4 |
1.52 |
<940 |
|
|
|
|
|
Source: from Bolz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 251.
*The diffusion coefficient D at a temperature T(K) is given by the following: D=Doe- E/kT
For Do in m2/s, multiply values in cm2/s by 10–4.
a |
Values obtained at the low concentration limit. |
|
©2001 CRC Press LLC
Shackelford, James F. & Alexander, W. “Thermal Properties of Materials”
Materials Science and Engineering Handbook
Ed. James F. Shackelford and W. Alexander Boca Raton: CRC Press LLC, 2001

CHAPTER 5
Thermal Properties
of Materials
List of Tables |
Specific Heat & Heat Capacity |
|
Specific Heat of the Elements at 25 ˚C |
|
Heat Capacity of Ceramics |
|
Specific Heat of Polymers |
|
Specific Heat of Fiberglass Reinforced Plastics |
Thermal Conductivity
Thermal Conductivity of Metals (Part 1)
Thermal Conductivity of Metals (Part 2)
Thermal Conductivity of Metals (Part 3)
Thermal Conductivity of Metals (Part 4)
Thermal Conductivity of Alloy Cast Irons
Thermal Conductivity of Iron and Iron Alloys
Thermal Conductivity of Aluminum and aluminum alloys
Thermal Conductivity of Copper and Copper Alloys
Thermal Conductivity of Magnesium
and Magnesium Alloys
Thermal Conductivity of Nickel and Nickel Alloys
Thermal Conductivity of Lead and Lead Alloys
Thermal Conductivity of Tin, Titanium, Zinc and their Alloys
Thermal Conductivity of Pure Metals
(Continued)
©2001 CRC Press LLC
List of Tables
(Continued)
Thermal Conductivity of Ceramics
Thermal Conductivity of Glasses
Thermal Conductivity of Cryogenic Insulation
Thermal Conductivity of Cryogenic Supports
Thermal Conductivity of Special Concretes
Thermal Conductivity of SiC-Whisker-Reinforced Ceramics
Thermal Conductivity of Polymers
Thermal Conductivity of Fiberglass Reinforced Plastics
Thermal Expansion
Thermal Expansion of Wrought Stainless Steels Thermal Expansion of Wrought Titanium Alloys Thermal Expansion of Graphite Magnesium Castings Linear Thermal Expansion of Metals and Alloys Thermal Expansion of Ceramics
Thermal Expansion of SiC-Whisker-Reinforced Ceramics
Thermal Expansion of Glasses
Thermal Expansion of Polymers
Thermal Expansion Coefficients of
Materials for Integrated Circuits
Thermal Expansion of Silicon Carbide SCS–2–Al
Tempering & Softening
ASTM B 601 Temper Designation Codes for
Copper and Copper Alloys
Temper Designation System for Aluminum Alloys Tool Steel Softening After 100 Hours
Thermoplastic Polyester Softening with Temperature
Heat-Deflection Temperature of Carbonand
Glass-Reinforced Engineering Thermoplastics
©2001 CRC Press LLC

Table 87. SPECIFIC HEAT OF THE ELEMENTS AT 25 ˚C
(SHEET 1 OF 4) |
|
|
|
|
|
|
|
Cp |
Element |
|
(cal • g-l • K–1) |
|
|
|
|
|
|
Aluminum |
|
0.215 |
Antimony |
|
0.049 |
Argon |
|
0.124 |
Arsenic |
|
0.0785 |
Barium |
|
0.046 |
Beryllium |
|
0.436 |
Bismuth |
|
0.0296 |
Boron |
|
0.245 |
Bromine (Br2) |
|
0.113 |
Cadmium |
|
0.0555 |
Calcium |
|
0.156 |
Carbon, diamond |
|
0.124 |
Carbon, graphite |
|
0.170 |
Cerium |
|
0.049 |
Cesium |
|
0.057 |
Chlorine (Cl2) |
|
0.114 |
Chromium |
|
0.107 |
Cobalt |
|
0.109 |
Columbium (see Niobium) |
|
|
Copper |
|
0.092 |
Dysprosium |
|
0.0414 |
Erbium |
|
0.0401 |
Europium |
|
0.0421 |
Fluorine (F2) |
|
0.197 |
Gadolinium |
|
0.055 |
Gallium |
|
0.089 |
Germanium |
|
0.077 |
Gold |
|
0.0308 |
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-144., Kelly, K. K., Bulletin 592, Bureau of Mines, Washington, D. C., 1961.and Hultgren, R., Orr, R L., Anderson, P. D., and Kelly, K. K., Selected Values of Thermodynamic Properties of Metals and Alloys, John Wiley & Sons, New York, (1963).
©2001 CRC Press LLC

Table 87. SPECIFIC HEAT OF THE ELEMENTS AT 25 ˚C
|
(SHEET 2 OF 4) |
|
|
|
|
|
|
Cp |
Element |
|
(cal • g-l • K–1) |
|
|
|
|
|
|
Hafnium |
|
0.035 |
Helium |
|
1.24 |
Hollnium |
|
0.0393 |
Hydrogen (H2) |
|
3.41 |
Indium |
|
0.056 |
lodine (I2) |
|
0.102 |
Iridium |
|
0.0317 |
Iron (α) |
|
0.106 |
Krypton |
|
0.059 |
Lanthanum |
|
0.047 |
Lead |
|
0.038 |
Lithium |
|
0.85 |
Lutetium |
|
0.037 |
Magnesium |
|
0.243 |
Manganese, α |
|
0.114 |
Manganese, β |
|
1.119 |
Mercury |
|
0.0331 |
Molybdenum |
|
0.599 |
Neodymium |
|
0.049 |
Neon |
|
0.246 |
Nickel |
|
0.106 |
Niobium |
|
0.064 |
Nitrogen (N2) |
|
0.249 |
Osmium |
|
0.03127 |
Oxygen (O2) |
|
0.219 |
Palladium |
|
0.0584 |
Phosphorus, white |
|
0.181 |
Phosphorus, red, triclinic |
|
0.160 |
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-144., Kelly, K. K., Bulletin 592, Bureau of Mines, Washington, D. C., 1961.and Hultgren, R., Orr, R L., Anderson, P. D., and Kelly, K. K., Selected Values of Thermodynamic Properties of Metals and Alloys, John Wiley & Sons, New York, (1963).
©2001 CRC Press LLC

Table 87. SPECIFIC HEAT OF THE ELEMENTS AT 25 ˚C
|
(SHEET 3 OF 4) |
|
|
|
|
|
|
Cp |
Element |
|
(cal • g-l • K–1) |
|
|
|
|
|
|
Platinum |
|
0.0317 |
Polonium |
|
0.030 |
Potassium |
|
0.180 |
Praseodymium |
|
0.046 |
Promethium |
|
0.0442 |
Protactinium |
|
0.029 |
Radium |
|
0.0288 |
Radon |
|
0.0224 |
Rhenium |
|
0.0329 |
Rhodium |
|
0.0583 |
Rubidium |
|
0.0861 |
Ruthenium |
|
0.057 |
Samarium |
|
0.043 |
Scandium |
|
0.133 |
Selenium (Se2) |
|
0.0767 |
Silicon |
|
0.168 |
Silver |
|
0.0566 |
Sodium |
|
0.293 |
Strontium |
|
0.0719 |
Sulfur, yellow |
|
0.175 |
Tantalum |
|
0.0334 |
Technetium |
|
0.058 |
Tellurium |
|
0.0481 |
Terbium |
|
0.0437 |
Thallium |
|
0.0307 |
Thorium |
|
0.0271 |
Thulium |
|
0.0382 |
Tin (α) |
|
0.0510 |
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-144., Kelly, K. K., Bulletin 592, Bureau of Mines, Washington, D. C., 1961.and Hultgren, R., Orr, R L., Anderson, P. D., and Kelly, K. K., Selected Values of Thermodynamic Properties of Metals and Alloys, John Wiley & Sons, New York, (1963).
©2001 CRC Press LLC

Table 87. SPECIFIC HEAT OF THE ELEMENTS AT 25 ˚C
|
(SHEET 4 OF 4) |
|
|
|
|
|
|
Cp |
Element |
|
(cal • g-l • K–1) |
|
|
|
|
|
|
Tin (β) |
|
0.0530 |
Titanium |
|
0.125 |
Tungsten |
|
0.0317 |
Uranium |
|
0.0276 |
Vanadium |
|
0.116 |
Xenon |
|
0.0378 |
Ytterbium |
|
0.0346 |
Yttrium |
|
0.068 |
Zinc |
|
0.0928 |
Zirconium |
|
0.0671 |
|
|
|
Source: data from Weast, R. C., Ed., Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, 1974, D-144., Kelly, K. K., Bulletin 592, Bureau of Mines, Washington, D. C., 1961.and Hultgren, R., Orr, R L., Anderson, P. D., and Kelly, K. K., Selected Values of Thermodynamic Properties of Metals and Alloys, John Wiley & Sons, New York, (1963).
©2001 CRC Press LLC

Table 88. HEAT CAPACITY OF CERAMICS
(SHEET 1 OF 2)
|
|
Heat Capacity, Cp |
|
|
(cal/mole/K) |
Class |
Ceramic |
|
|
|
|
|
|
|
Borides |
Chromium Diboride (CrB2) |
9.61 + 10.72x10-3T cal/mole |
|
|
at 494-1010K |
|
Hafnium Diboride (HfB2) |
9.61 + 10.72x10-3T cal/mole |
|
|
at 494-1010K |
|
Tantalum Diboride (TaB2) |
0.04 cal/g˚C |
|
Titanium Diboride (TiB2) |
10.93 + 7.08x10-3T cal/mole |
|
|
at 420-1180 K |
|
Zirconium Diboride (ZrB2) |
15.81T + 4.20x10-3T - 3.52x105T–2 |
|
|
for 429-1171K |
Carbides |
Hafnium Monocarbide (HfC) |
0.05 at room temp. |
|
|
15 ± 0.15 at 925˚C |
|
|
16 ± 0.16 at 1525˚C |
|
Silicon Carbide (SiC) |
0.26 at 540˚C |
|
|
0.27 at 700˚C |
|
|
0.30 at 1000˚C |
|
|
0.32 at 1200˚C |
|
|
0.33 at 1350˚C |
|
|
0.35 at 1550˚C |
|
Titanium MonoCarbide (TiC) |
0.150-0.170 cal/g at 150˚C |
|
|
0.170-0.187 cal/g at 300˚C |
|
|
0.183-0.196 cal/g at 450˚C |
|
|
0.192-0.201 cal/g at 600˚C |
|
|
0.20-0.207 cal/g at 750˚C |
|
|
0.209 cal/g at 900˚C |
|
|
0.210 cal/g at 1000˚C |
|
|
0.211 cal/g at 1100˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); SmithellsBrandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 88. HEAT CAPACITY OF CERAMICS
|
(SHEET 2 OF 2) |
|
|
|
|
|
|
Heat Capacity, Cp |
|
|
(cal/mole/K) |
Class |
Ceramic |
|
|
|
|
|
|
|
Nitrides |
Aluminum Nitride (AlN) |
0.1961 cal/g/˚C ; 0-100˚C |
|
|
0.2277 cal/g/˚C ; 0-420˚C |
|
|
0.2399 cal/g/˚C ; 0-598˚C |
|
Trisilicon tetranitride (Si3N4) |
0.17 cal/g/˚C |
Oxides |
Cerium Dioxide (CeO2) |
14.24T + 5.62x10-3T 491-1140K |
Silicides |
Molybdenum Disilicide (MoSi2) |
10-14 cal/g/˚C; 425-1000˚C |
|
Tungsten Disilicide (WSi2) |
8 cal/g/˚C; 425-1450˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); SmithellsBrandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 89. SPECIFIC HEAT OF POLYMERS
(SHEET 1 OF 4)
|
|
Specific heat |
Polymer Class |
Polymer Subclass |
(Btu/lb/°F) |
|
|
|
|
|
|
ABS Resins; Molded, Extruded |
Medium impact |
0.36—0.38 |
|
High impact |
0.36—0.38 |
|
Very high impact |
0.36—0.38 |
|
Low temperature impact |
0.35—0.38 |
|
Heat resistant |
0.37—0.39 |
Acrylics; Cast, Molded, Extruded |
Cast Resin Sheets, Rods: |
|
|
General purpose, type I |
0.35 |
|
General purpose, type II |
0.35 |
|
Moldings: |
|
|
Grades 5, 6, 8 |
0.35 |
|
High impact grade |
0.34 |
Thermoset Carbonate |
Allyl diglycol carbonate |
0.3 |
Cellulose Acetate; Molded, |
ASTM Grade: |
|
Extruded |
|
|
|
|
|
|
H6—1 |
0.3—0.42 |
|
H4—1 |
0.3—0.42 |
|
H2—1 |
0.3—0.42 |
|
MH—1, MH—2 |
0.3—0.42 |
|
MS—1, MS—2 |
0.3—0.42 |
|
S2—1 |
0.3—0.42 |
Cellulose Acetate Butyrate; |
ASTM Grade: |
|
Molded, Extruded |
|
|
|
|
|
|
H4 |
0.3—0.4 |
|
MH |
0.3—0.4 |
|
S2 |
0.3—0.4 |
Cellusose Acetate Propionate; |
ASTM Grade: |
|
Molded, Extruded |
|
|
|
|
|
|
1 |
0.3—0.4 |
|
3 |
0.3—0.4 |
|
6 |
0.3—0.4 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 89. SPECIFIC HEAT OF POLYMERS
(SHEET 2 OF 4)
|
|
Specific heat |
|
Polymer Class |
Polymer Subclass |
(Btu/lb/°F) |
|
|
|
|
|
|
|
|
|
Chlorinated polyvinyl chloride |
Chlorinated polyvinyl chloride |
0.3 |
|
Polycarbonate |
|
0.3 |
|
Fluorocarbons; Molded,Extruded |
Polytrifluoro chloroethylene |
0.22 |
|
(PTFCE) |
|||
|
|
||
|
Polytetrafluoroethylene (PTFE) |
0.25 |
|
|
Fluorinated ethylene |
0.28 |
|
|
propylene(FEP) |
||
|
|
||
|
Polyvinylidene— fluoride |
0.33 |
|
|
(PVDF) |
||
|
|
||
Epoxies; Cast, Molded, Reinforced |
Standard epoxies (diglycidyl |
|
|
|
ethers of bisphenol A) |
|
|
|
Cast rigid |
0.4-0.5 |
|
|
High strength laminate |
0.21 |
|
|
Filament wound composite |
0.24 |
|
Nylons; Molded, Extruded |
Type 6 |
|
|
|
General purpose |
0.4 |
|
|
Cast |
0.4 |
|
|
Type 8 |
0.4 |
|
|
Type 11 |
0.58 |
|
|
Type 12 |
0.28 |
|
Nylons; Molded, Extruded |
6/6 Nylon |
|
|
|
General purpose molding |
0.3—0.5 |
|
|
General purpose extrusion |
0.3—0.5 |
|
|
6/10 Nylon |
|
|
|
General purpose |
0.3—0.5 |
|
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 89. SPECIFIC HEAT OF POLYMERS
(SHEET 3 OF 4)
|
|
Specific heat |
Polymer Class |
Polymer Subclass |
(Btu/lb/°F) |
|
|
|
|
|
|
Phenolics; Molded |
Type and filler: |
|
|
General: woodflour and flock |
0.35—0.40 |
|
Shock: paper, flock, or pulp |
— |
|
High shock: chopped fabric or |
0.30—0.35 |
|
cord |
|
|
|
|
|
Very high shock: glass fiber |
0.28—0.32 |
Phenolics: Molded |
Arc resistant—mineral |
0.27—0.37 |
|
Rubber phenolic—woodflour or |
0.33 |
|
flock |
|
|
|
|
|
PVC—Acrylic Alloy |
|
|
PVC—acrylic sheet |
0.293 |
Polymides |
Unreinforced |
0.31 |
|
Unreinforced 2nd value |
0.25—0.35 |
|
Glass reinforced |
0.15—0.27 |
Polyacetals |
Standard |
0.35 |
|
Copolymer: |
|
|
Standard |
0.35 |
|
High flow |
0.35 |
Polyesters: Thermosets |
Cast polyyester |
|
|
Rigid |
0.30—0.55 |
|
Reinforced polyester moldings |
|
|
High strength (glass fibers) |
0.25—0.35 |
|
Sheet molding compounds, |
0.20—0.25 |
|
general purpose |
|
|
|
|
Phenylene oxides (Noryl) |
Standard |
0.24 |
Polypropylene: |
General purpose |
0.45 |
|
High impact |
0.45—0.48 |
Polyphenylene sulfide: |
Standard |
0.26 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 89. SPECIFIC HEAT OF POLYMERS
(SHEET 4 OF 4)
|
|
Specific heat |
|
Polymer Class |
Polymer Subclass |
(Btu/lb/°F) |
|
|
|
|
|
|
|
|
|
Polyethylenes; Molded, Extruded |
Type I—lower density (0.910— |
|
|
0.925) |
|
||
|
|
||
|
Melt index 0.3—3.6 |
0.53—0.55 |
|
|
Melt index 6—26 |
0.53—0.55 |
|
|
Melt index 200 |
0.53—0.55 |
|
|
Type II—medium density |
|
|
|
(0.926—0.940) |
|
|
|
Melt index 20 |
0.53—0.55 |
|
|
Melt index l.0—1.9 |
0.53—0.55 |
|
|
Type III—higher density (0.941— |
|
|
|
0.965) |
|
|
|
Melt index 0.2—0.9 |
0.46—0.55 |
|
|
Melt Melt index 0.l—12.0 |
0.46—0.55 |
|
|
Melt index 1.5—15 |
0.46—0.55 |
|
Polystyrenes; Molded |
Polystyrenes |
|
|
|
General purpose |
0.30—0.35 |
|
|
Medium impact |
0.30—0.35 |
|
|
High impact |
0.30—0.35 |
|
|
Glass fiber -30% reinforced |
0.256 |
|
|
Styrene acrylonitrile (SAN) |
0.33 |
|
Polyvinyl Chloride And |
Vinylidene chloride |
0.32 |
|
Copolymers; Molded, Extruded |
|||
|
|
||
Silicones; Molded, Laminated |
Woven glass fabric/ silicone |
0.246 |
|
laminate |
|||
|
|
||
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 90. SPECIFIC HEAT OF
FIBERGLASS REINFORCED PLASTICS
|
|
Glass |
Specific |
|
|
|
fiber content |
heat |
|
Class |
Material |
(wt%) |
(Btu/lb–˚F) |
|
|
|
|
|
|
|
|
|
|
|
Glass fiber reinforced |
Sheet molding compound (SMC) |
15 to 30 |
0.30 to 0.35 |
|
thermosets |
||||
|
|
|
||
|
Bulk molding compound(BMC) |
15 to 35 |
0.30 to 0.35 |
|
|
Preform/mat(compression molded) |
25 to 50 |
0.30 to 0.33 |
|
|
Cold press molding–polyester |
20 to 30 |
0.30 to 0.33 |
|
|
Spray–up–polyester |
30 to 50 |
0.30 to 0.34 |
|
|
Filament wound–epoxy |
30 to 80 |
0.23 to 0.25 |
|
|
Rod stock–polyester |
40 to 80 |
0.22 to 0.25 |
|
|
Molding compound–phenolic |
5 to 25 |
0.20 to 0.30 |
|
Glass–fiber–reinforced |
Nylon |
6 to 60 |
0.30 to 0.35 |
|
thermoplastics |
||||
|
|
|
||
|
Polystyrene |
20 to 35 |
0.23 to 0.35 |
|
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p106, (1994).
©2001 CRC Press LLC

Table 91. THERMAL CONDUCTIVITY OF METALS (PART 1)
(SHEET 1 OF 2)
T (K) |
Aluminum |
Cadmium |
Chromium |
Copper |
Gold |
|
|
|
|
|
|
1 |
7.8 |
48.7 |
0.401 |
28.7 |
4.4 |
2 |
15.5 |
89.3 |
0.802 |
57.3 |
8.9 |
3 |
23.2 |
104 |
1.20 |
85.5 |
13.1 |
4 |
30.8 |
92.0 |
1.60 |
113 |
17.1 |
5 |
38.1 |
69.0 |
1.99 |
138 |
20.7 |
6 |
45.1 |
44.2 |
2.38 |
159 |
23.7 |
7 |
51.5 |
28.0 |
2.77 |
177 |
26.0 |
8 |
57.3 |
18.0 |
3.14 |
189 |
27.5 |
9 |
62.2 |
12.2 |
3.50 |
195 |
28.2 |
10 |
66.1 |
8.87 |
3.85 |
196 |
28.2 |
11 |
69.0 |
6.91 |
4.18 |
193 |
27.7 |
12 |
70.8 |
5.56 |
4.49 |
185 |
26.7 |
13 |
71.5 |
4.67 |
4.78 |
176 |
25.5 |
14 |
71.3 |
4.01 |
5.04 |
166 |
24.1 |
15 |
70.2 |
3.55 |
5.27 |
156 |
22.6 |
16 |
68.4 |
3.16 |
5.48 |
145 |
20.9 |
18 |
63.5 |
2.62 |
5.81 |
124 |
17.7 |
20 |
56.5 |
2.26 |
6.01 |
105 |
15.0 |
25 |
40.0 |
1.79 |
6.07 |
68 |
10.2 |
30 |
28.5 |
1.56 |
5.58 |
43 |
7.6 |
35 |
21.0 |
1.41 |
5.03 |
29 |
6.1 |
40 |
16.0 |
1.32 |
4.30 |
20.5 |
5.2 |
45 |
12.5 |
1.25 |
3.67 |
15.3 |
4.6 |
50 |
10.0 |
1.20 |
3.17 |
12.2 |
4.2 |
60 |
6.7 |
1.13 |
2.48 |
8.5 |
3.8 |
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
Source: data from Ho, C. Y., Powell, R. W., and Liley, P. E., Thermal Conductictivity of Selected Materials, NSRDS–NBS–8 and NSRD-NBS-16, Part 2 , National Standard Reference Data System–National Bureau of Standards, Part 1, 1966; Part 2, 1968.
©2001 CRC Press LLC

Table 91. THERMAL CONDUCTIVITY OF METALS (PART 1)
(SHEET 2 OF 2)
T (K) |
Aluminum |
Cadmium |
Chromium |
Copper |
Gold |
|
|
|
|
|
|
70 |
5.0 |
1.08 |
2.08 |
6.7 |
3.58 |
80 |
4.0 |
1.06 |
1.82 |
5.7 |
3.52 |
90 |
3.4 |
1.04 |
1.68 |
5.14 |
3.48 |
100 |
3.0 |
1.03 |
1.58 |
4.83 |
3.45 |
200 |
2.37 |
0.993 |
1.11 |
4.13 |
3.27 |
273 |
2.36 |
0.975 |
0.948 |
4.01 |
3.18 |
300 |
2.37 |
0.968 |
0.903 |
3.98 |
3.15 |
400 |
2.4 |
0.947 |
0.873 |
3.92 |
3.12 |
500 |
2.37 |
0.92 |
0.848 |
3.88 |
3.09 |
600 |
2.32 |
(0.42) |
0.805 |
3.83 |
3.04 |
700 |
2.26 |
(0.49) |
0.757 |
3.77 |
2.98 |
800 |
2.2 |
(0.559) |
0.713 |
3.71 |
2.92 |
900 |
2.13 |
|
0.678 |
3.64 |
2.85 |
1000 |
(0.93) |
|
0.653 |
3.57 |
2.78 |
1100 |
(0.96) |
|
0.636 |
3.5 |
2.71 |
1200 |
(0.99) |
|
0.624 |
3.42 |
2.62 |
1400 |
|
|
0.611 |
|
|
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
Source: data from Ho, C. Y., Powell, R. W., and Liley, P. E., Thermal Conductictivity of Selected Materials, NSRDS–NBS–8 and NSRD-NBS-16, Part 2 , National Standard Reference Data System–National Bureau of Standards, Part 1, 1966; Part 2, 1968.
©2001 CRC Press LLC

Table 92. THERMAL CONDUCTIVITY OF METALS (PART 2)
(SHEET 1 OF 2)
T (K) |
Iron |
Lead |
Magnesium |
Mercury |
Molybdenum |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.75 |
27.7 |
1.30 |
|
0.146 |
2 |
1.49 |
42.4 |
2.59 |
|
0.292 |
3 |
2.24 |
34.0 |
3.88 |
|
0.438 |
4 |
2.97 |
22.4 |
5.15 |
|
0.584 |
5 |
3.71 |
13.8 |
6.39 |
|
0.730 |
6 |
4.42 |
8.2 |
7.60 |
|
0.876 |
7 |
5.13 |
4.9 |
8.75 |
|
1.02 |
8 |
5.80 |
3.2 |
9.83 |
|
1.17 |
9 |
6.45 |
2.3 |
10.8 |
|
1.31 |
10 |
7.05 |
1.78 |
11.7 |
|
1.45 |
11 |
7.62 |
1.46 |
12.5 |
|
1.60 |
12 |
8.13 |
1.23 |
13.1 |
|
1.74 |
13 |
8.58 |
1.07 |
13.6 |
|
1.88 |
14 |
8.97 |
0.94 |
14.0 |
|
2.01 |
15 |
9.30 |
0.84 |
14.3 |
|
2.15 |
16 |
9.56 |
0.77 |
14.4 |
|
2.28 |
18 |
9.88 |
0.66 |
14.3 |
|
2.53 |
20 |
9.97 |
0.59 |
13.9 |
|
2.77 |
25 |
9.36 |
0.507 |
12.0 |
|
3.25 |
30 |
8.14 |
0.477 |
9.5 |
|
3.55 |
35 |
6.81 |
0.462 |
7.4 |
|
3.62 |
40 |
5.55 |
0.451 |
5.7 |
|
3.51 |
45 |
4.50 |
0.442 |
4.57 |
|
3.26 |
50 |
3.72 |
0.435 |
3.75 |
|
3.00 |
60 |
2.65 |
0.424 |
2.74 |
|
2.60 |
70 |
2.04 |
0.415 |
2.23 |
|
2.30 |
80 |
1.68 |
0.407 |
1.95 |
|
2.09 |
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
©2001 CRC Press LLC

Table 92. THERMAL CONDUCTIVITY OF METALS (PART 2)
(SHEET 2 OF 2)
T (K) |
Iron |
Lead |
Magnesium |
Mercury |
Molybdenum |
|
|
|
|
|
|
|
|
|
|
|
|
90 |
1.46 |
0.401 |
1.78 |
|
1.92 |
100 |
1.32 |
0.396 |
1.69 |
|
1.79 |
200 |
0.94 |
0.366 |
1.59 |
|
1.43 |
273 |
0.835 |
0.355 |
1.57 |
(0.078) |
1.39 |
300 |
0.803 |
0.352 |
1.56 |
(0.084) |
1.38 |
400 |
0.694 |
0.338 |
1.53 |
(0.098) |
1.34 |
500 |
0.613 |
0.325 |
1.51 |
(0.109) |
1.3 |
600 |
0.547 |
0.312 |
1.49 |
(0.12) |
1.26 |
700 |
0.487 |
(0.174) |
1.47 |
(0.127) |
1.22 |
800 |
0.433 |
(0.19) |
1.46 |
(0.13) |
1.18 |
900 |
0.38 |
(0.203) |
1.45 |
|
1.15 |
1000 |
0.326 |
(0.215) |
(0.84) |
|
1.12 |
1100 |
0.297 |
|
(0.91) |
|
1.08 |
1200 |
0.282 |
|
(0.98) |
|
1.05 |
1400 |
0.309 |
|
|
|
0.996 |
1600 |
0.327 |
|
|
|
0.946 |
1800 |
|
|
|
|
0.907 |
2000 |
|
|
|
|
0.88 |
2200 |
|
|
|
|
0.858 |
2600 |
|
|
|
|
0.825 |
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
©2001 CRC Press LLC

Table 93. THERMAL CONDUCTIVITY OF METALS (PART 3)
(SHEET 1 OF 2)
T (K) |
Nickel |
Niobium |
Platinum |
Silver |
Tantalum |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.64 |
0.251 |
2.31 |
39.4 |
0.115 |
2 |
1.27 |
0.501 |
4.60 |
78.3 |
0.230 |
3 |
1.91 |
0.749 |
6.79 |
115 |
0.345 |
4 |
2.54 |
0.993 |
8.8 |
147 |
0.459 |
5 |
3.16 |
1.23 |
10.5 |
172 |
0.571 |
6 |
3.77 |
1.46 |
11.8 |
187 |
0.681 |
7 |
4.36 |
1.67 |
12.6 |
193 |
0.788 |
8 |
4.94 |
1.86 |
12.9 |
190 |
0.891 |
9 |
5.49 |
2.04 |
12.8 |
181 |
0.989 |
10 |
6.00 |
2.18 |
12.3 |
168 |
1.08 |
11 |
6.48 |
2.30 |
11.7 |
154 |
1.16 |
12 |
6.91 |
2.39 |
10.9 |
139 |
1.24 |
13 |
7.30 |
2.46 |
10.1 |
124 |
1.30 |
14 |
7.64 |
2.49 |
9.3 |
109 |
1.36 |
15 |
7.92 |
2.50 |
8.4 |
96 |
1.40 |
16 |
8.15 |
2.49 |
7.6 |
85 |
1.44 |
18 |
8.45 |
2.42 |
6.1 |
66 |
1.47 |
20 |
8.56 |
2.29 |
4.9 |
51 |
1.47 |
25 |
8.15 |
1.87 |
3.15 |
29.5 |
1.36 |
30 |
6.95 |
1.45 |
2.28 |
19.3 |
1.16 |
35 |
5.62 |
1.16 |
1.80 |
13.7 |
0.99 |
40 |
4.63 |
0.97 |
1.51 |
10.5 |
0.87 |
45 |
3.91 |
0.84 |
1.32 |
8.4 |
0.78 |
50 |
3.36 |
0.76 |
1.18 |
7.0 |
0.72 |
60 |
2.63 |
0.66 |
1.01 |
5.5 |
0.651 |
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
©2001 CRC Press LLC

Table 93. THERMAL CONDUCTIVITY OF METALS (PART 3)
(SHEET 2 OF 2)
T (K) |
Nickel |
Niobium |
Platinum |
Silver |
Tantalum |
|
|
|
|
|
|
|
|
|
|
|
|
70 |
2.21 |
0.61 |
0.90 |
4.97 |
0.616 |
80 |
1.93 |
0.58 |
0.84 |
4.71 |
0.603 |
90 |
1.72 |
0.563 |
0.81 |
4.60 |
0.596 |
100 |
1.58 |
0.552 |
0.79 |
4.50 |
0.592 |
200 |
1.06 |
0.526 |
0.748 |
4.3 |
0.575 |
273 |
0.94 |
0.533 |
0.734 |
4.28 |
0.574 |
300 |
0.905 |
0.537 |
0.73 |
4.27 |
0.575 |
400 |
0.801 |
0.552 |
0.722 |
4.2 |
0.578 |
500 |
0.721 |
0.567 |
0.719 |
4.13 |
0.582 |
600 |
0.655 |
0.582 |
0.72 |
4.05 |
0.586 |
700 |
0.653 |
0.598 |
0.723 |
3.97 |
0.59 |
800 |
0.674 |
0.613 |
0.729 |
3.89 |
0.594 |
900 |
0.696 |
0.629 |
0.737 |
3.82 |
0.598 |
1000 |
0.718 |
0.644 |
0.748 |
3.74 |
0.602 |
1100 |
0.739 |
0.659 |
0.76 |
3.66 |
0.606 |
1200 |
0.761 |
0.675 |
0.775 |
3.58 |
0.610 |
1400 |
0.804 |
0.705 |
0.807 |
|
0.618 |
1600 |
|
0.735 |
0.842 |
|
0.626 |
1800 |
|
0.764 |
0.877 |
|
0.634 |
2000 |
|
0.791 |
0.913 |
|
0.640 |
2200 |
|
0.815 |
|
|
0.647 |
2600 |
|
|
|
|
0.658 |
3000 |
|
|
|
|
0.665 |
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
©2001 CRC Press LLC

Table 94. THERMAL CONDUCTIVITY OF METALS (PART 4)
(SHEET 1 OF 2)
T (K) |
Tin |
Titanium |
Tungsten |
Zinc |
Zirconium |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
0.0144 |
14.4 |
19.0 |
0.111 |
2 |
|
0.0288 |
28.7 |
37.9 |
0.223 |
3 |
297 |
0.0432 |
42.6 |
55.5 |
0.333 |
4 |
181 |
0.0576 |
55.6 |
69.7 |
0.442 |
5 |
117 |
0.0719 |
67.1 |
77.8 |
0.549 |
6 |
76 |
0.0863 |
76.2 |
78.0 |
0.652 |
7 |
52 |
0.101 |
82.4 |
71.7 |
0.748 |
8 |
36 |
0.115 |
85.3 |
61.8 |
0.837 |
9 |
26 |
0.129 |
85.1 |
51.9 |
0.916 |
10 |
19.3 |
0.144 |
82.4 |
43.2 |
0.984 |
11 |
14.8 |
0.158 |
77.9 |
36.4 |
1.04 |
12 |
11.6 |
0.172 |
72.4 |
30.8 |
1.08 |
13 |
9.3 |
0.186 |
66.4 |
26.1 |
1.11 |
14 |
7.6 |
0.200 |
60.4 |
22.4 |
1.13 |
15 |
6.3 |
0.214 |
54.8 |
19.4 |
1.13 |
16 |
5.3 |
0.227 |
49.3 |
16.9 |
1.12 |
18 |
4.0 |
0.254 |
40.0 |
13.3 |
1.08 |
20 |
3.2 |
0.279 |
32.6 |
10.7 |
1.01 |
25 |
2.22 |
0.337 |
20.4 |
6.9 |
0.85 |
30 |
1.76 |
0.382 |
13.1 |
4.9 |
0.74 |
35 |
1.50 |
0.411 |
8.9 |
3.72 |
0.65 |
40 |
1.35 |
0.422 |
6.5 |
2.97 |
0.58 |
45 |
1.23 |
0.416 |
5.07 |
2.48 |
0.535 |
50 |
1.15 |
0.401 |
4.17 |
2.13 |
0.497 |
60 |
1.04 |
0.377 |
3.18 |
1.71 |
0.442 |
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
©2001 CRC Press LLC

Table 94. THERMAL CONDUCTIVITY OF METALS (PART 4)
(SHEET 2 OF 2)
T (K) |
Tin |
Titanium |
Tungsten |
Zinc |
Zirconium |
|
|
|
|
|
|
|
|
|
|
|
|
70 |
0.96 |
0.356 |
2.76 |
1.48 |
0.403 |
80 |
0.91 |
0.339 |
2.56 |
1.38 |
0.373 |
90 |
0.88 |
0.324 |
2.44 |
1.34 |
0.350 |
100 |
0.85 |
0.312 |
2.35 |
1.32 |
0.332 |
200 |
0.733 |
0.245 |
1.97 |
1.26 |
0.252 |
273 |
0.682 |
0.224 |
1.82 |
1.22 |
0.232 |
300 |
0.666 |
0.219 |
1.78 |
1.21 |
0.227 |
400 |
0.622 |
0.204 |
1.62 |
1.16 |
0.216 |
500 |
0.596 |
0.197 |
1.49 |
1.11 |
0.210 |
600 |
(0.323) |
0.194 |
1.39 |
1.05 |
0.207 |
700 |
(0.343) |
0.194 |
1.33 |
(0.499) |
0.209 |
800 |
(0.364) |
0.197 |
1.28 |
(0.557) |
0.216 |
900 |
(0.384) |
0.202 |
1.24 |
(0.615) |
0.226 |
1000 |
(0.405) |
0.207 |
1.21 |
(0.673) |
0.237 |
1100 |
(0.425) |
0.213 |
1.18 |
(0.73) |
0.248 |
1200 |
(0.446) |
0.220 |
1.15 |
|
0.257 |
1400 |
(0.487) |
0.236 |
1.11 |
|
0.275 |
1600 |
|
0.253 |
1.07 |
|
0.290 |
1800 |
|
0.271 |
1.03 |
|
0.302 |
2000 |
|
|
1.00 |
|
0.313 |
2200 |
|
|
0.98 |
|
|
2600 |
|
|
0.94 |
|
|
3000 |
|
|
0.915 |
|
|
|
|
|
|
|
|
Values are in watt • cm-1 • K-1.
Note: Values in parentheses are for liquid state
These data apply only to metals of purity of at least 99.9%.
The third significant figure may not be accurate.
©2001 CRC Press LLC

Table 95. THERMAL CONDUCTIVITY OF ALLOY CAST IRONS
|
|
Thermal Conductivity |
Description |
Description |
W/(m • K) |
|
|
|
|
|
|
Abrasion–Resistant White Irons |
Low–C white iron |
22 |
|
Martensitic nickel–chromium |
30 |
|
iron |
|
|
|
|
Corrosion–Resistant Irons |
High–nickel gray iron |
38 to 40 |
|
High–nickel ductile iron |
13.4 |
Heat–Resistant Gray Irons |
Medium–silicon iron |
37 |
|
High–chromium iron |
20 |
|
High–nickel iron |
37 to 40 |
|
Nickel–chromium–silicon iron |
30 |
Heat–Resistant Ductile Iron |
High–nickel ductile (20 Ni) |
13 |
|
|
|
Source: Data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p172, (1984).
©2001 CRC Press LLC
Table 96. THERMAL CONDUCTIVITY OF IRON
AND IRON ALLOYS
|
Thermal Conductivity |
|
near room temperature |
Metal or alloy |
(cal / cm2 • cm • s • °C) |
|
|
|
|
Pure iron |
0.178 |
Cast iron (3.16C, 1.54Si, 0.57Mn) |
0.112 |
Carbon steel(0.23C, 0.64Mn) |
0.124 |
Carbon steel(1.22 C, 0.35 Mn) |
0.108 |
Alloy steel (0.34 C, 0.55 Mn, 0.78 Cr, 3.53 Ni, 0.39 Mo, 0.05 Cu) |
0.079 |
Type 410 |
0.057 |
Type 304 |
0.036 |
T1 tool steel |
0.058 |
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 97. THERMAL CONDUCTIVITY OF ALUMINUM
AND ALUMINUM ALLOYS (SHEET 1 OF 2)
|
|
Thermal Conductivity |
|
|
near room temperature |
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
Wrought alloys |
EC(O) |
0.57 |
|
1060(O) |
0.56 |
|
1100 |
0.53 |
|
2011(T3) |
0.34 |
|
2014(O) |
0.46 |
|
2024(O) |
0.45 |
|
2218(T72) |
0.37 |
|
3003(O) |
0.46 |
|
4032(O) |
0.37 |
|
5005 |
0.48 |
|
5050(O) |
0.46 |
|
5052(O) |
0.33 |
|
5056(O) |
0.28 |
|
5083 |
0.28 |
|
5086 |
0.30 |
|
5154 |
0.30 |
|
5357 |
0.40 |
|
5456 |
0.28 |
|
6061(O) |
0.41 |
|
6063(O) |
0.52 |
|
6101(T6) |
0.52 |
|
6151(O) |
0.49 |
|
7075(T6) |
0.29 |
|
7079(T6) |
0.29 |
|
7178 |
0.29 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 97. THERMAL CONDUCTIVITY OF ALUMINUM
AND ALUMINUM ALLOYS (SHEET 2 OF 2)
|
|
Thermal Conductivity |
|
|
near room temperature |
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
Casting alloys |
A13 |
0.29 |
|
43(F) |
0.34 |
|
108(F) |
0.29 |
|
A108 |
0.34 |
|
A132(T551) |
0.28 |
|
D132(T5) |
0.25 |
|
F132 |
0.25 |
|
138 |
0.24 |
|
142 (T21, sand) |
0.40 |
|
195 (T4, T62) |
0.33 |
|
B195 (T4, T6) |
0.31 |
|
214 |
0.33 |
|
200(T4) |
0.21 |
|
319 |
0.26 |
|
355(T51, sand) |
0.40 |
|
356(T51, sand) |
0.40 |
|
360 |
0.35 |
|
380 |
0.23 |
|
750 |
0.44 |
|
40E |
0.33 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 98. THERMAL CONDUCTIVITY OF COPPER
AND COPPER ALLOYS (SHEET 1 OF 3)
|
|
Thermal Conductivity |
|
|
near room temperature |
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
Wrought coppers |
Pure Copper |
0.941 |
|
Electrolytic tough pitch copper (ETP) |
0.934 |
|
Deoxidized copper high residual |
0.81 |
|
phosphorus (DHP) |
|
|
|
|
|
Free–machining copper (0.5% Te) |
0.88 |
|
Free–machining copper (1% Pb) |
0.92 |
Wrought alloys |
Gilding, 95% |
0.56 |
|
Commercial bronze, 90% |
0.45 |
|
Jewelry bronze, 87.5% |
0.41 |
|
Red brass, 85% |
0.38 |
|
Low brass, 80% |
0.33 |
|
Cartridge brass, 70% |
0.29 |
|
Yellow brass |
0.28 |
|
Muntz metal |
0.29 |
|
Leaded commercial bronze |
0.43 |
|
Low–leaded brass (tube) |
0.28 |
|
Medium leaded brass |
0.28 |
|
High–leaded brass (tube) |
0.28 |
|
High–leaded brass |
0.28 |
|
Extra–high–leaded brass |
0.28 |
|
Leaded Muntz metal |
0.29 |
|
Forging brass |
0.28 |
|
Architectual bronze |
0.29 |
|
Inhibited admiralty |
0.26 |
|
Naval brass |
0.28 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 98. THERMAL CONDUCTIVITY OF COPPER
AND COPPER ALLOYS (SHEET 2 OF 3)
|
|
Thermal Conductivity |
|
|
|
near room temperature |
|
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
|
|
|
Wrought alloys |
Leaded naval brass |
0.28 |
|
(Con’t) |
|||
|
|
||
|
Manganese bronze |
0.26 |
|
|
Phosphor bronze,5% |
0.17 |
|
|
Pbosphor bronze, 8% |
0.15 |
|
|
Phosphor bronze, 10% |
0.12 |
|
|
Phosphor bronze, 1.25% |
0.49 |
|
|
Free cutting phosphor bronze |
0.18 |
|
|
Cupro-nickel,30% |
0.07 |
|
|
Cupro-nickel,10% |
0.095 |
|
|
Nickel silver, 65–18 |
0.08 |
|
|
Nickel silver, 55–18 |
0.07 |
|
|
Nickel silver, 65–12 |
0.10 |
|
|
High–silicon bronze |
0.09 |
|
|
Low–silicon bronze |
0.14 |
|
|
Aluminum bronze, 5%Al |
0.198 |
|
|
Aluminum bronze |
0.18 |
|
|
Aluminum–silicon bronze |
0.108 |
|
|
Aluminum bronze |
0.144 |
|
|
Aluminum bronze |
0.091 |
|
|
Beryllium copper |
0.20 |
|
Casting alloys |
Chromium copper (1% Cr) |
0.4 |
|
|
89cu–11Sn |
0.121 |
|
|
88Cu–6Sn–1.5Pb–4.5Zn |
18% of Cu |
|
|
87Cu–8Sn–1Pb–4Zn |
12% of Cu |
|
|
87Cu–10Sn–1Pb–2Zn |
12% of Cu |
|
|
80Cu–10Sn–10Pb |
12% of Cu |
|
|
Manganese bronze, 110 ksi |
9.05% of Cu |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 98. THERMAL CONDUCTIVITY OF COPPER
AND COPPER ALLOYS (SHEET 3 OF 3)
|
|
Thermal Conductivity |
|
|
|
near room temperature |
|
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
|
|
|
Casting alloys |
Aluminum bronze, Alloy 9A |
15% of Cu |
|
(Con’t) |
|||
|
|
||
|
Aluminum bronze, Alloy 9B |
16% of Cu |
|
|
Aluminum bronze, Alloy 9C |
18% of Cu |
|
|
Aluminum bronze, Alloy 9D |
12% of Cu |
|
|
Propeller bronze |
11% of Cu |
|
|
Nickel silver, 12% Ni |
7% of Cu |
|
|
Nickel silver, 16% Ni |
7% of Cu |
|
|
Nickel silver, 20% Ni |
6% of Cu |
|
|
Nickel silver. 25% Ni |
6.5% of Cu |
|
|
Silicon bronze |
7% of Cu |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 99. THERMAL CONDUCTIVITY OF
MAGNESIUM AND MAGNESIUM ALLOYS
|
|
Thermal Conductivity |
|
|
near room temperature |
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
Pure |
Magnesium (99.8%) |
0.367 |
Casting alloys |
AM100A |
0.17 |
|
AZ63A |
0.18 |
|
AZ81A(T4) |
0.12 |
|
AZ91A, B, C |
0.17 |
|
AZ92A |
0.17 |
|
HK31A (T6, sand cast) |
0.22 |
|
HZ32A |
0.26 |
|
ZH42 |
0.27 |
|
ZH62A |
0.26 |
|
ZK51A |
0.26 |
|
ZE41A(T5) |
0.27 |
|
EZ33A |
0.24 |
|
EK30A |
0.26 |
|
EK41A(T5) |
0.24 |
Wrought alloys |
M1A |
0.33 |
|
AZ31B |
0.23 |
|
AZ61A |
0.19 |
|
AZ80A |
0.18 |
|
ZK60A,B(F) |
0.28 |
|
ZE10A(O) |
0.33 |
|
HM21A(O) |
0.33 |
|
HM31A |
0.25 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 100. THERMAL CONDUCTIVITY OF
NICKEL AND NICKEL ALLOYS
|
Thermal Conductivity |
|
near room temperature |
Metal or alloy |
(cal / cm2 • cm • s • °C) |
|
|
|
|
Nickel (99.95% Ni + Co) |
0.22 |
“A” nickel |
0.145 |
“D” nickel |
0.115 |
Monel |
0.062 |
“K” Monel |
0.045 |
Inconel |
0.036 |
Hastelloy B |
0.027 |
Hastelloy C |
0.03 |
Hastelloy D |
0.05 |
Illium G |
0.029 |
Illium R |
0.031 |
60Ni–24Fe–16Cr |
0.032 |
35Ni–45Fe–20Cr |
0.031 |
Constantan |
0.051 |
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 101. THERMAL CONDUCTIVITY OF LEAD
AND LEAD ALLOYS
|
Thermal Conductivity |
|
near room temperature |
Metal or alloy |
(cal / cm2 • cm • s • °C) |
|
|
|
|
Corroding lead (99.73 + % Pb) |
0.083 |
5–95 solder |
0.085 |
20–80 solder |
0.089 |
50-50 solder |
0.111 |
1% antimonial lead |
0.080 |
Hard lead (96Pb-4Sb) |
0.073 |
Hard lead (94Pb–6Sb) |
0.069 |
8% antimonial lead |
0.065 |
9% antimonial lead |
0.064 |
Lead-base babbitt (SAE 14) |
0.057 |
Lead-base babbitt (alloy 8) |
0.058 |
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 102. THERMAL CONDUCTIVITY OF TIN, TITANIUM, ZINC
AND THEIR ALLOYS
|
|
Thermal Conductivity |
|
|
near room temperature |
Metal or alloy |
Designation |
(cal / cm2 • cm • s • °C) |
|
|
|
|
|
|
Tin and Tin Alloys |
Pure tin |
0.15 |
|
Soft solder (63Sn–37Pb) |
0.12 |
|
Tin foil (92Sn–8Zn) |
0.14 |
Titanium and Titanium Alloys |
Titanium(99.0%) |
0.043 |
|
Ti–5Al–2.5Sn |
0.019 |
|
Ti–2Fe-2Cr–2Mo |
0.028 |
|
Ti–8Mn |
0.026 |
Zinc and Zinc Alloys |
Pure zinc |
0.27 |
|
AG40A alloy |
0.27 |
|
AC41A alloy |
0.26 |
|
Commercial rolled zinc 0.08 Pb |
0.257 |
|
Commercial rolled zinc 0.06 Pb, 0.06 Cd |
0.257 |
|
Rolled zinc alloy (1 CU, 0.010 Mg) |
0.25 |
|
Zn-Cu–Ti alloy (0.8 Cu, 0.15 Ti) |
0.25 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 103. THERMAL CONDUCTIVITY OF PURE METALS
|
Thermal Conductivity |
|
near room temperature |
Metal or alloy |
(cal / cm2 • cm • s • °C) |
|
|
|
|
Beryllium |
0.35 |
Cadmium |
0.22 |
Chromium |
0.16 |
Cobalt |
0.165 |
Germanium |
0.14 |
Gold |
0.71 |
Indium |
0.057 |
Iridium |
0.14 |
Lithium |
0.17 |
Molybdenum |
0.34 |
Niobium |
0.13 |
Palladium |
0.168 |
Platinum |
0.165 |
Plutonium |
0.020 |
Rhenium |
0.17 |
Rhodium |
0.21 |
Silicon |
0.20 |
Silver |
1.0 |
Sodium |
0.32 |
Tantalum |
0.130 |
Thallium |
0.093 |
Thorium |
0.090 |
Tungsten |
0.397 |
Uranium |
0.071 |
Vanadium |
0.074 |
Yttrium |
0.035 |
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p156, (1993).
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 1 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
Borides |
Chromium Diboride (CrB2) |
0.049-0.076 at room temp. |
|
Hafnium Diboride (HfB2) |
0.015 at room temp. |
|
Tantalum Diboride (TaB2) |
0.026 at room temp. |
|
|
0.033 at 200 oC. |
|
Titanium Diboride (TiB2) |
0.058-0.062 at room temp. |
|
|
0.063 at 200 oC |
|
Zirconium Diboride (ZrB2) |
0.055-0.058 at room temp. |
|
|
0.055-0.060 at 200 oC |
Carbides |
Boron Carbide (B4C) |
0.065-0.069 at room temp. |
|
|
0.198 at 425 oC |
|
Hafnium Monocarbide (HfC) |
0.053 at room temp. |
|
|
0.15 + 1.20x10 T watts cm-1 K-1 |
|
|
from 1000-2000K |
|
Silicon Carbide (SiC) |
|
|
(with 1 wt% Be addictive) |
0.621 |
|
(with 1 wt% B addictive) |
0.406 |
|
(with 1 wt% Al addictive) |
0.143 |
|
(with 2 wt% BN addictive) |
0.263 |
|
(with 1.6 wt% BeO addictive) |
0.645 at room temp. |
|
(with 3.2 wt% BeO addictive) |
0.645 at room temp. |
|
|
0.098-0.10 at 20oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 2 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
(cubic, CVD) |
0.289 at 127oC |
|
|
0.049-0.080 at 600oC |
|
|
0.061 at 800oC |
|
|
0.051 at 1000oC |
|
|
0.0059 at 1250oC |
|
|
0.0827 at 1327oC |
|
|
0.0032 at 1530oC |
|
Tantalum Monocarbide (TaC) |
0.053 at room temp. |
|
Titanium Monocarbide (TiC) |
0.041-0.074 at room temp. |
|
|
0.0135 at 1000 oC |
|
Trichromium Dicarbide (Cr3C2) |
0.454 |
|
Tungsten Monocarbide (WC) |
0.201 at 20 oC |
|
(6% Co, 1-3μm grain size) |
0.239 |
|
(12% Co, 1-3μm grain size) |
0.251 |
|
(24% Co, 1-3μm grain size) |
0.239 |
|
(6% Co, 2-4μm grain size) |
0.251 |
|
(6% Co, 3-6μm grain size) |
0.256 |
|
Zirconium Monocarbide (ZrC) |
0.049 at room temp. |
|
|
0.098 at 50oC |
|
|
0.069 at 150oC |
|
|
0.065 at 188oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
|
(SHEET 3 OF 12) |
|
|
|
|
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
|
0.061 at 288oC |
|
|
0.080 at 600oC |
|
|
0.083 at 800oC |
|
|
0.086 at 1000oC |
|
|
0.089 at 1200oC |
|
|
0.092 at 1400oC |
|
|
0.096 at 1600oC |
|
|
0.099 at 1800oC |
|
|
0.103 at 2000oC |
|
|
0.105 at 2200oC |
Nitrides |
Aluminum Nitride (AlN) |
0.072 at 25oC |
|
|
0.060 at 200oC |
|
|
0.053 at 400oC |
|
|
0.048 at 600oC |
|
|
0.042 at 800oC |
|
Boron Nitride (BN) |
|
|
parallel to c axis |
0.0687 at 300oC |
|
|
0.0646 at 700oC |
|
|
0.0637 at 1000oC |
|
parallel to a axis |
0.0362 at 300oC |
|
|
0.0318 at 700oC |
|
|
0.0295 at 1000oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 4 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
Titanium Mononitride (TiN) |
0.069 at 25 oC |
|
|
0.057 at 127 oC |
|
|
0.040 at 200 oC |
|
|
0.027 at 650 oC |
|
|
0.020 at 1000 oC |
|
|
0.162 at 1500 oC |
|
|
0.136 at 2300 oC |
|
Trisilicon tetranitride (Si3N4) |
|
|
(pressureless sintered) |
0.072 at room temp. |
|
|
0.022-0.072 at 127 oC |
|
|
0.041 at 200-750 oC |
|
|
0.036-0.042 at 500 oC |
|
(pressureless sintered) |
0.038 at 1000 oC |
|
|
0.033-0.034 at 1200 oC |
|
Zirconium Mononitride (ZrN) |
0.040 at 200 oC |
|
|
0.025 at 425 oC |
|
|
0.018 at 650 oC |
|
|
0.016 at 875 oC |
|
|
0.015 at 1100 oC |
Oxides |
Aluminum Oxide (Al2O3) |
0.06 at room temp. |
|
|
0.04-0.069 at 100oC |
|
|
0.03-0.064 at 200oC |
|
|
0.037 at 315oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
|
(SHEET 5 OF 12) |
|
|
|
|
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
|
0.02-0.031 at 400oC |
|
|
0.035 at 500oC |
|
|
0.021-0.022 at 600oC |
|
|
0.015-0.017 at 800oC |
|
|
0.014-0.016 at 1000oC |
|
|
0.013-0.015 at 1200oC |
|
|
0.013 at 1400oC |
|
|
0.014 at 1600oC |
|
|
0.017 at 1800oC |
|
Aluminum Oxide (Al2O3) (single crystal) |
0.103 at 20oC |
|
|
0.047 at 300oC |
|
|
0.029 at 800oC |
|
Beryllium Oxide (BeO) |
0.038-0.47 at 20oC |
|
|
0.032-0.34 at 100oC |
|
|
0.14-0.16 at 400oC |
|
|
0.089-0.1137 at 600oC |
|
|
0.060-0.093 at 800oC |
|
|
0.043 at 1100oC |
|
|
0.041-0.054 at 1200oC |
|
|
0.038 at 1300oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
|
(SHEET 6 OF 12) |
|
|
|
|
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
|
0.036 at 1400oC |
|
|
0.034 at 1500oC |
|
|
0.033-0.039 at 1600oC |
|
|
0.033 at 1700oC |
|
|
0.036 at 1800oC |
|
|
0.036 at 1900oC |
|
|
0.036 at 2000oC |
|
Calcium Oxide (CaO) |
0.037 at 100oC |
|
|
0.027 at 200oC |
|
|
0.022 at 400oC |
|
|
0.020 at 600oC |
|
|
0.019 at 800oC |
|
|
0.0186-0.019 at 1000oC |
|
Cerium Dioxide (CeO2) |
0.0229 at 400K |
|
|
0.00287 at 1400K |
|
Dichromium Trioxide (Cr2O3) |
0.0239-0.0788 |
|
Hafnium Dioxide (HfO2) |
0.0273 at 25-425oC |
|
Magnesium Oxide (MgO) |
0.097 at room temp. |
|
|
0.078-0.082 at 100oC |
|
|
0.064-0.065 at 200oC |
|
|
0.038-0.045 at 400oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
|
(SHEET 7 OF 12) |
|
|
|
|
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
|
0.0198-0.026 at 800oC |
|
|
0.016-0.020 at 1000oC |
|
|
0.0139-0.0148 at 1200oC |
|
|
0.012-0.014 at 1400oC |
|
|
0.0108-0.016 at 1600oC |
|
|
0.0096-0.0191 at 1800oC |
|
Nickel monoxide (NiO) |
|
|
(0% porosity) |
0.029 at 100oC |
|
(0% porosity) |
0.024 at 200oC |
|
(0% porosity) |
0.017 at 400oC |
|
(0% porosity) |
0.012 at 800oC |
|
(0% porosity) |
0.011 at 1000oC |
|
Silicon Dioxide (SiO2) |
0.0025 at 200oC |
|
|
0.003 at 400oC |
|
|
0.004 at 800oC |
|
|
0.005 at 1200oC |
|
|
0.006 at 1600oC |
|
Thorium Dioxide (ThO2) |
|
|
(0% porosity) |
0.024 at room temp. |
|
(0% porosity) |
0.020 at 100oC |
|
(0% porosity) |
0.019 at 200oC |
|
(0% porosity) |
0.014 at 400oC |
|
(0% porosity) |
0.010 at 600oC |
|
(0% porosity) |
0.008 at 800oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 8 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
(0% porosity) |
0.007-0.0074 at 1000oC |
|
(0% porosity) |
0.006-0.0076 at 1200oC |
|
(0% porosity) |
0.006 at 1400oC |
|
Titanium Oxide (TiO2) |
|
|
(0% porosity) |
0.016 at 100oC |
|
(0% porosity) |
0.012 at 200oC |
|
(0% porosity) |
0.009 at 400oC |
|
(0% porosity) |
0.008 at 600oC |
|
(0% porosity) |
0.008 at 800oC |
|
(0% porosity) |
0.008 at 1000oC |
|
(0% porosity) |
0.008 at 1200oC |
|
Uranium Dioxide (UO2) |
|
|
(0% porosity) |
0.025 at 100oC |
|
(0% porosity) |
0.020 at 200oC |
|
(0% porosity) |
0.015 at 400oC |
|
(0% porosity) |
0.010 at 600oC |
|
(0% porosity) |
0.009 at 800oC |
|
(0% porosity) |
0.008 at 1000oC |
|
|
0.018 at 100oC |
|
|
0.012 at 400oC |
|
|
0.008 at 600oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
|
(SHEET 9 OF 12) |
|
|
|
|
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
|
0.008 at 700oC |
|
|
0.006 at 1000oC |
|
|
0.006 at 1200oC |
|
Zirconium Oxide (ZrO2) |
|
|
(stabilized, 0% porosity) |
0.005 at 100oC |
|
(stabilized, 0% porosity) |
0.005 at 200oC |
|
(stabilized, 0% porosity) |
0.005 at 400oC |
|
(stabilized, 0% porosity) |
0.0055 at 800oC |
|
(stabilized, 0% porosity) |
0.006 at 1200oC |
|
(stabilized, 0% porosity) |
0.0065 at 1400oC |
|
(stabilized) |
0.004 at 100oC |
|
(stabilized) |
0.0044 at 500oC |
|
(stabilized) |
0.0048-0.0055 at 1000oC |
|
(stabilized) |
0.0049-0.0050 at 1200oC |
|
(MgO stabilized) |
0.0076 at room temp. |
|
(MgO stabilized) |
0.0057 at 800oC |
|
(Y2O3 stabilized) |
0.0055 at room temp. |
|
(Y2O3 stabilized) |
0.0053 at 800oC |
|
(plasma sprayed) |
0.0019-0.0031 at room temp. |
|
(plasma sprayed) |
0.0019-0.0022 at 800oC |
|
(plasma sprayed and coated with Cr2O3) |
0.0033 at room temp. |
|
(plasma sprayed and coated with Cr2O3) |
0.0033 at 800oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 10 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
(5-10% CaO stabilized) |
0.0045 at 400oC |
|
(5-10% CaO stabilized) |
0.0049 at 800oC |
|
(5-10% CaO stabilized) |
0.0057 at 1200oC |
|
Cordierite (2MgO 2Al2O3 5SiO2) |
|
|
(ρ=2.3g/cm3) |
0.0077 at 20oC |
|
(ρ=2.3g/cm3) |
0.0062 at 300oC |
|
(ρ=2.3g/cm3) |
0.0055 at 500oC |
|
(ρ=2.3g/cm3) |
0.0055 at 800oC |
|
(ρ=2.1g/cm3) |
0.0043 at 20oC |
|
(ρ=2.1g/cm3) |
0.0041 at 300oC |
|
(ρ=2.1g/cm3) |
0.0040 at 500oC |
|
(ρ=2.1g/cm3) |
0.0038 at 800oC |
|
Mullite (3Al2O3 2SiO2) |
|
|
(0% porosity) |
0.0145 at 100oC |
|
(0% porosity) |
0.013 at 200oC |
|
(0% porosity) |
0.011 at 400oC |
|
(0% porosity) |
0.010 at 600oC |
|
(0% porosity) |
0.0095 at 800oC |
|
(0% porosity) |
0.009 at 1000oC |
|
(0% porosity) |
0.009 at 1200oC |
|
(0% porosity) |
0.009 at 1400oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 11 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
|
Sillimanite (Al2O3 SiO2) |
|
|
(0% porosity) |
0.0042 at 100oC |
|
(0% porosity) |
0.004 at 400oC |
|
(0% porosity) |
0.0035 at 800oC |
|
(0% porosity) |
0.0035 at 1200oC |
|
(0% porosity) |
0.003 at 1500oC |
|
Spinel (Al2O3 MgO) |
|
|
(0% porosity) |
0.035 at 100oC |
|
(0% porosity) |
0.031 at 200oC |
|
(0% porosity) |
0.024 at 400oC |
|
(0% porosity) |
0.019 at 600oC |
|
(0% porosity) |
0.015 at 800oC |
|
(0% porosity) |
0.013-0.0138 at 1000oC |
|
(0% porosity) |
0.013 at 1200oC |
|
Zircon (SiO2 ZrO2) |
|
|
(0% porosity) |
0.0145 at 100oC |
|
(0% porosity) |
0.0135 at 200oC |
|
(0% porosity) |
0.012 at 400oC |
|
(0% porosity) |
0.010 at 800oC |
|
(0% porosity) |
0.0095 at 1200oC |
|
(0% porosity) |
0.0095 at 1400oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 104. THERMAL CONDUCTIVITY OF CERAMICS
(SHEET 12 OF 12)
|
|
Thermal Conductivity |
Class |
Ceramic |
(cal • cm-1 • sec-1 • K-1) |
|
|
|
|
|
|
Silicides |
Molybdenum Disilicide (MoSi2) |
0.129 at 150oC |
|
|
0.074 at 425oC |
|
|
0.053 at 540oC |
|
|
0.057 at 650oC |
|
|
0.046 at 875oC |
|
|
0.041 at 1100oC |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 105. THERMAL CONDUCTIVITY OF GLASSES
(SHEET 1 OF 5)
|
|
Thermal |
|
Temperature Range |
Glass |
Description |
Conductivity |
Units |
of Validity |
|
|
|
|
|
|
|
|
|
|
SiO2 glass |
|
0.00329 |
cal/cm s K |
20˚C |
|
|
0.59 |
W/m K |
80˚C |
|
|
0.67 |
W/m K |
100˚C |
|
|
0.88 |
W/m K |
150˚C |
|
|
1.10 |
W/m K |
200˚C |
|
|
1.28 |
W/m K |
250˚C |
|
|
1.32 |
W/m K |
273.1˚C |
|
|
1.36 |
W/m K |
300˚C |
|
|
1.43 |
W/m K |
350˚C |
|
|
1.50 |
W/m K |
400˚C |
|
|
1.62 |
W/m K |
500˚C |
|
|
1.72 |
W/m K |
600˚C |
|
|
1.80 |
W/m K |
700˚C |
|
|
|
|
|
Source: compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko-Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 105. THERMAL CONDUCTIVITY OF GLASSES
(SHEET 2 OF 5)
|
|
Thermal |
|
Temperature Range |
Glass |
Description |
Conductivity |
Units |
of Validity |
SiO2-Na2O glass |
(22% mol Na2O) |
0.70 |
kcal/m hr K |
450˚C |
|
(22% mol Na2O) |
0.90 |
kcal/m hr K |
850˚C |
|
(22% mol Na2O) |
1.20 |
kcal/m hr K |
1050˚C |
|
(22% mol Na2O) |
1.55 |
kcal/m hr K |
1250˚C |
|
(22% mol Na2O) |
2.25 |
kcal/m hr K |
1500˚C |
|
(25% mol Na2O) |
0.15 |
W/m K |
35 K |
|
(25% mol Na2O) |
0.25 |
W/m K |
60 K |
|
(25% mol Na2O) |
0.40 |
W/m K |
80 K |
|
(25% mol Na2O) |
0.50 |
W/m K |
100 K |
|
(25% mol Na2O) |
0.60 |
W/m K |
140 K |
|
(25% mol Na2O) |
0.65 |
W/m K |
150 K |
|
(25% mol Na2O) |
0.80 |
W/m K |
190 K |
|
(25% mol Na2O) |
0.85 |
W/m K |
240 K |
Source: compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko-Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 105. THERMAL CONDUCTIVITY OF GLASSES
(SHEET 3 OF 5)
|
|
Thermal |
|
Temperature Range |
Glass |
Description |
Conductivity |
Units |
of Validity |
SiO2-Na2O glass (Con’t) |
(25% mol Na2O) |
0.90 |
W/m K |
280 K |
|
(25% mol Na2O) |
0.95 |
W/m K |
300 K |
|
(27% mol Na2O) |
0.68 |
kcal/m hr K |
450˚C |
|
(27% mol Na2O) |
0.85 |
kcal/m hr K |
850˚C |
|
(27% mol Na2O) |
1.10 |
kcal/m hr K |
1050˚C |
|
(27% mol Na2O) |
1.45 |
kcal/m hr K |
1250˚C |
|
(27% mol Na2O) |
1.80 |
kcal/m hr K |
1500˚C |
|
(34.05% mol Na2O) |
0.5 |
kcal/m hr K |
450˚C |
|
(34.05% mol Na2O) |
0.75 |
kcal/m hr K |
850˚C |
|
(34.05% mol Na2O) |
0.75 |
kcal/m hr K |
1050˚C |
|
(34.05% mol Na2O) |
1.20 |
kcal/m hr K |
1250˚C |
|
(34.05% mol Na2O) |
1.5 |
kcal/m hr K |
1500˚C |
Source: compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko-Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 105. THERMAL CONDUCTIVITY OF GLASSES
(SHEET 4 OF 5)
|
|
Thermal |
|
Temperature Range |
Glass |
Description |
Conductivity |
Units |
of Validity |
|
|
|
|
|
|
|
|
|
|
SiO2-PbO glass |
(51.9% mol PbO) |
0.00089 |
cal/cm s K |
-150˚C |
|
(51.9% mol PbO) |
0.00100 |
cal/cm s K |
-100˚C |
|
(51.9% mol PbO) |
0.00111 |
cal/cm s K |
-50˚C |
|
(51.9% mol PbO) |
0.00123 |
cal/cm s K |
0˚C |
|
(51.9% mol PbO) |
0.00134 |
cal/cm s K |
50˚C |
|
(51.9% mol PbO) |
0.00146 |
cal/cm s K |
100˚C |
|
(49.3% mol PbO) |
0.00130 |
cal/cm s K |
40˚C |
|
(66.2% mol PbO) |
0.00112 |
cal/cm s K |
40˚C |
B2O3 glass |
|
0.5 |
mW/cm K |
2K |
|
|
0.75 |
mW/cm K |
5K |
|
|
1.5 |
mW/cm K |
20K |
B2O3-Na2O glass |
(3% mol Na2O) |
1.7 + 0.0054 (T-900) |
W/m K |
1173-1373 K |
|
(7% mol Na2O) |
1.5 + 0.0045 (T-900) |
W/m K |
1173-1373 K |
|
(11% mol Na2O) |
1.25 + 0.0037 (T-900) |
W/m K |
1173-1373 K |
|
|
|
|
|
Source: compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko-Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 105. THERMAL CONDUCTIVITY OF GLASSES
(SHEET 5 OF 5)
|
|
Thermal |
|
Temperature Range |
Glass |
Description |
Conductivity |
Units |
of Validity |
B2O3-Na2O glass (Con’t) |
(14% mol Na2O) |
1.15 + 0.0020 (T-900) |
W/m K |
1173-1373 K |
|
(19% mol Na2O) |
1.0 + 0.0012 (T-900) |
W/m K |
1173-1373 K |
|
(25% mol Na2O) |
0.85 + 0.00075 (T-900) |
W/m K |
1173-1373 K |
|
(31% mol Na2O) |
0.9 + 0.00080 (T-900) |
W/m K |
1173-1373 K |
B2O3-PbO glass |
(27.6% mol PbO) |
0.522±0.022 |
W/m K |
30˚C |
|
(31.9% mol PbO) |
0.483±0.016 |
W/m K |
30˚C |
|
(36.7% mol PbO) |
0.464±0.010 |
W/m K |
30˚C |
|
(42.1% mol PbO) |
0.433±0.018 |
W/m K |
30˚C |
|
(48.3% mol PbO) |
0.406±0.020 |
W/m K |
30˚C |
|
(55.5% mol PbO) |
0.381±0.015 |
W/m K |
30˚C |
|
(64.0% mol PbO) |
0.351±0.011 |
W/m K |
30˚C |
Source: compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko-Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

z
Table 106. THERMAL CONDUCTIVITY OF
CRYOGENIC INSULATION
|
|
Thermal Conductivity |
Interspace |
|
|
|
Range |
||
|
Cryogenic |
Pressure |
||
Class* |
(mW • m–1 • K–1) |
|||
Insulation |
(mm Hg) |
|||
|
|
|
|
|
|
|
|
|
|
2 |
Multilayer |
0.04—0.2 |
10–4 |
|
3 |
Opacified powder |
0.26—0.7 |
10–4 |
|
4 |
Evacuated powder |
1.0—2.0 |
10–4 |
|
5 |
Vacuum flask |
5.0 |
10–6 |
|
6 |
Gas–filled powder |
1.7—7.0 |
760 |
|
7 |
Expanded foam |
5.0—35 |
760 |
|
8 |
Fiber blanket |
35—45 |
760 |
|
|
|
|
|
To convert mm Hg to N • m–2 multiply by 133.32.
Source: From Boltz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 529.
*1. Liquid and vapor shields – Very low–temperature, valuable, or dangerous liquids such as helium or fluorine are often shielded by an intermediate cryogenic liquid or vapor container that must in turn be insulated by one of the methods described below.
2.Multilayer reflecting shields – Foil or aluminized plastic alternated with paper-thin glass or plastic-fiber sheets; lowest conductivity, low density, and heat storage; good stability; minimum support structure.
3.Opacified evacuated powders - Contain metallic flakes to reduce radiation; conform to irregular shapes.
4.Evacuated dielectric powders - Very fine powders of low-conductivity adsorbent; moderate vacuum requirement; minimum fire hazard in oxygen.
5.Vacuum flasks (Dewar) - Tight shield-space with highly. reflecting walls and high vacuum; minimum heat capacity; rugged; small thickness.
6.Gas-filled powders – Same powders as Class 4 but with air or inert gas; low cost; easy application; no vacuum requirement.
7.Expanded foams – Very light foamed plastic; inexpensive; minimum weight but bulky; self supporting.
8.Porous fiber blankets – Blanket material of fine fibers, usually glass; minimum cost and easy installation but not an adequate insulation for most cryogenic applications.
©2001 CRC Press LLC
Table 107. THERMAL CONDUCTIVITY OF
CRYOGENIC SUPPORTS
Insulation |
Mean Thermal Conductivity * |
|
(W • m–1 • K–1) |
||
Support |
||
|
|
|
|
|
|
Aluminum alloy |
86 |
|
“K” Monel® |
17 |
|
Stainbss steel |
9.3 |
|
Titanhm alloy |
6.1 |
|
Nylon |
0.29 |
|
Teflon |
0.24 |
|
|
|
Source: From Boltz, R. E. and Tuve, G. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, 529.
*Range of Validity is 20–300 K.
©2001 CRC Press LLC

Table 108. THERMAL CONDUCTIVITY OF SPECIAL CONCRETES*
|
Thermal Conductivity |
Description; type of aggregate |
Btu / (hr • ft • ˚F) |
|
|
|
|
Frost resisting; 1% CaCl2; normal aggregates |
1.0 |
Frost-resisting porous;6% air entrainment |
0.85 |
Lightweight; with expanded shale or clay |
0.25 |
Lightweight; with foamed slag |
0.20 |
Cinder concrete; fine and coarse |
0.25 |
Pulverized fuel ash |
0.25 |
Lightweight refractory concrete with aluminous cement |
0.20 |
Lightweight; insulating, with perlite |
0.15 |
Lightweight; insulating, with expanded vermiculite |
0.10 |
|
|
Source: from Bolz, R. E. and Tuve, C. L., Eds., Handbook of Tables for Applied Engineering Science, 2nd ed., CRC Press, Cleveland, 1973, p.645.
*A great many varieties of aggregates have been used for concrete, dependent largely on the materials available. In general, high density concretes have high strength and high thermal conductivity, although such variables as water/cement ratio, percentage of fines, and curing conditions may result in wide differences in properties with the same materials.
©2001 CRC Press LLC
Table 109. THERMAL CONDUCTIVITY OF
SIC-WHISKER-REINFORCED CERAMICS
|
Thermal Conductivity |
||
|
|
(W/m • K) |
|
|
|
|
|
Composite |
at 22 °C |
|
at 600 °C |
|
|
|
|
|
|
|
|
Alumina |
36 ± 5 |
|
12 ± 3 |
Alumina with 20 vol% SiC whiskers |
32 |
|
16 |
SiC |
95 |
|
50 |
Mullite with 20 vol% SiC whiskers |
7.2 |
|
— |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p173,(1994).
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 1 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
ABS Resins; Molded, Extruded |
Medium impact |
0.08—0.18 |
|
High impact |
0.12—0.16 |
|
Very high impact |
0.01—0.14 |
|
Low temperature impact |
0.08—0.14 |
|
Heat resistant |
0.12—0.20 |
Acrylics; Cast, Molded, Extruded |
Cast Resin Sheets, Rods: |
|
|
General purpose, type I |
0.12 |
|
General purpose, type II |
0.12 |
|
Moldings: |
|
|
Grades 5, 6, 8 |
0.12 |
|
High impact grade |
0.12 |
Thermoset Carbonate |
Allyl diglycol carbonate |
1.45 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 2 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Alkyds; Molded |
Putty (encapsulating) |
0.35—0.60 |
|
Rope (general purpose) |
0.35—0.60 |
|
Granular (high speed molding) |
0.35—0.60 |
|
Glass reinforced (heavy duty parts) |
0.20—0.30 |
Cellulose Acetate; Molded, Extruded |
ASTM Grade: |
|
|
H6—1 |
0.10—0.19 |
|
H4—1 |
0.10—0.19 |
|
H2—1 |
0.10—0.19 |
|
MH—1, MH—2 |
0.10—0.19 |
|
MS—1, MS—2 |
0.10—0.19 |
|
S2—1 |
0.10—0.19 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 3 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Cellulose Acetate Butyrate; |
ASTM Grade: |
|
Molded, Extruded |
|
|
|
|
|
|
H4 |
0.10—0.19 |
|
MH |
0.10—0.19 |
|
S2 |
0.10—0.19 |
Cellulose Acetate Propionate; Molded, Extruded |
ASTM Grade: |
|
|
1 |
0.10—0.19 |
|
3 |
0.10—0.19 |
|
6 |
0.10—0.19 |
Chlorinated Polymers |
Chlorinated polyether |
0.91 |
|
Chlorinated polyvinyl chloride |
0.95 |
Polycarbonates |
Polycarbonate |
0.11 |
|
Polycarbonate (40% glass fiber reinforced) |
0.13 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 4 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Fluorocarbons; Molded,Extruded |
Polytrifluoro chloroethylene (PTFCE) |
0.145 |
|
Polytetrafluoroethylene (PTFE) |
0.14 |
|
Fluorinated ethylene propylene(FEP) |
0.12 |
|
Polyvinylidene— fluoride (PVDF) |
0.14 |
Epoxies; Cast, Molded, Reinforced |
Standard epoxies (diglycidyl ethers of bisphenol |
|
A) |
|
|
|
|
|
|
Cast rigid |
0.1—0.3 |
|
Molded |
0.1—0.5 |
|
High strength laminate |
2.35 |
Melamines; Molded |
Filler & type |
|
|
Cellulose electrical |
0.17—0.20 |
|
Glass fiber |
0.28 |
Nylons; Molded, Extruded |
Type 6 |
|
|
General purpose |
1.2—1.69 |
|
Glass fiber (30%) reinforced |
1.69—3.27 |
|
Cast |
1.2—1.7 |
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 5 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Nylons; Molded, Extruded (Con’t) |
Type 11 |
1.5 |
|
Type 12 |
1.7 |
|
6/6 Nylon |
|
|
General purpose molding |
1.69—1.7 |
|
Glass fiber reinforced |
1.5— 3.3 |
|
General purpose extrusion |
1.7 |
|
6/10 Nylon |
|
|
General purpose |
1.5 |
|
Glass fiber (30%) reinforced |
3.5 |
Phenolics; Molded |
Type and filler |
|
|
General: woodflour and flock |
0.097—0.3 |
|
Shock: paper, flock, or pulp |
0.1—0.16 |
|
High shock: chopped fabric or cord |
0.097—0.170 |
|
Very high shock: glass fiber |
0.2 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 6 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Phenolics: Molded |
Arc resistant—mineral |
0.24—0.34 |
|
Rubber phenolic—woodflour or flock |
0.12 |
|
Rubber phenolic—chopped fabric |
0.05 |
|
Rubber phenolic—asbestos |
0.04 |
|
ABS–Polycarbonate Alloy |
2.46 (per ft) |
PVC–Acrylic Alloy |
PVC–acrylic sheet |
1.01 |
|
PVC–acrylic injection molded |
0.98 |
Polymides |
Unreinforced |
6.78 |
|
Unreinforced 2nd value |
3.8 |
|
Glass reinforced |
3.59 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 7 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Polyacetals |
Homopolymer: |
|
|
Standard |
0.13 |
|
Copolymer: |
|
|
Standard |
0.16 |
|
High flow |
1.6 |
Polyester; Thermoplastic |
Injection Moldings: |
|
|
General purpose grade |
0.36—0.55 |
Polyesters: Thermosets |
Cast polyyester |
|
|
Rigid |
0.10—0.12 |
|
Reinforced polyester moldings |
|
|
High strength (glass fibers) |
1.32—1.68 |
Phenylene Oxides |
SE—100 |
1.1 |
|
SE—1 |
1.5 |
|
Glass fiber reinforced |
1.15,1.1 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 8 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Phenylene oxides (Noryl) |
Standard |
1.8 |
|
Polyarylsulfone |
1.1 |
Polypropylene: |
General purpose |
1.21—1.36 |
|
High impact |
1.72 |
|
Polyphenylene sulfide: |
|
|
Standard |
2 |
|
40% glass reinforced |
2 |
Polyethylenes; Molded, Extruded |
Type I—lower density (0.910—0.925) |
|
|
Melt index 0.3—3.6 |
0.19 |
|
Melt index 6—26 |
0.19 |
|
Melt index 200 |
0.19 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 9 OF 10)
|
|
Thermal Conductivity |
|
|
(ASTM C177) |
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
Polyethylenes; Molded, Extruded (Con’t) |
Type II—medium density (0.926—0.940) |
|
|
Melt index 20 |
0.19 |
|
Melt index l.0—1.9 |
0.19 |
|
Type III—higher density (0.941—0.965) |
|
|
Melt index 0.2—0.9 |
0.19 |
|
Melt Melt index 0.l—12.0 |
0.19 |
|
Melt index 1.5—15 |
0.19 |
|
High molecular weight |
0.19 |
Polystyrenes; Molded |
Polystyrenes |
|
|
General purpose |
0.058—0.090 |
|
Medium impact |
0.024—0.090 |
|
High impact |
0.024—0.090 |
|
Glass fiber -30% reinforced |
0.117 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 110. THERMAL CONDUCTIVITY OF POLYMERS
(SHEET 10 OF 10)
|
|
Thermal Conductivity |
|
|
|
(ASTM C177) |
|
Class |
Polymer |
Btu / (hr • ft • ˚F) |
|
|
|
|
|
|
|
|
|
Polyvinyl Chloride And Copolymers; Molded, |
Nonrigid—general |
0.07—0.10 |
|
Extruded |
|||
|
|
||
|
Nonrigid—electrical |
0.07—0.10 |
|
|
Rigid—normal impact |
0.07—0.10 |
|
|
Vinylidene chloride |
0.053 |
|
Silicones; Molded, Laminated |
Fibrous (glass) reinforced silicones |
0.18 |
|
|
Granular (silica) reinforced silicones |
0.25—0.5 |
|
|
Woven glass fabric/ silicone laminate |
0.075—0.125 |
|
Ureas; Molded |
Alpha—cellulose filled (ASTM Type l) |
0.17—0.244 |
|
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC
Table 111. THERMAL CONDUCTIVITY OF
FIBERGLASS REINFORCED PLASTICS
|
|
Glass |
Thermal |
||
|
|
conductivity |
|||
|
|
fiber content |
|||
|
|
(Btu • in/ft2 • h •˚F) |
|||
Class |
Material |
(wt%) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
Glass fiber reinforced |
Sheet molding compound (SMC) |
15 to 30 |
1.3 to |
1.7 |
|
thermosets |
|||||
|
|
|
|
||
|
Bulk molding compound(BMC) |
15 to 35 |
1.3 to |
1.7 |
|
|
Preform/mat(compression molded) |
25 to 50 |
1.3 to |
1.8 |
|
|
Cold press molding–polyester |
20 to 30 |
1.3 to |
1.8 |
|
|
Spray–up–polyester |
30 to 50 |
1.2 to |
1.6 |
|
|
Filament wound–epoxy |
30 to 80 |
1.92 to |
2.28 |
|
|
Rod stock–polyester |
40 to 80 |
1.92 to |
2.28 |
|
|
Molding compound–phenolic |
5 to 25 |
1.1 to |
2.0 |
|
Glass–fiber–reinforced |
Thermoplastic polyester |
20 to 35 |
1.3 |
|
|
thermoplastic |
|
||||
|
|
|
|
||
|
|
|
|
|
|
To convert (Btu • in/ft2 • h •˚F) to (W/m • K), multiply by 0.144
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p106, (1994).
©2001 CRC Press LLC

Table 112. THERMAL EXPANSION OF
WROUGHT STAINLESS STEELS* (SHEET 1 OF 2)
|
|
Coefficient of Thermal Expansion |
||
|
|
|
(µm/m • °C) |
|
|
|
|
|
|
Type |
UNS Designation |
0-100°C |
100-315°C |
0-538°C |
|
|
|
|
|
|
|
|
|
|
201 |
S20100 |
15.7 |
17.5 |
18.4 |
202 |
S20200 |
17.5 |
18.4 |
19.2 |
205 |
S20500 |
— |
17.9 |
19.1 |
301 |
S30100 |
17.0 |
17.2 |
18.2 |
302 |
S30200 |
17.2 |
17.8 |
18.4 |
302B |
S30215 |
16.2 |
18.0 |
19.4 |
303 |
S30300 |
17.2 |
17.8 |
18.4 |
304 |
S30400 |
17.2 |
17.8 |
18.4 |
S30430 |
S30430 |
17.2 |
17.8 |
— |
305 |
S30500 |
17.2 |
17.8 |
18.4 |
308 |
S30800 |
17.2 |
17.8 |
18.4 |
309 |
S30900 |
15.0 |
16.6 |
17.2 |
310 |
S31000 |
15.9 |
16.2 |
17.0 |
314 |
S31400 |
— |
15.1 |
— |
316 |
S31600 |
15.9 |
16.2 |
17.5 |
317 |
S31700 |
15.9 |
16.2 |
17.5 |
317L |
S31703 |
16.5 |
— |
18.1 |
321 |
S32100 |
16.6 |
17.2 |
18.6 |
330 |
N08330 |
14.4 |
16.0 |
16.7 |
347 |
S34700 |
16.6 |
17.2 |
18.6 |
384 |
S38400 |
17.2 |
17.8 |
18.4 |
405 |
S40500 |
10.8 |
11.6 |
12.1 |
409 |
S40900 |
11.7 |
— |
— |
410 |
S41000 |
9.9 |
11.4 |
11.6 |
414 |
S41400 |
10.4 |
11.0 |
12.1 |
416 |
S41600 |
9.9 |
11.0 |
11.6 |
420 |
S42000 |
10.3 |
10.8 |
11.7 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p360, (1993).
©2001 CRC Press LLC

Table 112. THERMAL EXPANSION OF
WROUGHT STAINLESS STEELS* (SHEET 2 OF 2)
|
|
Coefficient of Thermal Expansion |
||
|
|
|
(µm/m • °C) |
|
|
|
|
|
|
Type |
UNS Designation |
0-100°C |
100-315°C |
0-538°C |
|
|
|
|
|
|
|
|
|
|
422 |
S42200 |
11.2 |
11.4 |
11.9 |
429 |
S42900 |
10.3 |
— |
— |
430 |
S43000 |
10.4 |
11.0 |
11.4 |
430F |
S43020 |
10.4 |
11.0 |
11.4 |
431 |
S43100 |
10.2 |
12.1 |
— |
434 |
S43400 |
10.4 |
11.0 |
11.4 |
436 |
S43600 |
9.3 |
— |
— |
440A |
S44002 |
10.2 |
— |
— |
440C |
S44004 |
10.2 |
— |
— |
444 |
S44400 |
10.0 |
10.6 |
11.4 |
446 |
S44600 |
10.4 |
10.8 |
11.2 |
PH 13–8 Mo |
S13800 |
10.6 |
11.2 |
11.9 |
15–5 PH |
S15500 |
10.8 |
11.4 |
— |
17–4 PH |
S17400 |
10.8 |
11.6 |
— |
17–7 PH |
S17700 |
11.0 |
11.6 |
— |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p360, (1993).
* Annealed Condition.
©2001 CRC Press LLC

Table 113. THERMAL EXPANSION OF WROUGHT TITANIUM ALLOYS
(SHEET 1 OF 2)
|
|
|
Coefficient of Linear Thermal Expansion (µm/m • K) |
|
||||
|
|
|
|
|
|
|
|
|
Class |
Metal or Alloy |
20-100 °C |
20-205 °C |
20-315 °C |
20-425 °C |
20-540 °C |
20-650 °C |
20-815 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commercially Pure |
99.5Ti |
8.6 |
— |
9.2 |
— |
9.7 |
10.1 |
10.1 |
|
99.2Ti |
8.6 |
— |
9.2 |
— |
9.7 |
10.1 |
10.1 |
|
99.1Ti |
8.6 |
— |
9.2 |
— |
9.7 |
10.1 |
10.1 |
|
99.0Ti |
8.6 |
— |
9.2 |
— |
9.7 |
10.1 |
10.1 |
|
99.2 Ti–0.2Pd |
8.6 |
— |
9.2 |
— |
9.7 |
10.1 |
10.1 |
Alpha Alloys |
Ti-5Al-2.5Sn |
9.4 |
— |
9.5 |
— |
9.5 |
9.7 |
10.1 |
|
Ti-5Al-2.5Sn (low O2) |
9.4 |
— |
9.5 |
— |
9.7 |
9.9 |
10.1 |
Near Alpha Alloys |
Ti-8Al-1Mo-1V |
8.5 |
— |
9.0 |
— |
10.1 |
10.3 |
— |
|
Ti-11Sn-1Mo-2.25Al-5.0Zr-1Mo-0.2Si |
8.5 |
— |
9.2 |
— |
9.4 |
— |
— |
|
Ti-6Al-2Sn-4Zr-2Mo |
7.7 |
— |
8.1 |
— |
8.1 |
— |
— |
|
Ti-5Al-5Sn-2Zr-2Mo-0.25Si |
— |
— |
— |
— |
— |
— |
10.3 |
|
Ti-6Al-2Nb-1Ta-1Mo |
— |
— |
— |
— |
— |
9.0 |
— |
|
|
|
|
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p511, (1993).
©2001 CRC Press LLC

Table 113. THERMAL EXPANSION OF WROUGHT TITANIUM ALLOYS
(SHEET 2 OF 2)
|
|
|
Coefficient of Linear Thermal Expansion (µm/m • K) |
|
||||
|
|
|
|
|
|
|
|
|
Class |
Metal or Alloy |
20-100 °C |
20-205 °C |
20-315 °C |
20-425 °C |
20-540 °C |
20-650 °C |
20-815 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alpha-Beta Alloys |
Ti-8Mn |
8.6 |
9.2 |
9.7 |
10.3 |
10.8 |
11.7 |
12.6 |
|
Ti-3Al-2.5V |
9.5 |
— |
9.9 |
— |
9.9 |
— |
— |
|
Ti-6Al-4V |
8.6 |
9.0 |
9.2 |
9.4 |
9.5 |
9.7 |
— |
|
Ti-6Al-4V (low O2) |
8.6 |
9.0 |
9.2 |
9.4 |
9.5 |
9.7 |
— |
|
Ti-6Al-6V-2Sn |
9.0 |
— |
9.4 |
— |
9.5 |
— |
— |
|
Ti-7Al-4Mo |
9.0 |
9.2 |
9.4 |
9.7 |
10.1 |
10.4 |
11.2 |
|
Ti-6Al-2Sn-4Zr-6Mo |
9.0 |
9.2 |
9.4 |
9.5 |
9.5 |
— |
— |
|
Ti-6Al-2Sn-2Zr-2Mo-2Cr-0.25Si |
— |
— |
9.2 |
— |
— |
— |
— |
Beta Alloys |
Ti-13V-11Cr-3Al |
9.4 |
— |
10.1 |
— |
10.6 |
— |
— |
|
Ti-8Mo-8V-2Fe-3Al |
— |
— |
— |
— |
— |
— |
— |
|
Ti-3Al-8V-6Cr-4Mo-4Zr |
— |
— |
— |
9.68 |
— |
— |
— |
|
(to 900 •F) |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p511, (1993). |
|
|
||||||
|
|
|
|
|
|
|
|
|
©2001 CRC Press LLC
Table 114. THERMAL EXPANSION OF
GRAPHITE MAGNESIUM CASTINGS*
|
|
|
|
|
Coefficient |
|
|
|
|
|
of Thermal |
|
|
|
|
Fiber Preform |
Expansion |
|
|
|
|
(10-6/K) |
|
Fiber Type |
Fiber content |
Fiber orientation |
Casting |
Method |
|
|
|
|
|
|
|
|
|
|
|
|
|
P75 |
40% |
±16° |
Hollow cylinder |
Filament wound |
1.3 |
|
plus 9% |
90° |
Hollow cylinder |
Filament wound |
1.3 |
P100 |
40% |
± 16° |
Hollow cylinder |
Filament wound |
–0.07 |
P55 |
40% |
0° |
Plate |
Prepreg |
2.3 |
|
30% |
0° plus |
Plate |
Prepreg |
4.5 |
|
10% |
90° |
Plate |
Prepreg |
4.5 |
|
|
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p148,(1994).
*Pitch-base fibers
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 1 OF 8)
|
|
|
Coefficient of |
|
|
|
Temperature |
Thermal Expansion |
|
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
|
|
Aluminum and |
Aluminum (99.996%) |
20 to 100 |
23.6 |
|
Aluminum Alloys |
||||
|
|
|
||
Wrought Alloys |
EC, 1060, 1100 |
20 to 100 |
23.6 |
|
|
2011, 2014 |
20 to 100 |
23.0 |
|
|
2024 |
20 to 100 |
22.8 |
|
|
2218 |
20 to 100 |
22.3 |
|
|
3003 |
20 to 100 |
23.2 |
|
|
4032 |
20 to 100 |
19.4 |
|
|
5005, 5050, 5052 |
20 to 100 |
23.8 |
|
|
5056 |
20 to 100 |
24.1 |
|
|
5083 |
20 to 100 |
23.4 |
|
|
5086 |
60 to 300 |
23.9 |
|
|
5154 |
20 to 100 |
23.9 |
|
|
5357 |
20 to 100 |
23.7 |
|
|
5456 |
20 to 100 |
23.9 |
|
|
6061, 6063 |
20 to 100 |
23.4 |
|
|
6101, 6151 |
20 to 100 |
23.0 |
|
|
7075 |
20 to 100 |
23.2 |
|
|
7079, 7178 |
20 to 100 |
23.4 |
|
Casting Alloys |
A13 |
20 to 100 |
20.4 |
|
|
43 and 108 |
20 to 100 |
22.0 |
|
|
A108 |
20 to 100 |
21.5 |
|
|
A132 |
20 to 100 |
19.0 |
|
|
D132 |
20 to 100 |
20.05 |
|
|
F132 |
20 to 100 |
20.7 |
|
|
138 |
20 to 100 |
21.4 |
|
|
142 |
20 to 100 |
22.5 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 2 OF 8)
|
|
|
Coefficient of |
|
|
Temperature |
Thermal Expansion |
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
195 |
20 to 100 |
23.0 |
|
B195 |
20 to 100 |
22.0 |
|
214 |
20 to 100 |
24.0 |
|
220 |
20 to 100 |
25.0 |
|
319 |
20 to 100 |
21.5 |
|
355 |
20 to 100 |
22.0 |
|
356 |
20 to 100 |
21.5 |
|
360 |
20 to 100 |
21.0 |
|
750 |
20 to 100 |
23.1 |
|
40E |
21 to 93 |
24.7 |
Copper and Copper |
|
|
|
Alloys |
|
|
|
Wrought Coppers |
Pure copper |
20 |
16.5 |
|
Electrolytic tough pitch |
20 to 100 |
16.8 |
|
copper (ETP) |
||
|
|
|
|
|
Deoxidized copper, high |
|
|
|
residual phosphorus |
20 to 300 |
17.7 |
|
(DHP) |
|
|
|
Oxygen-free copper |
20 to 300 |
17.7 |
|
Free machining copper, |
20 to 300 |
17.7 |
|
0.5% Te or 1% Pb |
||
|
|
|
|
Wrought Alloys |
Gilding, 95% |
20 to 300 |
18.1 |
|
Commercial bronze, 90% |
20 to 300 |
18.4 |
|
Jewelry bronze, 87.5% |
20 to 300 |
18.6 |
|
Red brass, 85% |
20 to 300 |
18.7 |
|
Low brass, 80% |
20 to 300 |
19.1 |
|
Cartridge brass, 70% |
20 to 300 |
19.9 |
|
Yellow brass |
20 to 300 |
20.3 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 3 OF 8)
|
|
|
Coefficient of |
|
|
Temperature |
Thermal Expansion |
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
Muntz metal |
20 to 300 |
20.8 |
|
Leaded commercial bronze |
20 to 300 |
18.4 |
|
Low-leaded brass |
20 to 300 |
20.2 |
|
Medium-leaded brass |
20 to 300 |
20.3 |
|
High-leaded brass |
20 to 300 |
20.3 |
|
Extra-high-leaded brass |
20 to 300 |
20.5 |
|
Free-cutting brass |
20 to 300 |
20.5 |
|
Leaded Muntz metal |
20 to 300 |
20.8 |
|
Forging brass |
20 to 300 |
20.7 |
|
Architectural bronze |
20 to 300 |
20.9 |
|
Inhibited admiralty |
20 to 300 |
20.2 |
|
Naval brass |
20 to 300 |
21.2 |
|
Leaded naval brass |
20 to 300 |
21.2 |
|
Manganese bronze |
20 to 300 |
21.2 |
|
(longitudinal) |
||
|
|
|
|
|
Manganese bronze |
20 to 300 |
23.4 |
|
(transverse) |
||
|
|
|
|
|
Phosphor bronze, 5% |
20 to 300 |
17.8 |
|
(longitudinal) |
||
|
|
|
|
|
Phosphor bronze, 5% |
20 to 300 |
23.4 |
|
(transverse) |
||
|
|
|
|
|
Phosphor bronze, 8% |
20 to 300 |
18.2 |
|
(longitudinal) |
||
|
|
|
|
|
Phosphor bronze, 8% |
20 to 300 |
19.4 |
|
(transverse) |
||
|
|
|
|
|
Phosphor bronze, 10% |
20 to300 |
18.4 |
|
(longitudinal) |
||
|
|
|
|
|
Phosphor bronze, 1.25% |
20 to 300 |
17.8 |
|
Free-cutting phosphor |
20 to 300 |
17.3 |
|
bronze |
||
|
|
|
|
|
Cupro-nickel, 30% |
20 to 300 |
16.2 |
|
Cupro-nickel, 10% |
20 to 300 |
17.1 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 4 OF 8)
|
|
|
Coefficient of |
|
|
Temperature |
Thermal Expansion |
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
Nickel silver, 65-18 |
20 to 300 |
16.2 |
|
Nickel silver, 55-18 |
20 to 300 |
16.7 |
|
Nickel silver, 65-12 |
20 to 300 |
16.2 |
|
High-silicon bronze |
20 to 300 |
18.0 |
|
(longitudinal) |
||
|
|
|
|
|
High-silicon bronze |
20 to 300 |
23.4 |
|
(transverse) |
||
|
|
|
|
|
Low-silicon bronze |
20 to 300 |
17.9 |
|
(longitudinal) |
||
|
|
|
|
|
Low-silicon bronze |
20 to 300 |
21.1 |
|
(transverse) |
||
|
|
|
|
|
Aluminum bronze |
20 to 300 |
16.4 |
|
Aluminum-silicon bronze |
20 to 300 |
18.0 |
|
Aluminum bronze |
20 to 300 |
16.8 |
|
Beryllium copper |
20 to 300 |
17.8 |
Casting Alloys |
88Cu-8Sn-4Zn |
21 to 177 |
18.0 |
|
89Cu-11Sn |
20 to 300 |
18.4 |
|
88Cu-6Sn-1.5Pb-4 .5Zn |
21 to 260 |
18.5 |
|
87Cu-8Sn-1Pb-4Zn |
21 to 177 |
18.0 |
|
87Cu-10Sn-1Pb-2Zn |
21 to 177 |
18.0 |
|
80Cu-10Sn-10Pb |
21 to 204 |
18.5 |
|
78Cu-7Sn-15Pb |
21 to 204 |
18.5 |
|
85Cu-5Sn-5Pb- 5Zn |
21 to 204 |
18.1 |
|
72Cu-1Sn-3Pb-24Zn |
21 to 93 |
20.7 |
|
67Cu-1Sn-3Pb-29Zn |
21 to 93 |
20.2 |
|
61Cu-1Sn-1Pb-37Zn |
21 to 260 |
21.6 |
Manganese bronze |
Manganese bronze, 60 ksi |
21 to 204 |
20.5 |
|
Manganese bronze, 65 ksi |
21 to 93 |
21.6 |
|
Manganese bronze, 110 ksi |
21 to 260 |
19.8 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 5 OF 8)
|
|
|
Coefficient of |
|
|
|
Temperature |
Thermal Expansion |
|
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
|
|
Iron and Iron Alloys |
Pure iron |
20 |
11.7 |
|
|
Fe-C alloy 0.06% C |
20 to 100 |
11.7 |
|
|
Fe-C alloy 0.22% C |
20 to 100 |
11.7 |
|
|
Fe-C alloy 0.40% C |
20 to 100 |
11.3 |
|
|
Fe-C alloy 0.56% C |
20 to 100 |
11.0 |
|
|
Fe-C alloy 1.08% C |
20 to 100 |
10.8 |
|
|
Fe-C alloy 1.45% C |
20 to 100 |
10.1 |
|
|
Invar (36% Ni) |
20 |
0-2 |
|
|
13Mn-1.2C |
20 |
18.0 |
|
|
13Cr-0.35C |
20 to 100 |
10.0 |
|
|
12.3Cr-0.4Ni-0.09C |
20 to 100 |
9.8 |
|
|
17.7Cr-9.6Ni-0.06C |
20 to 100 |
16.5 |
|
|
18W-4Cr-1V |
0 to 100 |
11.2 |
|
|
Gray cast iron |
0 to 100 |
10.5 |
|
|
Malleable iron (pearlitic) |
20 to 400 |
12 |
|
Lead and Lead Alloys |
Corroding lead |
17 to 100 |
29.3 |
|
(99.73 + % Pb) |
||||
|
|
|
||
|
5-95 solder |
15 to 110 |
28.7 |
|
|
20-80 solder |
15 to 110 |
26.5 |
|
|
50-50 solder |
15 to 110 |
23.4 |
|
|
1% antimonial lead |
20 to 100 |
28.8 |
|
|
8% antimonial lead |
20 to 100 |
26.7 |
|
|
9% antimonial lead |
20 to 100 |
26.4 |
|
|
Hard lead(96Pb-4Sb) |
20 to 100 |
27.8 |
|
|
Hard lead(94Pb-6Sb) |
20 to 100 |
27.2 |
|
|
Lead-base babbitt SAE 14 |
20 to 100 |
19.6 |
|
|
Lead-base babbitt Alloy 8 |
20 to 100 |
24.0 |
|
Magnesium and |
Magnesium (99.8%) |
20 |
25.2 |
|
Magnesium Alloys |
||||
|
|
|
||
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 6 OF 8)
|
|
|
Coefficient of |
|
|
|
Temperature |
Thermal Expansion |
|
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
|
|
Casting alloys |
AM100A |
18 to 100 |
25.2 |
|
|
AZ63A |
20 to 100 |
26.1 |
|
|
AZ91A,B,C |
20 to 100 |
26 |
|
|
AZ92A |
18 to 100 |
25.2 |
|
|
HZ32A |
20 to 200 |
26.7 |
|
|
ZH42 |
20 to 200 |
27 |
|
|
ZH62A |
20 to 200 |
27.1 |
|
|
ZK51A |
20 |
26.1 |
|
|
EZ33A |
20 to 100 |
26.1 |
|
|
EK30A, EK41A |
20 to 100 |
26.1 |
|
Wrought Alloys |
M1A, A3A |
20 to 100 |
26 |
|
|
AZ31B,PE |
20 to 100 |
26 |
|
|
AZ61A, Z80A |
20 to 100 |
26 |
|
|
ZK60A, B |
20 to 100 |
26 |
|
|
HM31A |
20 to 93 |
26.1 |
|
Nickel and Nickel |
Nickel (99.95% Ni + Co) |
0 to 100 |
13.3 |
|
Alloys |
||||
|
|
|
||
|
Duranickel |
0 to 100 |
13.0 |
|
|
Monel |
0 to 100 |
14.0 |
|
|
Monel (cast) |
25 to 100 |
12.9 |
|
|
Inconel |
20 to 100 |
11.5 |
|
|
Ni-o-nel |
27 to 93 |
12.9 |
|
|
Hastelloy B |
0 to 100 |
10.0 |
|
|
Hastelloy C |
0 to 100 |
11.3 |
|
|
Hastelloy D |
0 to 100 |
11.0 |
|
|
Hastelloy F |
20 to 100 |
14.2 |
|
|
Hastelloy N |
21 to 204 |
10.4 |
|
|
Hastelloy W |
23 to 100 |
11.3 |
|
|
Hastelloy X |
26 to 100 |
13.8 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 7 OF 8)
|
|
|
Coefficient of |
|
|
|
Temperature |
Thermal Expansion |
|
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
|
|
|
Illium G |
0 to 100 |
12.19 |
|
|
Illium R |
0 to 100 |
12.02 |
|
|
80Ni-20Cr |
20 to 1000 |
17.3 |
|
|
60Ni-24Fc-l6Cr |
20 to 1000 |
17.0 |
|
|
35Ni-45Fe-20Cr |
20 to 500 |
15.8 |
|
|
Constantan |
20 to 1000 |
18.8 |
|
Tin and Tin Alloys |
Pure tin |
0 to 100 |
23 |
|
|
Solder (70Sn-30Pb) |
15 to 110 |
21.6 |
|
|
Solder (63Sn-37Pb) |
15 to 110 |
24.7 |
|
Titanium and |
99.9% Ti |
20 |
8.41 |
|
Titanium Alloys |
||||
|
|
|
||
|
99.0% Ti |
93 |
8.55 |
|
|
Ti-5Al-2.5Sn |
93 |
9.36 |
|
|
Ti-8Mn |
93 |
8.64 |
|
Zinc and Zinc Alloys |
Pure zinc |
20 to 250 |
39.7 |
|
|
AG40A alloy |
20 to 100 |
27.4 |
|
|
AC41A alloy |
20 to 100 |
27.4 |
|
|
Commercial rolled zinc |
20 to 40 |
32.5 |
|
|
0.08 Pb |
|||
|
|
|
||
|
Commercial rolled zinc 0.3 |
20 to 98 |
33.9 |
|
|
Pb, 0.3 Cd |
|||
|
|
|
||
|
Rolled zinc alloy (1 Cu, |
20 to 100 |
34.8 |
|
|
0.010 Mg) |
|||
|
|
|
||
|
Zn-Cu-Ti alloy (0.8 Cu, |
20 to 100 |
24.9 |
|
|
0.15 Ti) |
|||
|
|
|
||
Pure Metals |
Beryllium |
25 to 100 |
11.6 |
|
|
Cadmium |
20 |
29.8 |
|
|
Calcium |
0 to 400 |
22.3 |
|
|
Chromium |
20 |
6.2 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 115. LINEAR THERMAL EXPANSION OF
METALS AND ALLOYS (SHEET 8 OF 8)
|
|
|
Coefficient of |
|
|
Temperature |
Thermal Expansion |
Class |
Metal or Alloy |
(°C) |
(µm/m • °C) |
|
|
|
|
|
|
|
|
|
Cobalt |
20 |
13.8 |
|
Gold |
20 |
14.2 |
|
Iridium |
20 |
6.8 |
|
Lithium |
20 |
56 |
|
Manganese |
0 to 100 |
22 |
|
Palladium |
20 |
11.76 |
|
Platinum |
20 |
8.9 |
|
Rhenium |
20 to 500 |
6.7 |
|
Rhodium |
20 to 100 |
8.3 |
|
Ruthenium |
20 |
9.1 |
|
Silicon |
0 to 1400 |
5 |
|
Silver |
0 to 100 |
19.68 |
|
Tungsten |
27 |
4.6 |
|
Vanadium |
23 to 100 |
8.3 |
|
Zirconium |
— |
5.85 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154-155, (1993).
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 1 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Borides |
Chromium Diboride (CrB2) |
4.6–11.1 x 10–6 for 20–1000˚C |
|
Hafnium Diboride (HfB2) |
5.5 –5.54 x 10–6 for room temp.–1000˚C |
|
Tantalum Diboride (TaB2) |
5.1 x 10–6 at room temp. |
|
Titanium Diboride (TiB2) |
4.6–8.1 x 10–6 |
|
Zirconium Diboride (ZrB2) |
5.69 x 10–6 for 25–500˚C |
|
|
5.5–6.57 x 10–6 ˚C for 25–1000˚C |
|
|
6.98 x 10–6 for 20–1500˚C |
Carbides |
Boron Carbide (B4C) |
4.5 x 10–6 for room temp.–800˚C |
|
|
4.78 x 10–6 for 25–500˚C |
|
|
5.54 x 10–6 for 25–1000˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 2 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Carbides (Con’t) |
Boron Carbide (B4C) (Con’t) |
6.02 x 10–6 for 25–1500˚C |
|
|
6.53 x 10–6 for 25–2000˚C |
|
|
7.08 x 10–6 for 25–2500˚C |
|
Hafnium Monocarbide (HfC) |
6.27–6.59 x 10–6 for 25–650˚C |
|
|
6.25 x 10–6 for 25–1000˚C |
|
Silicon Carbide (SiC) |
4.63 x 10–6 for 25–500˚C |
|
|
5.12 x 10–6 for 25–1000˚C |
|
|
5.48 x 10–6 for 25–1500˚C |
|
|
5.77 x 10–6 for 25–2000˚C |
|
|
5.94 x 10–6 for 25–2500˚C |
|
|
4.70 x 10–6 for 20–1500˚C |
|
|
4.70 x 10–6 for 0–1700˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 3 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Carbides (Con’t) |
Tantalum Monocarbide (TaC) |
6.29–6.32 x 10–6 for 25–500˚C |
|
|
6.67 x 10–6 for 25–1000˚C |
|
|
7.12 x 10–6 for 25–1500˚C |
|
|
7.64 x 10–6 for 25–2000˚C |
|
|
8.40 x 10–6 for 25–2500˚C |
|
|
6.50 x 10–6 for 0–1000˚C |
|
|
6.64 x 10–6 for 0–1200˚C |
|
Titanium Monocarbide (TiC) |
6.52–7.15 x 10–6 for 25–500˚C |
|
|
7.18–7.45 x 10–6 for 25–750˚C |
|
|
7.40–8.82 x 10–6 for 25–1000˚C |
|
|
9.32 x 10–6 for 25–1250˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 4 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Carbides (Con’t) |
Titanium Monocarbide (TiC) (Con’t) |
8.15–9.45 x 10–6 for 25–1500˚C |
|
|
8.81 x 10–6 for 25–2000˚C |
|
|
7.90 x 10–6 for 0–2500˚C |
|
|
7.08 x 10–6 for 0–750˚C |
|
|
7.85–7.86 x 10–6 for 0–1000˚C |
|
|
8.02 x 10–6 for 0–1275˚C |
|
|
8.29 x 10–6 for 0–1400˚C |
|
|
8.26 x 10–6 for 0–1525˚C |
|
|
8.40 x 10–6 for 0–1775˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 5 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Carbides (Con’t) |
Trichromium Dicarbide (Cr3C2) |
8.00 x 10–6 for 25–500˚C |
|
|
9.95 x 10–6 for 25–500˚C |
|
|
8.8 x 10–6 for 25–120˚C |
|
|
10.9 x 10–6 for 150–980˚C |
|
Tungsten Monocarbide (WC) |
4.42 x 10–6 for 25–500˚C |
|
|
4.84–4.92 x 10–6 for 25–1000˚C |
|
|
5.35–5.8 x 10–6 for 25–1500˚C |
|
|
5.82–7.4 x 10–6 for 25–2000˚C |
|
Zirconium Monocarbide (ZrC) |
6.10x 10–6 for 25–500˚C |
|
|
6.65x 10–6 for 25–800˚C |
|
|
6.56x 10–6 for 25–1000˚C |
|
|
7.06x 10–6 for 25–1500˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 6 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Carbides (Con’t) |
Zirconium Monocarbide (ZrC) (Con’t) |
7.65x 10–6 for 25–650˚C |
|
|
6.10–6.73 x 10–6 for 25–650˚C |
|
|
6.32x 10–6 for 0–750˚C |
|
|
6.46–6.66x 10–6 for 0–1000˚C |
|
|
6.68x 10–6 for 0–1275˚C |
|
|
6.83x 10–6 for 0–1525˚C |
|
|
6.98x 10–6 for 0–1775˚C |
|
|
9.0x 10–6 for 1000–2000˚C |
Nitrides |
Aluminum Nitride (AlN) |
4.03 x 10–6 for 25 to 200˚C |
|
|
4.84 x 10–6 for 25 to 500˚C |
|
|
4.83 x 10–6 for 25 to 600˚C |
|
|
5.54–5.64 x 10–6 for 25 to 1000˚C |
|
|
6.09 x 10–6 for 25 to 1350˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 7 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Nitrides (Con’t) |
Boron Nitride (BN) |
12.2 x 10–6 for 25 to 500˚C |
|
|
13.3 x 10–6 for 25 to 1000˚C |
|
parallel to c axis |
10.15 x 10–6 for 25 to 350˚C |
|
|
8.06 x 10–6 for 25 to 700˚C |
|
|
7.15 x 10–6 for 25 to 1000˚C |
|
parallel to a axis |
0.59 x 10–6 for 25 to 350˚C |
|
|
0.89 x 10–6 for 25 to 700˚C |
|
|
0.77 x 10–6 for 25 to 1000˚C |
|
Titanium Mononitride (TiN) |
9.35 x 10–6 |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 8 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Nitrides (Con’t) |
Trisilicon tetranitride (Si3N4) |
2.11 x 10–6 for 25 to 500˚C |
|
|
2.87 x 10–6 for 25 to 1000˚C |
|
|
3.66 x 10–6 for 25 to 1500˚C |
|
(hot pressed) |
3–3.9 x 10–6 for 20 to 1000˚C |
|
(sintered) |
3.5 x 10–6 for 20 to 1000˚C |
|
(reaction sintered) |
2.9 x 10–6 for 20 to 1000˚C |
|
(pressureless sintered) |
3.7 x 10–6 for 40 to 1000˚C |
|
Zirconium Mononitride (TiN) |
6.13 x 10–6 for 20–450˚C |
|
|
7.03 x 10–6 for 20–680˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 9 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides |
Aluminum Oxide (Al2O3) |
|
|
parallel to c axis |
1.95 x 10–6 for 0 to –273˚C |
|
|
3.01 x 10–6 for 0 to –173˚C |
|
|
4.39 x 10–6 for 0 to –73˚C |
|
|
5.31 x 10–6 for 0 to 27˚C |
|
|
6.26 x 10–6 for 0 to 127˚C |
|
|
6.86 x 10–6 for 0 to 227˚C |
|
|
7.31 x 10–6 for 0 to 327˚C |
|
|
7.68 x 10–6 for 0 to 427˚C |
|
|
7.96 x 10–6 for 0 to 527˚C |
|
|
8.19 x 10–6 for 0 to 627˚C |
|
|
8.38 x 10–6 for 0 to 727˚C |
|
|
8.52 x 10–6 for 0 to 827˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 10 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Aluminum Oxide (Al2O3) parallel to c axis (Con’t) |
8.65 x 10–6 for 0 to 927˚C |
|
|
8.75 x 10–6 for 0 to 1027˚C |
|
|
8.84 x 10–6 for 0 to 1127˚C |
|
|
8.92 x 10–6 for 0 to 1227˚C |
|
|
8.98 x 10–6 for 0 to 1327˚C |
|
|
9.02 x 10–6 for 0 to 1427˚C |
|
|
9.08 x 10–6 for 0 to 1527˚C |
|
|
9.13 x 10–6 for 0 to 1627˚C |
|
|
9.18 x 10–6 for 0 to 1727˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 11 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Aluminum Oxide (Al2O3) (single crystal) |
|
|
perpendicular to c axis |
1.65 x 10–6 for 0 to –273˚C |
|
|
2.55 x 10–6 for 0 to –173˚C |
|
|
3.75 x 10–6 for 0 to –73˚C |
|
|
4.78 x 10–6 for 0 to 27˚C |
|
|
5.51 x 10–6 for 0 to 127˚C |
|
|
6.10 x 10–6 for 0 to 227˚C |
|
|
6.52 x 10–6 for 0 to 327˚C |
|
|
6.88 x 10–6 for 0 to 427˚C |
|
|
7.15 x 10–6 for 0 to 527˚C |
|
|
7.35 x 10–6 for 0 to 627˚C |
|
|
7.53 x 10–6 for 0 to 727˚C |
|
|
7.67 x 10–6 for 0 to 827˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 12 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Aluminum Oxide (Al2O3) (single crystal) (Con’t) |
7.80 x 10–6 for 0 to 927˚C |
|
perpendicular to c axis (Con’t) |
7.88 x 10–6 for 0 to 1027˚C |
|
|
7.96 x 10–6 for 0 to 1127˚C |
|
|
8.05 x 10–6 for 0 to 1227˚C |
|
|
8.12 x 10–6 for 0 to 1327˚C |
|
|
8.16 x 10–6 for 0 to 1427˚C |
|
|
8.20 x 10–6 for 0 to 1527˚C |
|
|
8.26 x 10–6 for 0 to 1627˚C |
|
|
8.30 x 10–6 for 0 to 1727˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 13 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Aluminum Oxide (Al2O3) (Con’t) |
1.89 x 10–6 for 0 to –273˚C |
|
(polycrystalline) |
2.91 x 10–6 for 0 to –173˚C |
|
|
4.10 x 10–6 for 0 to –73˚C |
|
|
5.60 x 10–6 for 0 to 27˚C |
|
|
6.03 x 10–6 for 0 to 127˚C |
|
|
6.55 x 10–6 for 0 to 227˚C |
|
|
6.93 x 10–6 for 0 to 327˚C |
|
|
7.24 x 10–6 for 0 to 427˚C |
|
|
7.50 x 10–6 for 0 to 527˚C |
|
|
7.69 x 10–6 for 0 to 627˚C |
|
|
7.83 x 10–6 for 0 to 727˚C |
|
|
7.97 x 10–6 for 0 to 827˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 14 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Aluminum Oxide (Al2O3) (Con’t) |
8.08 x 10–6 for 0 to 927˚C |
|
(polycrystalline) (Con’t) |
8.18 x 10–6 for 0 to 1027˚C |
|
|
8.25 x 10–6 for 0 to 1127˚C |
|
|
8.32 x 10–6 for 0 to 1227˚C |
|
|
8.39 x 10–6 for 0 to 1327˚C |
|
|
8.45 x 10–6 for 0 to 1427˚C |
|
|
8.49 x 10–6 for 0 to 1527˚C |
|
|
8.53 x 10–6 for 0 to 1627˚C |
|
|
8.58 x 10–6 for 0 to 1727˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 15 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Beryllium Oxide (BeO) (single crystal) |
6.3 x 10–6 for 28 to 252˚C |
|
parallel to c axis |
6.7 x 10–6 for 28 to 474˚C |
|
|
|
|
|
7.8 x 10–6 for 28 to 749˚C |
|
|
8.2 x 10–6 for 28 to 872˚C |
|
|
8.9 x 10–6 for 28 to 1132˚C |
|
Beryllium Oxide (BeO) (single crystal) |
|
|
perpendicular to c axis |
7.1 x 10–6 for 28 to 252˚C |
|
|
7.8 x 10–6 for 28 to 474˚C |
|
|
8.5 x 10–6 for 28 to 749˚C |
|
|
9.2 x 10–6 for 28 to 872˚C |
|
|
9.9 x 10–6 for 28 to 1132˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 16 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Beryllium Oxide (BeO) (single crystal) |
6.83 x 10–6 for 28 to 252˚C |
|
average for (2a+c)/3 |
7.43 x 10–6 for 28 to 474˚C |
|
|
8.27 x 10–6 for 28 to 749˚C |
|
|
8.87 x 10–6 for 28 to 872˚C |
|
|
9.57 x 10–6 for 28 to 1132˚C |
|
Beryllium Oxide (BeO) (polycrystalline) |
2.4 x 10–6 for 25–200˚C |
|
|
6.3–6.4 x 10–6 for 25–300˚C |
|
|
7.59 x 10–6 for 25–500˚C |
|
|
8.4–8.5 x 10–6 for 25–800˚C |
|
|
9.03 x 10–6 for 25–1000˚C |
|
|
9.18 x 10–6 for 25–1250˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 17 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Beryllium Oxide (BeO) (polycrystalline) (Con’t) |
10.3 x 10–6 for 25–1500˚C |
|
|
11.1 x 10–6 for 25–2000˚C |
|
|
9.40 x 10–6 for 500–1200˚C |
|
Cerium Dioxide (CeO2) |
8.22 x 10–6 for 25–500˚C |
|
|
8.92 x 10–6 for 25–1000˚C |
|
|
8.5 + 0.54T for 0–1000˚C |
|
Dichromium Trioxide (Cr2O3) |
8.43 x 10–6 for 25–500˚C |
|
|
8.62 x 10–6 for 25–1000˚C |
|
|
8.82 x 10–6 for 25–1500˚C |
|
|
9.55 x 10–6 for 20–1400˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 18 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Hafnium Dioxide (HfO2) (monoclinic single crystal) |
6.8x10–6 for 28–262˚C |
|
parallel to a axis |
6.2x10–6 for 28–494˚C |
|
|
6.7x10–6 for 28–697˚C |
|
|
7.5x10–6 for 28–903˚C |
|
|
7.9x10–6 for 28–1098˚C |
|
parallel to b axis |
0 for 28–262˚C |
|
|
0.9x10–6 for 28–494˚C |
|
|
1.3x10–6 for 28–697˚C |
|
|
1.4x10–6 for 28–903˚C |
|
|
2.1x10–6 for 28–1098˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 19 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Hafnium Dioxide (HfO2) (monoclinic single crystal) |
11x10–6 for 28–262˚C |
|
parallel to c axis |
11.4x10–6 for 28–494˚C |
|
|
10.8x10–6 for 28–697˚C |
|
|
11.9x10–6 for 28–903˚C |
|
|
12.1x10–6 for 28–1098˚C |
|
Hafnium Dioxide (HfO2) |
5.47 x 10–6 for 25–500˚C |
|
(monoclinic polycrystalline) |
5.85 x 10–6 for 25–1000˚C |
|
|
5.8 x 10–6 for 25–1300˚C |
|
|
6.30 x 10–6 for 25–1500˚C |
|
|
6.45 x 10–6 for 20–1700˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 20 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Hafnium Dioxide (HfO2) |
1.31 x 10–6 for 25–1700˚C |
|
(tetragonal polycrystalline) |
3.03 x 10–6 for 25–2000˚C |
|
Magnesium Oxide (MgO) |
12.83 x 10–6 for 25–500˚C |
|
|
13.63 x 10–6 for 25–1000˚C |
|
|
15.11 x 10–6 for 25–1500˚C |
|
|
15.89 x 10–6 for 25–1800˚C |
|
|
14.0 x 10–6 for 20–1400˚C |
|
|
14.2–14.9 x 10–6 for 20–1700˚C |
|
|
13.3 x 10–6 for 20–1700˚C |
|
|
13.90 x 10–6 for 0–1000˚C |
|
|
14.46 x 10–6 for 0–1200˚C |
|
|
15.06 x 10–6 for 0–1400˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 21 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Silicon Dioxide (SiO2) |
19.35 x 10–6 for 25–500˚C |
|
α quartz |
22.2 x 10–6 for 25–575˚C |
|
β quartz |
27.8 x 10–6 for 25–575˚C |
|
|
14.58 x 10–6 for 25–1000˚C |
|
α tridymite |
18.5 x 10–6 for 25–117˚C |
|
β1 tridymite |
25.0 x 10–6 for 25–117˚C |
|
|
27.5 x 10–6 for 25–163˚C |
|
β2 tridymite |
31.9 x 10–6 for 25–163˚C |
|
|
19.35 x 10–6 for 25–500˚C |
|
|
10.45 x 10–6 for 25–1000˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 22 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Silicon Dioxide (SiO2) (Con’t) |
0.527 x 10–6 for 25–500˚C |
|
Vitreous |
0.564 x 10–6 for 25–1000˚C |
|
|
|
|
|
0.5 x 10–6 for 20–1250˚C |
|
Thorium Dioxide (ThO2) |
3.67 x 10–6 for 0 to –273˚C |
|
|
5.32 x 10–6 for 0 to –173˚C |
|
|
6.47 x 10–6 for 0 to –73˚C |
|
|
8.10 x 10–6 for 0 to 27˚C |
|
|
8.06 x 10–6 for 0 to 127˚C |
|
|
8.31 x 10–6 for 0 to 227˚C |
|
|
8.53 x 10–6 for 0 to 327˚C |
|
|
8.71 x 10–6 for 0 to 427˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 23 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Thorium Dioxide (ThO2) (Con’t) |
8.87 x 10–6 for 0 to 527˚C |
|
|
9.00 x 10–6 for 0 to 627˚C |
|
|
9.14 x 10–6 for 0 to 727˚C |
|
|
9.24 x 10–6 for 0 to 827˚C |
|
|
9.34 x 10–6 for 0 to 927˚C |
|
|
9.42 x 10–6 for 0 to 1027˚C |
|
|
9.53 x 10–6 for 0 to 1127˚C |
|
|
9.60 x 10–6 for 0 to 1227˚C |
|
|
9.68 x 10–6 for 0 to 1327˚C |
|
|
9.76 x 10–6 for 0 to 1427˚C |
|
|
9.83 x 10–6 for 0 to 1527˚C |
|
|
9.91 x 10–6 for 0 to 1627˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 24 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Thorium Dioxide (ThO2) (Con’t) |
9.97 x 10–6 for 0 to 1727˚C |
|
|
8.63 x 10–6 for 25 to 500˚C |
|
|
9.44 x 10–6 for 25 to 1000˚C |
|
|
10.17 x 10–6 for 25 to 1500˚C |
|
|
10.43 x 10–6 for 25 to 1700˚C |
|
|
9.55 x 10–6 for 20 to 800˚C |
|
|
9.55 x 10–6 for 20 to 1400˚C |
|
|
7.8 x 10–6 for 27 to 223˚C |
|
|
8.7 x 10–6 for 27 to 498˚C |
|
|
8.9 x 10–6 for 27 to 755˚C |
|
|
9.2 x 10–6 for 27 to 994˚C |
|
|
9.1 x 10–6 for 27 to 1087˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC
Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 25 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Thorium Dioxide (ThO2) (Con’t) |
8.96 x 10–6 for 0 to 1000˚C |
|
|
9.35 x 10–6 for 0 to 1200˚C |
|
|
9.84 x 10–6 for 0 to 1400˚C |
|
αl (linear expansion coefficient) |
0.6216x10–5 +3.541x10–9T–0.1124T–2 |
|
|
from 298–1073K |
|
αv (volume expansion coefficient) |
1.85x10–5 +10.96x10–9T–0.3375T–2 |
|
|
from 298–1073K |
|
Titanium Oxide (TiO2) (polycrystalline) |
8.22 x 10–6 for 25–500˚C |
|
|
8.83 x 10–6 for 25–1000˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 26 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Titanium Oxide (TiO2) (polycrystalline) (Con’t) |
9.50 x 10–6 for 25–1500˚C |
|
|
7.8 x 10–6 for 20–600˚C |
|
|
8.98 x 10–6 for 0–1000˚C |
|
Titanium Oxide (TiO2) (single crystal) |
9.8 x 10–6 for 26 to 240˚C |
|
parallel to c axis |
10.5 x 10–6 for 26 to 455˚C |
|
|
|
|
|
10.6 x 10–6 for 26 to 670˚C |
|
|
10.5 x 10–6 for 26 to 940˚C |
|
|
10.8 x 10–6 for 26 to 1110˚C |
|
Titanium Oxide (TiO2) (single crystal)) |
7.9 x 10–6 for 26 to 240˚C |
|
perpendicular to a axis |
8.2 x 10–6 for 26 to 455˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 27 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Titanium Oxide (TiO2) (single crystal)) (Con’t) |
8.1 x 10–6 for 26 to 670˚C |
|
perpendicular to a axis (Con’t) |
8.2 x 10–6 for 26 to 940˚C |
|
|
8.3 x 10–6 for 26 to 1110˚C |
|
Titanium Oxide (TiO2) (single crystal)) |
|
|
average for (2a+c)/3 |
8.53 x 10–6 for 26 to 240˚C |
|
|
8.97 x 10–6 for 26 to 455˚C |
|
|
8.93 x 10–6 for 26 to 670˚C |
|
|
8.97 x 10–6 for 26 to 940˚C |
|
|
9.13 x 10–6 for 26 to 1110˚C |
|
Uranium Dioxide (UO2) |
9.47 x 10–6 for 25 to 500˚C |
|
|
11.19 x 10–6 for 25 to 1000˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 28 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Uranium Dioxide (UO2) (Con’t) |
12.19 x 10–6 for 25 to 1200˚C |
|
|
11.15 x 10–6 for 25 to 1750˚C |
|
|
9.18 x 10–6 for 27 to 400˚C |
|
(heating) |
9.07 x 10–6 for 27 to 400˚C |
|
|
11.1 x 10–6 for 400 to 800˚C |
|
|
13.0 x 10–6 for 800 to 1200˚C |
|
(cooling) |
9.28 x 10–6 for 27 to 400˚C |
|
|
10.8 x 10–6 for 400 to 800˚C |
|
|
10.8 x 10–6 for 400 to 800˚C |
|
|
12.6 x 10–6 for 800 to 1250˚C |
|
|
12.9 x 10–6 for 800 to 1200˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 29 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Zirconium Oxide (ZrO2) (monoclinic) |
6.53 x 10–6 for 25 to 500˚C |
|
|
7.59 x 10–6 for 25 to 1000˚C |
|
|
7.72 x 10–6 for 25 to 1050˚C |
|
|
8.0 x 10–6 for 25 to 1080˚C |
|
Zirconium Oxide (ZrO2) (tetragonal) |
–21.7 x 10–6 for 25 to 1050˚C |
|
|
–11.11 x 10–6 for 25 to 1500˚C |
|
|
–9.53 x 10–6 for 25 to 1600˚C |
|
|
4.0 x 10–6 for 0 to 500˚C |
|
|
10.5 x 10–6 for 0 to 1000˚C |
|
|
10.52 x 10–6 for 0 to 1000˚C (MgO) |
|
|
10.6 x 10–6 for 0 to 1200˚C (CaO) |
|
|
5.0 x 10–6 for 0 to 1400˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 30 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Zirconium Oxide (ZrO2) (tetragonal) (Con’t) |
11.0 x 10–6 for 0 to 1500˚C |
|
|
5.5–5.58 x 10–6 for 20 to 1200˚C |
|
|
7.2 x 10–6 for –10 to 1000˚C |
|
|
8.64 x 10–6 for –20 to 600˚C |
|
Zirconium Oxide (ZrO2) (tetragonal, single crystal) |
8.4 x 10–6 for 27 to 264˚C |
|
parallel to a axis |
7.5 x 10–6 for 27 to 504˚C |
|
|
6.8 x 10–6 for 27 to 759˚C |
|
|
7.8 x 10–6 for 27 to 964˚C |
|
|
8.7 x 10–6 for 27 to 1110˚C |
|
Zirconium Oxide (ZrO2) (tetragonal, single crystal) |
3 x 10–6 for 27 to 264˚C |
|
parallel to b axis |
2 x 10–6 for 27 to 504˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC
Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 31 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Zirconium Oxide (ZrO2) (tetragonal, single crystal) |
1.1 x 10–6 for 27 to 759˚C |
|
(Con’t) |
|
|
parallel to b axis |
1.5 x 10–6 for 27 to 964˚C |
|
|
1.9 x 10–6 for 27 to 1110˚C |
|
Zirconium Oxide (ZrO2) (tetragonal, single crystal) |
14 x 10–6 for 27 to 264˚C |
|
parallel to c axis |
13 x 10–6 for 27 to 504˚C |
|
|
11.9 x 10–6 for 27 to 759˚C |
|
|
12.8 x 10–6 for 27 to 964˚C |
|
|
13.6 x 10–6 for 27 to 1110˚C |
|
Cordierite (2MgO 2Al2O3 5SiO2) |
|
|
(ρ=2.51g/cm3) |
2.7 x 10–6 for 25 to 1100˚C |
|
(ρ=2.3g/cm3) |
2.3 x 10–6 for 25 to 400˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 32 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
(ρ=2.3g/cm3) |
3.3 x 10–6 for 25 to 700˚C |
|
(ρ=2.3g/cm3) |
3.7 x 10–6 for 25 to 900˚C |
|
(ρ=2.1g/cm3) |
2.2 x 10–6 for 25 to 400˚C |
|
(ρ=2.1g/cm3) |
2.8 x 10–6 for 25 to 700˚C |
|
(ρ=2.1g/cm3) |
2.8 x 10–6 for 25 to 900˚C |
|
(ρ=1.8g/cm3) |
0.6 x 10–6 for 25 to 400˚C |
|
(ρ=1.8g/cm3) |
1.5 x 10–6 for 25 to 700˚C |
|
(ρ=1.8g/cm3) |
1.7 x 10–6 for 25 to 900˚C |
|
(glass) |
3.7–3.8 x 10–6 for 25 to 900˚C |
|
Mullite (3Al2O3 2SiO2) |
4.5 x 10–6 for 20 to 1325˚C |
|
|
4.63 x 10–6 for 25 to 500˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 33 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Oxides (Con’t) |
Mullite (3Al2O3 2SiO2) (Con’t) |
5.0 x 10–6 for 25 to 800˚C |
|
|
5.13 x 10–6 for 25 to 1000˚C |
|
|
5.62 x 10–6 for 20 to 1500˚C |
|
Sillimanite (Al2O3 SiO2) |
6.58 x 10–6 at 20˚C |
|
Spinel (Al2O3 MgO) |
7.79 x 10–6 for 25 to 500˚C |
|
|
8.41 x 10–6 for 25 to 1000˚C |
|
|
9.17 x 10–6 for 25 to 1500˚C |
|
|
9.0 x 10–6 for 20 to 1250˚C |
|
Zircon (SiO2 ZrO2) |
5.5 x 10–6 for 20 to 1200˚C |
|
|
3.79 x 10–6 for 25 to 500˚C |
|
|
4.62 x 10–6 for 25 to 1000˚C |
|
|
5.63 x 10–6 for 20 to 1500˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC

Table 116. THERMAL EXPANSION OF CERAMICS
(SHEET 34 OF 34)
|
|
Thermal Expansion |
Class |
Ceramic |
(˚C–1) |
|
|
|
|
|
|
Silicides |
Molybdenum Disilicide (MoSi2) |
7.79 x 10–6 for 25–500˚C |
|
Molybdenum Disilicide (MoSi2) |
8.51 x 10–6 for 25–1000˚C |
|
|
9.00–9.18 x 10–6 for 25–1500˚C |
|
|
8.41 x 10–6 for 0–1000˚C |
|
|
8.56 x 10–6 for 0–1400˚C |
|
Tungsten Disilicide (WSi2) |
7.79 x 10–6 for 25–500˚C |
|
|
8.31 x 10–6 for 25–1000˚C |
|
|
8.21 x 10–6 for 0–1000˚C |
|
|
8.81 x 10–6 for 0–1400˚C |
|
|
|
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991)
©2001 CRC Press LLC
Table 117. THERMAL EXPANSION OF
SIC-WHISKER-REINFORCED CERAMICS
|
Linear Coefficient of Thermal Expansion |
|
at 22 to 1100 °C |
Composite |
(10-6/K) |
|
|
|
|
Alumina |
7.8 to 8.2 |
Alumina with 20 vol% SiC whiskers |
7.35 |
Alumina with 30 vol% SiC whiskers |
6.70 |
Alumina with 60 vol% SiC whiskers |
5.82 |
SiC |
4.8 |
Mullite with 20 vol% SiC whiskers |
5.60 |
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p173,(1994).
©2001 CRC Press LLC
Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 1 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
SiO2 glass |
Pure |
3.50x10–7/K |
–60—20˚C |
|
|
3.80x10–7/K |
–40—20˚C |
|
|
4.00x10–7/K |
–20—20˚C |
|
|
4.30x10–7/K |
0–20˚C |
|
|
5.35x10–7/K |
20–100˚C |
|
|
5.75x10–7/K |
20–150˚C |
|
|
5.85x10–7/K |
20–200˚C |
|
|
5.92x10–7/K |
20–250˚C |
|
|
5.94x10–7/K |
20–300˚C |
|
|
5.90x10–7/K |
20–350˚C |
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 2 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
SiO2–B2O3 glass |
(39.2% mol B2O3 ) |
47.5x10–7/K |
0–100˚C |
|
(39.2% mol B2O3 ) |
44.9x10–7/K |
100–200˚C |
|
(39.2% mol B2O3 ) |
301x10–7/K |
390–410˚C |
|
(44.2% mol B2O3 ) |
49.8x10–7/K |
0–100˚C |
|
(44.2% mol B2O3 ) |
50.8x10–7/K |
100–200˚C |
|
(44.2% mol B2O3 ) |
450x10–7/K |
380–400˚C |
|
(50.8% mol B2O3 ) |
57.6x10–7/K |
0–100˚C |
|
(50.8% mol B2O3 ) |
54.8x10–7/K |
100–200˚C |
|
(50.8% mol B2O3 ) |
579x10–7/K |
350–370˚C |
|
(58.4% mol B2O3 ) |
71.9x10–7/K |
0–100˚C |
|
(58.4% mol B2O3 ) |
70.1x10–7/K |
100–200˚C |
|
(58.4% mol B2O3 ) |
694x10–7/K |
320–340˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 3 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
SiO2–B2O3 glass (Con’t) |
(72.7% mol B2O3 ) |
87.0x10–7/K |
0–100˚C |
|
(72.7% mol B2O3 ) |
89.7x10–7/K |
100–200˚C |
|
(72.7% mol B2O3 ) |
899x10–7/K |
300–320˚C |
|
(83.2% mol B2O3 ) |
111.4x10–7/K |
0–100˚C |
|
(83.2% mol B2O3 ) |
116.6x10–7/K |
100–200˚C |
|
(83.2% mol B2O3 ) |
970x10–7/K |
280–300˚C |
|
(88.6% mol B2O3 ) |
118.1x10–7/K |
0–100˚C |
|
(88.6% mol B2O3 ) |
126.0x10–7/K |
100–200˚C |
|
(88.6% mol B2O3 ) |
1023x10–7/K |
280–300˚C |
|
(94.0% mol B2O3 ) |
131.7x10–7/K |
0–100˚C |
|
(94.0% mol B2O3 ) |
141.9x10–7/K |
100–200˚C |
|
(94.0% mol B2O3 ) |
1200x10–7/K |
270–290˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 4 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
SiO2–Al2O3 glass |
(13.9% mol Al2O3, 1000˚C |
22.7x10–7/K |
20–900˚C |
|
for 115 hr) |
|
|
|
(13.9% mol Al2O3, water |
17.2x10–7/K |
20–600˚C |
|
quenching) |
|
|
|
(17.4% mol Al2O3, 1000˚C |
28.3x10–7/K |
20–800˚C |
|
for 115 hr) |
|
|
|
(17.4% mol Al2O3, water |
20.7x10–7/K |
20–700˚C |
|
quenching) |
|
|
|
(3.1% mol Al2O3, 1000˚C |
6.2x10–7/K |
20–980˚C |
|
for 115 hr) |
|
|
|
(3.1% mol Al2O3, water |
6.2x10–7/K |
20–980˚C |
|
quenching) |
|
|
|
(5.4% mol Al2O3, 1130˚C |
12.2x10–7/K |
20–350˚C |
|
for 20 hr) |
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 5 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
SiO2–Al2O3 glass |
(8.2% mol Al2O3, 1000˚C |
14.5x10–7/K |
20–950˚C |
|
for 115 hr) |
|
|
|
(8.2% mol Al2O3, water |
8.8x10–7/K |
20–800˚C |
|
quenching) |
|
|
SiO2–CaO glass |
(30% mol CaO) |
66±5x10–6/K |
1700˚C |
|
(35% mol CaO) |
53±5x10–6/K |
1700˚C |
|
(40% mol CaO) |
64±4x10–6/K |
1700˚C |
|
(42.5% mol CaO) |
76±4x10–6/K |
1700˚C |
|
(45% mol CaO) |
85–100±4x10–6/K |
1700˚C |
|
(47.5% mol CaO) |
76±4x10–6/K |
1700˚C |
|
(50% mol CaO) |
84–85±4x10–6/K |
1700˚C |
|
(52.5% mol CaO) |
76–107±4x10–6/K |
1700˚C |
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 6 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
SiO2–CaO glass |
(55% mol CaO) |
94–95±4x10–6/K |
1700˚C |
|
(57.5% mol CaO) |
95±4x10–6/K |
1700˚C |
|
(60% mol CaO) |
103±4x10–6/K |
1700˚C |
SiO2–PbO glass |
(25.7% mol PbO) |
51.45–52.23x10–7/K |
20–170˚C |
|
(30.0% mol PbO) |
57.68–59.08x10–7/K |
20–170˚C |
|
(32.5% mol PbO) |
60.62–62.31x10–7/K |
20–170˚C |
|
(33.2% mol PbO) |
61.58–63.33x10–7/K |
20–170˚C |
|
(35.0% mol PbO) |
63.99–66.17x10–7/K |
20–170˚C |
|
(37.5% mol PbO) |
68.75–71.44x10–7/K |
20–170˚C |
|
(42.6% mol PbO) |
75.16–78.58x10–7/K |
20–170˚C |
|
(45.8% mol PbO) |
78.85–82.60x10–7/K |
20–170˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 7 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
SiO2–PbO glass |
(47.8% mol PbO) |
83.03–87.03x10–7/K |
20–170˚C |
|
(49.8% mol PbO) |
85.57–89.82x10–7/K |
20–170˚C |
|
(50% mol PbO) |
723x10–7/K |
1100˚C |
|
(53.8% mol PbO) |
90.62–95.25x10–7/K |
20–170˚C |
|
(57.5% mol PbO) |
95.64–100.45x10–7/K |
20–170˚C |
|
(59.0% mol PbO) |
97.00–101.90x10–7/K |
20–170˚C |
|
(61.0% mol PbO) |
100.66–105.58x10–7/K |
20–170˚C |
|
(61.75% mol PbO) |
101.36–106.30x10–7/K |
20–170˚C |
|
(66.7% mol PbO) |
867x10–7/K |
1100˚C |
|
(67.7% mol PbO) |
110.38–115.48x10–7/K |
20–170˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 8 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
SiO2–Na2O glass |
(20% mol Na2O) |
6.7x10–5/K |
liquidus temp. to 1400˚C |
|
(20% mol Na2O, |
120x10–7/K |
below Tg |
|
Tg = 478˚C) |
||
|
|
|
|
|
(20% mol Na2O, |
315x10–7/K |
above Tg |
|
Tg = 478˚C) |
||
|
|
|
|
|
(20.3% mol Na2O) |
97.5x10–7/K |
room temp–100˚C |
|
(20.3% mol Na2O) |
99.3x10–7/K |
100–200˚C |
|
(20.3% mol Na2O) |
100.6x10–7/K |
200–300˚C |
|
(20.3% mol Na2O) |
106.9x10–7/K |
300–400˚C |
|
(24.0% mol Na2O) |
109.7x10–7/K |
room temp–100˚C |
|
(24.0% mol Na2O) |
114.3x10–7/K |
100–200˚C |
|
(24.0% mol Na2O) |
116.6x10–7/K |
200–300˚C |
|
(24.0% mol Na2O) |
121.7x10–7/K |
300–400˚C |
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 9 OF 21)
|
|
Thermal |
Temperature Range |
|
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
|
|
|
SiO2–Na2O glass |
(30% mol Na2O, |
152x10–7/K |
below Tg |
|
Tg = 455˚C) |
||||
|
|
|
||
|
(30% mol Na2O, |
402x10–7/K |
above Tg |
|
|
Tg = 455˚C) |
|||
|
|
|
||
|
(31.1% mol Na2O) |
136.0x10–7/K |
room temp–100˚C |
|
|
(31.1% mol Na2O) |
142.5x10–7/K |
100–200˚C |
|
|
(31.1% mol Na2O) |
148.3x10–7/K |
200–300˚C |
|
|
(31.1% mol Na2O) |
160.0x10–7/K |
300–400˚C |
|
|
(33% mol Na2O, |
165x10–7/K |
below Tg |
|
|
Tg = 445˚C) |
|||
|
|
|
||
|
(33% mol Na2O, |
465x10–7/K |
above Tg |
|
|
Tg = 445˚C) |
|||
|
|
|
||
|
(33.3% mol Na2O) |
17.2x10–5/K |
liquidus temp.to 1400˚C |
|
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 10 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
SiO2–Na2O glass |
(33.8% mol Na2O) |
143.9x10–7/K |
room temp–100˚C |
|
(33.8% mol Na2O) |
153.6x10–7/K |
100–200˚C |
|
(33.8% mol Na2O) |
159.1x10–7/K |
200–300˚C |
|
(33.8% mol Na2O) |
173.6x10–7/K |
300–400˚C |
|
(37.2% mol Na2O) |
152.1x10–7/K |
room temp–100˚C |
|
(37.2% mol Na2O) |
160.9x10–7/K |
100–200˚C |
|
(37.2% mol Na2O) |
171.6x10–7/K |
200–300˚C |
|
(37.2% mol Na2O) |
187.7x10–7/K |
300–400˚C |
|
(40% mol Na2O) |
20.0x10–5/K |
liquidus temp. to 1400˚C |
|
(40% mol Na2O, |
179x10–7/K |
below Tg |
|
Tg = 421˚C) |
||
|
|
|
|
|
(40% mol Na2O, |
500x10–7/K |
above Tg |
|
Tg = 421˚C) |
||
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 11 OF 21)
|
|
Thermal |
Temperature Range |
|
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
|
|
|
SiO2–Na2O glass |
(45% mol Na2O, |
219x10–7/K |
below Tg |
|
Tg = 417˚C) |
||||
|
|
|
||
|
(45% mol Na2O, |
574x10–7/K |
above Tg |
|
|
Tg = 417˚C) |
|||
|
|
|
||
|
(50% mol Na2O) |
23.7x10–5/K |
liquidus temp. to 1400˚C |
|
B2O3 glass |
|
154.5–183x10–7/K |
0–100˚C |
|
|
|
154.5–169x10–7/K |
100–200˚C |
|
|
|
150±3–158±3x10–7/K |
20–200˚C |
|
B2O3–Na2O glass |
(0.01% mol Na2O) |
140x10–7/K |
–196—25˚C |
|
|
(0.01% mol Na2O) |
149.3x10–7/K |
20–50˚C |
|
|
(0.01% mol Na2O) |
149.0x10–7/K |
20–150˚C |
|
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC
Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 12 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
B2O3–Na2O glass |
(4.4% mol Na2O) |
94.6x10–7/K |
–196—25˚C |
|
(4.4% mol Na2O) |
103.0x10–7/K |
20–50˚C |
|
(4.4% mol Na2O) |
109.9x10–7/K |
20–150˚C |
|
(4.4% mol Na2O) |
116.0x10–7/K |
20–250˚C |
|
(5% mol Na2O, |
115x10–7/K |
below Tg |
|
Tg = 318˚C) |
||
|
|
|
|
|
(5% mol Na2O, |
1400x10–7/K |
above Tg |
|
Tg = 318˚C) |
||
|
|
|
|
|
(8.7% mol Na2O) |
98.8x10–7/K |
20–50˚C |
|
(8.7% mol Na2O) |
100.5x10–7/K |
20–150˚C |
|
(8.7% mol Na2O) |
105.3x10–7/K |
20–250˚C |
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 13 OF 21)
|
|
Thermal |
Temperature Range |
|
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
|
|
|
B2O3–Na2O glass |
(10% mol Na2O, |
77x10–7/K |
below Tg |
|
Tg = 354˚C) |
||||
|
|
|
||
|
(10% mol Na2O, |
1230x10–7/K |
above Tg |
|
|
Tg = 354˚C) |
|||
|
|
|
||
|
(11.5% mol Na2O) |
71.5x10–7/K |
–196—25˚C |
|
|
(11.5% mol Na2O) |
88.7x10–7/K |
20–50˚C |
|
|
(11.5% mol Na2O) |
94.9x10–7/K |
20–150˚C |
|
|
(11.5% mol Na2O) |
97.9x10–7/K |
20–250˚C |
|
|
(13.7% mol Na2O) |
69.3x10–7/K |
–196—25˚C |
|
|
(13.7% mol Na2O) |
87.5x10–7/K |
20–50˚C |
|
|
(13.7% mol Na2O) |
92.3x10–7/K |
20–150˚C |
|
|
(13.7% mol Na2O) |
90.9x10–7/K |
20–250˚C |
|
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 14 OF 21)
|
|
Thermal |
Temperature Range |
|
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
|
|
|
B2O3–Na2O glass |
(15% mol Na2O, |
69x10–7/K |
below Tg |
|
Tg = 407˚C) |
||||
|
|
|
||
|
(15% mol Na2O, |
761x10–7/K |
above Tg |
|
|
Tg = 407˚C) |
|||
|
|
|
||
|
(15.8% mol Na2O) |
67.4x10–7/K |
–196—25˚C |
|
|
(15.8% mol Na2O) |
80.7x10–7/K |
20–50˚C |
|
|
(15.8% mol Na2O) |
87.8x10–7/K |
20–150˚C |
|
|
(15.8% mol Na2O) |
93.3x10–7/K |
20–250˚C |
|
|
(15.8% mol Na2O) |
97.9x10–7/K |
20–350˚C |
|
|
(16.2% mol Na2O) |
65.9x10–7/K |
–196—25˚C |
|
|
(16.2% mol Na2O) |
86.0x10–7/K |
20–50˚C |
|
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 15 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
B2O3–Na2O glass |
(16.2% mol Na2O) |
87.7x10–7/K |
20–150˚C |
|
(16.2% mol Na2O) |
90.9x10–7/K |
20–250˚C |
|
(16.2% mol Na2O) |
96.9x10–7/K |
20–350˚C |
|
(17.4% mol Na2O) |
85.6x10–7/K |
20–50˚C |
|
(17.4% mol Na2O) |
89.1x10–7/K |
20–150˚C |
|
(17.4% mol Na2O) |
92.4x10–7/K |
20–250˚C |
|
(17.4% mol Na2O) |
96.3x10–7/K |
20–350˚C |
|
(18.4% mol Na2O) |
69.1x10–7/K |
–196—25˚C |
|
(18.4% mol Na2O) |
86.2x10–7/K |
20–50˚C |
|
(18.4% mol Na2O) |
89.2x10–7/K |
20–150˚C |
|
(18.4% mol Na2O) |
94.1x10–7/K |
20–250˚C |
|
(18.4% mol Na2O) |
96.2x10–7/K |
20–350˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 16 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
B2O3–Na2O glass |
(19.6% mol Na2O) |
86.8x10–7/K |
20–50˚C |
|
(19.6% mol Na2O) |
91.2x10–7/K |
20–150˚C |
|
(19.6% mol Na2O) |
95.3x10–7/K |
20–250˚C |
|
(19.6% mol Na2O) |
99.6x10–7/K |
20–350˚C |
|
(20.0% mol Na2O) |
87.6x10–7/K |
20–50˚C |
|
(20.0% mol Na2O) |
91.6x10–7/K |
20–150˚C |
|
(20.0% mol Na2O) |
97.6x10–7/K |
20–250˚C |
|
(20.0% mol Na2O) |
101.3x10–7/K |
20–350˚C |
|
(20% mol Na2O, |
86x10–7/K |
below Tg |
|
Tg = 456˚C) |
||
|
|
|
|
|
(20% mol Na2O, |
586x10–7/K |
above Tg |
|
Tg = 456˚C) |
||
|
|
|
|
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 17 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
B2O3–Na2O glass |
(22.5% mol Na2O) |
71.9x10–7/K |
–196—25˚C |
|
(22.5% mol Na2O) |
90.4x10–7/K |
20–50˚C |
|
(22.5% mol Na2O) |
94.7x10–7/K |
20–150˚C |
|
(22.5% mol Na2O) |
98.7x10–7/K |
20–250˚C |
|
(22.5% mol Na2O) |
104.0x10–7/K |
20–350˚C |
|
(23.6% mol Na2O) |
90.4x10–7/K |
20–50˚C |
|
(23.6% mol Na2O) |
96.7x10–7/K |
20–150˚C |
|
(23.6% mol Na2O) |
101.2x10–7/K |
20–250˚C |
|
(23.6% mol Na2O) |
106.5x10–7/K |
20–350˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 18 OF 21)
|
|
Thermal |
Temperature Range |
|
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
|
|
|
B2O3–Na2O glass |
(25% mol Na2O, |
95x10–7/K |
below Tg |
|
Tg = 466˚C) |
||||
|
|
|
||
|
(25% mol Na2O, |
834x10–7/K |
above Tg |
|
|
Tg = 466˚C) |
|||
|
|
|
||
|
(28.9% mol Na2O) |
81.4x10–7/K |
–196—25˚C |
|
|
(28.9% mol Na2O) |
102.1x10–7/K |
20–50˚C |
|
|
(28.9% mol Na2O) |
107.4x10–7/K |
20–150˚C |
|
|
(28.9% mol Na2O) |
112.8x10–7/K |
20–250˚C |
|
|
(28.9% mol Na2O) |
117.1x10–7/K |
20–350˚C |
|
|
(30% mol Na2O, |
128x10–7/K |
below Tg |
|
|
Tg = 468˚C) |
|||
|
|
|
||
|
(30% mol Na2O, |
1150x10–7/K |
above Tg |
|
|
Tg = 468˚C) |
|||
|
|
|
||
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 19 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
B2O3–CaO glass |
(29.3% mol CaO) |
54.9–56.4x10–7/K |
room temp. to 100˚C |
|
(29.3% mol CaO) |
60.2–60.8x10–7/K |
100–200˚C |
|
(29.3% mol CaO) |
63.9–65.4x10–7/K |
200–300˚C |
|
(29.3% mol CaO) |
71.3–71.6x10–7/K |
300–400˚C |
|
(29.3% mol CaO) |
76.9–77.1x10–7/K |
400–500˚C |
|
(29.3% mol CaO) |
80.9–86.8x10–7/K |
500–600˚C |
|
(31.4% mol CaO) |
57.3–58.2x10–7/K |
room temp. to 100˚C |
|
(31.4% mol CaO) |
63.5–65.1x10–7/K |
100–200˚C |
|
(31.4% mol CaO) |
67.4–68.1x10–7/K |
200–300˚C |
|
(31.4% mol CaO) |
76.5–76.7x10–7/K |
300–400˚C |
|
(31.4% mol CaO) |
79.2–81.0x10–7/K |
400–500˚C |
|
(31.4% mol CaO) |
83.1–88.5x10–7/K |
500–600˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 20 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
B2O3–CaO glass |
(34.9% mol CaO) |
60.1–66.2x10–7/K |
room temp. to 100˚C |
|
(34.9% mol CaO) |
67.5–67.6x10–7/K |
100–200˚C |
|
(34.9% mol CaO) |
74.7–75.2x10–7/K |
200–300˚C |
|
(34.9% mol CaO) |
77.8–78.5x10–7/K |
300–400˚C |
|
(34.9% mol CaO) |
83.8–95.0x10–7/K |
400–500˚C |
|
(34.9% mol CaO) |
91.8–92.1x10–7/K |
500–600˚C |
|
(37.1% mol CaO) |
63.1–64.0x10–7/K |
room temp. to 100˚C |
|
(37.1% mol CaO) |
68.4–70.4x10–7/K |
100–200˚C |
|
(37.1% mol CaO) |
74.6–75.8x10–7/K |
200–300˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 118. THERMAL EXPANSION OF GLASSES
(SHEET 21 OF 21)
|
|
Thermal |
Temperature Range |
Glass |
Composition |
Expansion |
of Validity |
|
|
|
|
|
|
|
|
B2O3–CaO glass |
(37.1% mol CaO) |
81.6–82.2x10–7/K |
300–400˚C |
|
(37.1% mol CaO) |
86.9–87.6x10–7/K |
400–500˚C |
|
(37.1% mol CaO) |
93.5–95.5x10–7/K |
500–600˚C |
|
|
|
|
Source: data compiled byJun S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 1 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
ABS Resins; Molded, Extruded |
Medium impact |
3.2—4.8 x 10–6 |
|
High impact |
5.5—6.0 x 10–6 |
|
Very high impact |
5.0—6.0 x 10–6 |
|
Low temperature impact |
5.0—6.0 x 10–6 |
|
Heat resistant |
3.0—4.0 x 10–6 |
Acrylics; Cast, Molded, Extruded |
Cast Resin Sheets, Rods: |
|
|
General purpose, type I |
4.5 x 10–6 |
|
General purpose, type II |
4.5 x 10–6 |
|
Moldings: |
|
|
Grades 5, 6, 8 |
3—4 x 10–6 |
|
High impact grade |
4—6 x 10–6 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 2 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Thermoset Carbonate |
Allyl diglycol carbonate |
6 x 10–5 |
Alkyds; Molded |
Putty (encapsulating) |
1.3 x 10–5 |
|
Rope (general purpose) |
1.3 x 10–5 |
|
Granular (high speed molding) |
1.3 x 10–5 |
|
Glass reinforced (heavy duty parts) |
1.3 x 10–5 |
Cellulose Acetate; Molded, Extruded |
ASTM Grade: |
|
|
H6—1 |
4.4—9.0 x 10–5 |
|
H4—1 |
4.4—9.0 x 10–5 |
|
H2—1 |
4.4—9.0 x 10–5 |
|
MH—1, MH—2 |
4.4—9.0 x 10–5 |
|
MS—1, MS—2 |
4.4—9.0 x 10–5 |
|
S2—1 |
4.4—9.0 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 3 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Cellulose Acetate Butyrate; Molded, Extruded |
ASTM Grade: |
|
|
H4 |
6—9 x 10–5 |
|
MH |
6—9 x 10–5 |
|
S2 |
6—9 x 10–5 |
Cellusose Acetate Propionate; Molded, Extruded |
ASTM Grade: |
|
|
1 |
6—9 x 10–5 |
|
3 |
6—9 x 10–5 |
|
6 |
6—9 x 10–5 |
Chlorinated Polymers |
Chlorinated polyether |
6.6 x 10–6 |
|
Chlorinated polyvinyl chloride |
4.4 x 10–6 |
Polycarbonates |
Polycarbonate |
3.75 x 10–6 |
|
Polycarbonate (40% glass fiber reinforced) |
1.0—1.1 x 10–6 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 4 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Diallyl Phthalates; Molded |
Orlon filled |
5.0 x 10–5 |
|
Dacron filled |
5.2 x 10–5 |
|
Asbestos filled |
4.0 x 10–5 |
|
Glass fiber filled |
2.2—2.6 x 10–5 |
Fluorocarbons; Molded,Extruded |
Polytrifluoro chloroethylene (PTFCE) |
3.88 x 10–5 |
|
Polytetrafluoroethylene (PTFE) |
55 x 10–5 |
|
Ceramic reinforced (PTFE) |
1.7—2.0 x 10–5 |
|
Fluorinated ethylene propylene(FEP) |
8.3—10.5 x 10–5 |
|
Polyvinylidene— fluoride (PVDF) |
8.5 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 5 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Epoxies; Cast, Molded, Reinforced |
Standard epoxies (diglycidyl ethers of bisphenol A) |
|
|
Cast rigid |
3.3 x 10–5 |
|
Cast flexible |
3—5 x 10–5 |
|
Molded |
1—2 x 10–5 |
|
General purpose glass cloth laminate |
3.3—4.8 x 10–6 |
|
High strength laminate |
3.3—4.8 x 10–6 |
|
Filament wound composite |
2—6 x 10–5 |
Epoxies—Molded, Extruded |
High performance resins |
|
|
(cycloaliphatic diepoxides) |
|
|
Molded |
1.7—2.2 x 10–6 |
|
Epoxy novolacs |
|
|
Cast, rigid |
1.6—3.0 x 10–6 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 6 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Melamines; Molded |
Filler & type |
|
|
Cellulose electrical |
1.11—2.78 x 10–5 |
|
Glass fiber |
0.82 x 10–5 |
Nylons; Molded, Extruded |
|
|
Type 6 Nylon |
General purpose |
4.8 x 10–5 |
|
Glass fiber (30%) reinforced |
1.2 x 10–5 |
|
Cast |
4.4 x 10–5 |
|
Type 11 |
5.5 x 10–5 |
|
Type 12 |
7.2 x 10–5 |
6/6 Nylon |
General purpose molding |
1.69—1.7 x 10–5 |
|
Glass fiber reinforced |
1.5–3.3 x 10–5 |
|
General purpose extrusion |
1.7 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 7 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
6/10 Nylon |
General purpose |
1.5 x 10–5 |
|
Glass fiber (30%) reinforced |
3.5 x 10–5 |
Phenolics; Molded |
Type and filler |
|
|
General: woodflour and flock |
1.66—2.50 x 10–5 |
|
Shock: paper, flock, or pulp |
1.6—2.3 x 10–5 |
|
High shock: chopped fabric or cord |
1.60—2.22 x 10–5 |
|
Very high shock: glass fiber |
0.88 x 10–5 |
Phenolics: Molded |
Rubber phenolic—woodflour or flock |
0.83—2.20 x 10–5 |
|
Rubber phenolic—chopped fabric |
1.7 x 10–5 |
|
Rubber phenolic—asbestos |
2.2 x 10–5 |
ABS–Polycarbonate Alloy |
ABS–Polycarbonate Alloy |
6.12 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 8 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
PVC–Acrylic |
PVC–Acrylic Alloy |
|
|
PVC–acrylic sheet |
3.5 x 10–5 |
Polymides |
Unreinforced |
2.5 x 10–6 |
|
Unreinforced 2nd value |
3.0—4.5 x 10–6 |
|
Glass reinforced |
0.8 x 10–6 |
Homopolymer |
Standard |
4.5 x 10–5 |
|
20% glass reinforced |
2.0—4.5 x 10–5 |
|
22% TFE reinforced |
4.5 x 10–5 |
|
Copolymer: |
|
|
Standard |
4.7 x 10–5 |
|
25% glass reinforced |
2.2—4.7 x 10–5 |
|
High flow |
4.7 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 9 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Polyester; Thermoplastic |
Injection Moldings: |
|
|
General purpose grade |
5.3 x 10–5 |
|
Glass reinforced grades |
2.7—3.3 x 10–5 |
|
Glass reinforced self extinguishing |
3.5 x 10–5 |
|
General purpose grade |
4.9—13.0 x 10–5 |
Polyesters: Thermosets |
Cast polyyester |
|
|
Rigid |
3.9—5.6 x 10–5 |
|
Reinforced polyester moldings |
|
|
High strength (glass fibers) |
13—19 x 10–6 |
Phenylene Oxides |
SE—100 |
3.8 x 10–5 |
|
SE—1 |
3.3 x 10–5 |
|
Glass fiber reinforced |
1.4–2.0 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 10 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Phenylene Oxides (Con’t) |
Phenylene oxides (Noryl) |
|
|
Standard |
3.1 x 10–5 |
|
Glass fiber reinforced |
1.2–1.6 x 10–5 |
Polyarylsulfone |
Polyarylsulfone |
2.6 x 10–5 |
Polypropylene |
General purpose |
3.8—5.8 x 10–5 |
|
High impact |
4.0—5.9 x 10–5 |
|
Asbestos filled |
2—3 x 10–5 |
|
Glass reinforced |
1.6—2.4 x 10–5 |
Polyphenylene sulfide |
Standard |
3.0—4.9 x 10–5 |
|
40% glass reinforced |
4 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 11 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Polyethylenes; Molded, Extruded |
Type I—lower density (0.910–0.925) |
|
|
Melt index 0.3—3.6 |
8.9—11.0 x 10–5 |
|
Melt index 6—26 |
8.9—11.0 x 10–5 |
|
Melt index 200 |
11 x 10–5 |
|
Type II—medium density (0.926—0.940) |
|
|
Melt index 20 |
8.3—16.7 x 10–5 |
|
Melt index l.0—1.9 |
8.3—16.7 x 10–5 |
|
Type III—higher density (0.941—0.965) |
|
|
Melt index 0.2—0.9 |
8.3—16.7 x 10–5 |
|
Melt Melt index 0.l—12.0 |
8.3—16.7 x 10–5 |
|
Melt index 1.5—15 |
8.3—16.7 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 12 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Polystyrenes; Molded |
General purpose |
3.3—4.8 x 10–5 |
|
Medium impact |
3.3—4.7 x 10–5 |
|
High impact |
2.2—5.6 x 10–5 |
|
Glass fiber -30% reinforced |
1.8 x 10–5 |
|
Styrene acrylonitrile (SAN) |
3.6—3.7 x 10–5 |
|
Glass fiber (30%) reinforced SAN |
1.6 x 10–5 |
Polyvinyl Chloride And Copolymers; Molded, Extruded |
|
|
|
Rigid—normal impact |
2.8—3 .3 x 10–5 |
Vinylidene chloride |
|
|
|
Vinylidene chloride |
8.78 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 119. THERMAL EXPANSION OF POLYMERS
(SHEET 13 OF 13)
|
|
Thermal Expansion Coefficient |
Type |
|
ASTM D696 |
|
(•F–1) |
|
|
Polymer |
|
|
|
|
|
|
|
Silicones; Molded, Laminated |
Fibrous (glass) reinforced silicones |
3.17—3.23 x 10–5 |
|
Granular (silica) reinforced silicones |
2.5—5.0 x 10–5 |
Ureas; Molded |
Alpha—cellulose filled (ASTM Type l) |
1.22—1 .50 x 10–5 |
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 120. THERMAL EXPANSION COEFFICIENTS OF
MATERIALS FOR INTEGRATED CIRCUITS
|
Coefficient |
Temperature |
Material |
Range |
Range (˚C) |
|
|
|
|
|
|
Aluminum oxide ceramic |
6.0–7.0 |
25–300 |
Brass |
17.7–21.2 |
25–300 |
Kanthal A |
13.9–15.1 |
20–900 |
Kovar |
5.0 |
25–300 |
Pyrex glass |
3.2 |
25–300 |
Pyroceram (#9608) |
420 |
25–300 |
Pyroceram cement (Vitreous #45) |
4 |
0–300 |
Pyroceram cement (Devitrified) |
2.4 |
25–300 |
Pyroceram cement (#89, #95) |
8–10 |
— |
Silicon carbide |
4.8 |
0–1,000 |
Silicon nitride (α) |
2.9 |
25–1,000 |
Silicon nitride (β) |
2.25 |
25–1,000 |
Solder glass (Kimble CV-101) |
809 |
0–300 |
|
|
|
Coefficient of Linear Thermal Expansion of Selected Materials per K
Note: Multiply all values by 10–6.
Source: from Beadles, R. L., Interconnections and Encapsulation, Integrated Silicon Device Technology, Vol. 14, Research Triangle Institute, Research Triangle Park, N. C., 1967. in CRC Handbook of Materials Science, Charles T. Lynch, Ed., CRC Press, Cleveland, (1974).
©2001 CRC Press LLC
Table 121. THERMAL EXPANSION OF
SILICON CARBIDE SCS–2–AL
|
|
Coefficient of Thermal Expansion, |
Fiber orientation |
No. of plies |
(10-6/K) |
|
|
|
|
|
|
0° |
6, 8, 12 |
6.6 |
90° |
6, 12,40 |
21.3 |
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p149,(1994).
©2001 CRC Press LLC

Table 122. ASTM B 601 TEMPER DESIGNATION CODES
FOR COPPER AND COPPER ALLOYS (SHEET 1 OF 2)
|
Temper |
Temper Name or Material |
Class |
Designation |
Condition |
|
|
|
|
|
|
Cold-worked tempers(a) |
H00 |
1/8 hard |
|
H01 |
1/4 hard |
|
H02 |
1/2 hard |
|
H03 |
3/4 hard |
|
H04 |
Hard |
|
H06 |
Extra hard |
|
H08 |
Spring |
|
H10 |
Extra spring |
|
H12 |
Special Spring |
|
H13 |
Ultra Spring |
|
H14 |
Super Spring |
Cold-worked tempers(b) |
H50 |
Extruded and drawn |
|
H52 |
Pierced and drawn |
|
H55 |
Light drawn, light cold rolled |
|
H58 |
Drawn general purpose |
|
H60 |
Cold heading; forming |
|
H63 |
Rivet |
|
H64 |
Screw |
|
H66 |
Bolt |
|
H70 |
Bending |
|
H80 |
Hard drawn |
|
H85 |
Medium-hard-drawn electrical wire |
|
H86 |
Hard-drawn electrical wire |
|
H90 |
As-finned |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p439, (1993).
©2001 CRC Press LLC

Table 122. ASTM B 601 TEMPER DESIGNATION CODES FOR COPPER AND COPPER ALLOYS (SHEET 2 OF 2)
|
Temper |
Temper Name or Material |
|
Class |
Designation |
Condition |
|
|
|
|
|
|
|
|
|
Cold-worked and stress-relieved |
HR01 |
H01 and stress relieved |
|
tempers |
|||
|
|
||
|
HR02 |
H02 and stress relieved |
|
|
HR04 |
H04 and stress relieved |
|
|
HR08 |
H08 and stress relieved |
|
|
HR10 |
H10 and stress relieved |
|
|
HR20 |
As-finned |
|
|
HR50 |
Drawn and stress relieved |
|
Cold-rolled and order- |
HT04 |
H04 and order heat treated |
|
strengthened tempers(c) |
|||
|
|
||
|
HT08 |
H08 and order heat treated |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p439, (1993).
(a)Cold-worked tempers to meet standard requirements based on cold rolling or cold drawing.
(b)Cold-worked tempers to meet standard requirements based on temper names applicable to specific processes.
(c)Tempers produced by controlled amounts of cold work following by thermal treatment to produce order strengthening.
©2001 CRC Press LLC

|
Table 123. TEMPER DESIGNATION SYSTEM FOR |
|
|
ALUMINUM ALLOYS |
|
|
|
|
Temper |
Definition |
|
|
|
|
|
|
|
F |
As fabricated |
|
O |
Annealed |
|
H1 |
Strain-hardened only |
|
H2 |
Strain-hardened and partially annealed |
|
H3 |
Strain-hardened and stabilized (mechanical properties stabilized by low- |
|
temperature thermal treatment) |
||
|
||
T1 |
Cooled from an elevated-temperature shaping process and naturally aged to a |
|
substantially stable condition |
||
|
||
T2 |
Cooled from an elevated temperature shaping process, cold-worked, and |
|
naturally aged to a substantially stable condition |
||
|
||
T3 |
Solution heat-treated, cold-worked, and naturally aged to a substantially stable |
|
condition |
||
|
||
T4 |
Solution heat-treated and naturally aged to a substantially stable condition |
|
T5 |
Cooled from an elevated-temperature shaping process and artificially aged |
|
T6 |
Solution heat-treated and artificially aged |
|
T7 |
Solution heat-treated and stabilized |
|
T8 |
Solution heat-treated, cold-worked, and artificially aged |
|
T9 |
Solution heat-treated, artificially aged, and cold-worked |
|
T10 |
Cooled from an elevated temperature shaping process, cold-worked, and |
|
artificially aged |
||
|
||
|
|
Source: data from Metals Handbook, 9th ed., Vol. 2, American Society for Metals, Metals Park, Ohio, 1979, 24-27.
©2001 CRC Press LLC
Table 124. TOOL STEEL SOFTENING AFTER 100 HOURS
|
Original |
|
|
Hardness (HRC) after 100 h at |
|
|
|
|
|
|
|
|
|
|
|
|
Hardness |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Type |
(HRC) |
480˚C |
540˚C |
600˚C |
650˚C |
700˚C |
760˚C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H13 |
60.2 |
48.7 |
46.3 |
29.0 |
22.7 |
20.1 |
13.9 |
|
41.7 |
38.6 |
39.3 |
27.7 |
23.7 |
20.2 |
13.2 |
H21 |
49.2 |
48.7 |
47.6 |
37.2 |
27.4 |
19.8 |
16.2 |
|
36.7 |
34.8 |
34.9 |
32.6 |
27.1 |
19.8 |
14.9 |
H23 |
40.8 |
40.0 |
40.6 |
40.8 |
38.6 |
33.2 |
26.8 |
|
38.9 |
38.9 |
38.0 |
38.0 |
37.1 |
32.6 |
26.6 |
H26 |
61.0 |
60.6 |
60.3 |
47.1 |
38.4 |
26.9 |
21.3 |
|
42.9 |
42.4 |
42.3 |
41.3 |
34.9 |
26.4 |
21.1 |
|
|
|
|
|
|
|
|
Source: Data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.426, (1984).
See also: Mechanical Properties of Tool Steels
©2001 CRC Press LLC

Table 125. THERMOPLASTIC POLYESTER SOFTENING WITH TEMPERATURE
|
|
Tensile strength |
|
|
|
Flexural modulus |
103 psi |
|
|
Polymer |
106 psi |
D638 |
212•F |
302• F |
|
|
|
|
|
|
|
|
|
|
Injection Molding Types: |
|
|
|
|
General purpose grade |
|
7.5—8 |
|
|
Glass reinforced grades |
|
17—25 |
7 |
5.5 |
Glass reinforced grades |
1.2—1.5 |
|
0.63 |
0.53 |
|
|
|
|
|
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
See also: Mechanical Properties of Polymers
©2001 CRC Press LLC

Table 126. HEAT-DEFLECTION TEMPERATURE
OF CARBON- AND GLASS-REINFORCED
ENGINEERING THERMOPLASTICS (SHEET 1 OF 2)
|
|
|
Heat-Deflection |
|
|
|
Temperature |
Class |
Resin Type |
Composition |
(°C) |
|
|
|
|
|
|
|
|
Amorphous |
Acrylonitrile-butadiene-styrene(ABS) |
30% glass fiber |
105 |
|
|
30% carbon fiber |
105 |
|
Nylon |
30% glass fiber |
140 |
|
|
30% carbon fiber |
145 |
|
Polycarbonate |
30% glass fiber |
150 |
|
|
30% carbon fiber |
150 |
|
Polyetherimide |
30% glass fiber |
215 |
|
|
30% carbon fiber |
215 |
|
Polyphenylene oxide (PPO) |
30% glass fiber |
155 |
|
|
30% carbon fiber |
155 |
|
Polysulfone |
30% glass fiber |
185 |
|
|
30% carbon fiber |
185 |
|
Styrene-maleic-anhydride (SMA) |
|
|
|
|
30% glass fiber |
120 |
|
Thermoplastic polyurethane |
30% glass fiber |
170 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p111–112, (1994).
©2001 CRC Press LLC

Table 126. HEAT-DEFLECTION TEMPERATURE
OF CARBON- AND GLASS-REINFORCED
ENGINEERING THERMOPLASTICS (SHEET 2 OF 2)
|
|
|
Heat-Deflection |
|
|
|
Temperature |
Class |
Resin Type |
Composition |
(°C) |
|
|
|
|
|
|
|
|
Crystalline |
Acetal |
30% glass fiber |
165 |
|
|
20% carbon fiber |
160 |
|
Nylon 66% |
30% glass fiber |
255 |
|
|
30% carbon fiber |
257 |
|
Polybutylene terephthalate (PBT) |
30% glass fiber |
210 |
|
|
30% carbon fiber |
210 |
|
Polythylene terephthalate (PET) |
30% glass fiber |
225 |
|
Polyphenylene sulfide (PPS) |
30% glass fiber |
260 |
|
|
30% carbon fiber |
265 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p111–112, (1994).
©2001 CRC Press LLC
Shackelford, James F. and Alexander, W. “Mechanical Properties of Materials”
Materials Science and Engineering Handbook
Ed. James F. Shackelford & W. Alexander Boca Raton: CRC Press LLC, 2001

CHAPTER 6
Mechanical Properties
of Materials
List of Tables |
Tensile Strength |
|
Tensile Strength of Tool Steels |
|
Tensile Strength of Gray Cast |
|
Tensile Strength of Gray Cast Iron Bars |
|
Tensile Strength of Ductile Irons |
|
Tensile Strength of Malleable Iron Castings |
|
Tensile Strength of Austenitic Stainless Steels |
|
Tensile Strength of Ferritic Stainless Steels |
|
Tensile Strength of |
|
Precipitation-Hardening Austenitic Stainless Steels |
|
Tensile Strength of |
|
High–Nitrogen Austenitic Stainless Steels |
|
Tensile Strength of Martensitic Stainless Steels |
|
Tensile Strength of Wrought Coppers and Copper Alloys |
|
Tensile Strength of Aluminum Casting Alloys |
|
Tensile Strength of Wrought Aluminum Alloys |
|
Tensile Strength of Cobalt-Base Superalloys |
|
Tensile Strength of Nickel-Base Superalloys |
|
Tensile Strength of |
|
Wrought Titanium Alloys at Room Temperature |
|
Tensile Strength of |
|
Wrought Titanium Alloys at High Temperature |
|
(continued) |
©2001 CRC Press LLC
List of Tables
(Continued)
Tensile Strength (continued)
Tensile Strength of Refractory Metal Alloys
Tensile Strength of Ceramics
Tensile Strength of Glass
Tensile Strength of Polymers
Tensile Strength of Fiberglass Reinforced Plastics
Tensile Strength of Carbonand
Glass-Reinforced Engineering Thermoplastics
Strength of Graphite Fiber Reinforced Metals
Tensile Strength of Graphite/Magnesium Castings
Tensile Strength of Graphite/Aluminum Composites
Tensile Strength of Graphite/Aluminum Composites
Tensile Strength of Silicon Carbide SCS–2–Al
Ultimate Tensile Strength of
Investment Cast Silicon Carbide SCS–Al
Ultimate Tensile Strength of
Silicon Carbide–Aluminum Alloy Composites
Tensile Strength of
SiC-Whisker–Reinforced Aluminum Alloy
Ultimate Tensile Strength of Aluminum Alloy Reinforced with SiC Whiskers vs. Temperature
Ultimate Tensile Strength of
Reinforced Aluminum Alloy vs. Temperature
Tensile Strength of
Polycrystalline–Alumina–Reinforced Aluminum Alloy
Tensile Strength of Boron/Aluminum Composites
Compressive Strength
Compressive Strength of Gray Cast Iron Bars
Compressive Strength of Ceramics
Compressive Strength of Fiberglass Reinforced Plastic
Ultimate Compressive Strength of
Investment Cast Silicon Carbide SCS–Al
©2001 CRC Press LLC
List of Tables
(Continued)
Yield Strength
Yield Strength of Tool Steels
Yield Strength of Ductile Irons
Yield Strength of Malleable Iron Castings Yield Strength of Austenitic Stainless Steels Yield Strength of Ferritic Stainless Steels Yield Strength of Martensitic Stainless Steels
Yield Strength of
Precipitation-Hardening Austenitic Stainless Steels
Yield Strength of
High–Nitrogen Austenitic Stainless Steels
Yield Strength of Wrought Coppers and Copper Alloys Yield Strength of Cast Aluminum Alloys
Yield Strength of Wrought Aluminum Alloys
Yield Strength of Wrought Titanium Alloys
at Room Temperature
Yield Strength of Wrought Titanium Alloys
at High Temperature
Yield Strength of Cobalt-Base Superalloys
Yield Strength of Nickel-Base Superalloys
Yield Strength of Commercially Pure Tin
Yield Strength of Polymers
Yield Strength of
SiC-Whisker–Reinforced Aluminum Alloy
Yield Strength of
Reinforced Aluminum Alloy vs. Temperature
Yield Strength of
Polycrystalline–Alumina–Reinforced Aluminum Alloy
Compressive Yield Strength of Polymers
Flextural Strength
Flexural Strength of Polymers
Flextural Strength of Fiberglass Reinforced Plastics
©2001 CRC Press LLC
List of Tables
(Continued)
Shear Strength
Shear Strength of Wrought Aluminum Alloys Torsion Shear Strength of Gray Cast Fe
Hardness
Hardness of Gray Cast Irons
Hardness of Gray Cast Iron Bars
Hardness of Malleable Iron Castings
Hardness of Ductile Irons
Hardness of Tool Steels
Hardness of Austenitic Stainless Steels
Hardness of Ferritic Stainless Steels
Hardness of Martensitic Stainless Steels
Hardness of
Precipitation-Hardening Austenitic Stainless Steels
Machinability Rating of
Wrought Coppers and Copper Alloys
Hardness of Wrought Aluminum Alloys
Hardness of
Wrought Titanium Alloys at Room Temperature
Hardness of Ceramics
Microhardness of Glass
Hardness of Polymers
Hardness of Si3N4 and Al2O3 Composites
Coefficient of Friction
Coefficient of Static Friction for Polymers
Abrasion Resistance
Abrasion Resistance of Polymers
Fatique
Fatigue Strength of Wrought Aluminum Alloys Reversed Bending Fatigue Limit of Gray Cast Iron Bars
©2001 CRC Press LLC
List of Tables
(Continued)
Impact
Impact Energy of Tool Steels
Impact Strength of
Wrought Titanium Alloys at Room Temperature
Impact Strength of Polymers
Impact Strength of Fiberglass Reinforced Plastics
Impact Strength of Carbonand
Glass-Reinforced Engineering Thermoplastics
Fracture Toughness
Fracture Toughness of Si3N4 and Al2O3 Composites
Tensile Modulus
Tensile Modulus of Gray Cast Irons Tension Modulus of Treated Ductile Irons
Tensile Modulus of Fiberglass Reinforced Plastics Tensile Modulus of Graphite/Aluminum Composites
Tensile Modulus of
Investment Cast Silicon Carbide SCS–Al
Tensile Modulus of Silicon Carbide SCS–2–Al
Young’s Modulus
Young’s Modulus of Ceramics
Young’s Modulus of Glass
(continues)
©2001 CRC Press LLC
List of Tables
(Continued)
Elastic Modulus
Elastic Modulus of Wrought Stainless Steels Modulus of Elasticity of Wrought Titanium Alloys Modulus of Elasticity in Tension for Polymers
Modulus of Elasticity of
55MSI Graphite/6061 Aluminum Composites Modulus of Elasticity of Graphite/Magnesium Castings
Modulus of Elasticity of Graphite/Aluminum Composites Modulus of Elasticity of Graphite Fiber Reinforced Metals
Modulus of Elasticity of
SiC-Whisker–Reinforced Aluminum Alloy
Modulus of Elasticity of
Polycrystalline–Alumina–Reinforced Aluminum Alloy
Modulus of Elasticity of Boron/Aluminum Composites
Compression Modulus
Compression Modulus of Treated Ductile Irons Modulus of Elasticity in Compression for Polymers
Bulk Modulus
Bulk Modulus of Glass
Sheer Modulus
Shear Modulus of Glass
Torsion Modulus
Torsional Modulus of Gray Cast Irons
Torsion Modulus of Treated Ductile Irons
Flexural Modulus
Modulus of Elasticity in Flexure for Polymers
Flexural Modulus of Fiberglass Reinforced Plastics
Flexural Modulus of Carbonand
Glass-Reinforced Engineering Thermoplastics
Modulus of Rupture
Modulus of Rupture for Ceramics
Rupture Strength of Refractory Metal Alloys
Rupture Strength of Superalloys
Modulus of Rupture for Si3N4 and Al2O3Composites
©2001 CRC Press LLC
List of Tables
(Continued)
Poisson’s Ratio
Poisson's Ratio of Wrought Titanium Alloys Poisson’s Ratio for Ceramics
Poisson’s Ratio of Glass
Poisson's Ratio of Silicon Carbide SCS–2–Al Compression Poisson’s Ratio of Treated Ductile Irons Torsion Poisson’s Ratio of Treated Ductile Irons
Elongation
Elongation of Tool Steels
Elongation of Ductile Irons
Elongation of Malleable Iron Castings
Elongation of Ferritic Stainless Steels
Elongation of Martensitic Stainless Steels
Elongation of
Precipitation-Hardening Austenitic Stainless Steels
Elongation of High–Nitrogen Austenitic Stainless Steels
Total Elongation of Cast Aluminum Alloys
Elongation of Wrought Coppers and Copper Alloys Elongation of Commercially Pure Tin
Elongation of Cobalt-Base Superalloys
Elongation of Nickel-Base Superalloys
Ductility of Refractory Metal Alloys
Elongation of Wrought Titanium Alloys
at Room Temperature
Elongation of Wrought Titanium Alloys
at High Temperature
Total Elongation of Polymers
Elongation at Yield for Polymers
Ultimate Tensile Elongation of
Fiberglass Reinforced Plastics
Total Strain of Silicon Carbide SCS–2–Al
©2001 CRC Press LLC
List of Tables
(Continued)
Area Reduction
Area Reduction of Tool Steels
Reduction in Area of Austenitic Stainless Steels
Reduction in Area of Ferritic Stainless Steels
Reduction in Area of
High–Nitrogen Austenitic Stainless Steels
Reduction in Area of
Precipitation-Hardening Austenitic Stainless Steels
Reduction in Area of Martensitic Stainless Steels
Reduction in Area of Commercially Pure Tin
Area Reduction of Wrought Titanium Alloys
at Room Temperature
Area Reduction of Wrought Titanium Alloys
at High Temperature
Ratios
Strength Density Ratio of
Graphite Fiber Reinforced Metals
Modulus Density Ratio of
Graphite Fiber Reinforced Metals
Viscosity
Viscosity of Glasses
Internal Friction of SiO2 Glass
Surface Tension
Surface Tension of Elements at Melting
Surface Tension of Liquid Elements
©2001 CRC Press LLC

Table 127. TENSILE STRENGTH OF TOOL STEELS
(SHEET 1 OF 2)
|
|
Tensile |
|
|
Strength |
Type |
Condition |
(MPa) |
|
|
|
|
|
|
L2 |
Annealed |
710 |
|
Oil quenched from 855 •C and single tempered at: |
|
|
205 •C |
2000 |
|
315 •C |
1790 |
|
425 •C |
1550 |
|
540 •C |
1275 |
|
650 •C |
930 |
L6 |
Annealed |
655 |
|
Oil quenched from 845 •C and single tempered at: |
|
|
315 •C |
2000 |
|
425 •C |
1585 |
|
540 •C |
1345 |
|
650 •C |
965 |
S1 |
Annealed |
690 |
|
Oil quenched from 930 •C and single tempered at: |
|
|
205 •C |
2070 |
|
315 •C |
2030 |
|
425 •C |
1790 |
|
540 •C |
1680 |
|
650 •C |
1345 |
S5 |
Annealed |
725 |
|
Oil quenched from 870 •C and single tempered at: |
|
|
205 •C |
2345 |
|
315 •C |
2240 |
|
425 •C |
1895 |
|
540 •C |
1520 |
|
650 •C |
1035 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p241, (1984).
©2001 CRC Press LLC
Table 127. TENSILE STRENGTH OF TOOL STEELS
(SHEET 2 OF 2)
|
|
Tensile |
|
|
Strength |
Type |
Condition |
(MPa) |
|
|
|
|
|
|
S7 |
Annealed |
640 |
|
Fan cooled from 940 •C and single tempered at: |
|
|
205 •C |
2170 |
|
315 •C |
1965 |
|
425 •C |
1895 |
|
540 •C |
1820 |
|
650 •C |
1240 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p241, (1984).
©2001 CRC Press LLC

Table 128. TENSILE STRENGTH OF GRAY CAST IRONS
|
Maximum Tensile Strength |
SAE grade |
(MPa) |
|
|
|
|
G1800 |
118 |
G2500 |
173 |
G2500a |
173 |
G3000 |
207 |
C3500 |
241 |
G3500b |
1241 |
G3500c |
1241 |
G4000 |
276 |
G4000d |
1276 |
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p166-167, (1984).
Table 129. TENSILE STRENGTH OF GRAY CAST IRON BARS
ASTM |
Tensile Strength |
Class |
(MPa) |
|
|
|
|
ASTM |
Tensile Strength |
Class |
(MPa) |
20 |
152 |
25 |
179 |
30 |
214 |
35 |
252 |
40 |
293 |
50 |
362 |
60 |
431 |
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p166-167, (1984).
©2001 CRC Press LLC

Table 130. TENSILE STRENGTH OF DUCTILE IRONS
|
|
Tensile Strength |
Specification Number |
Grade or Class |
(MPa) |
|
|
|
|
|
|
ASTM A395-76 |
|
|
ASME SA395 |
60-40-18 |
414 |
ASTM A476-70(d); |
|
|
SAE AMS5316 |
80-60-03 |
552 |
ASTM A536-72, |
|
|
MIL-1-11466B(MR) |
60-40-18 |
414 |
|
65-45-12 |
448 |
|
80-55-06 |
552 |
|
100-70-03 |
689 |
|
120-90-02 |
827 |
SAE J434c |
D4018 |
414 |
|
D4512 |
448 |
|
D5506 |
552 |
|
D7003 |
689 |
MlL-I-24137(Ships) |
Class A |
414 |
|
Class B |
379 |
|
Class C |
345 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p169, (1984).
©2001 CRC Press LLC

Table 131. TENSILE STRENGTH OF MALLEABLE IRON CASTINGS
|
|
Tensile Strength |
Specification Number |
Grade or Class |
(MPa) |
|
|
|
|
|
|
Ferritic |
|
|
ASTM A47, A338; ANSI G48.1; |
|
|
FED QQ–I–666c |
32510 |
345 |
|
35018 |
365 |
ASTM A197 |
|
276 |
Pearlitic and Martensitic |
|
|
ASTM A220; ANSI C48.2; |
|
|
MIL–I–11444B |
40010 |
414 |
|
45008 |
448 |
|
45006 |
448 |
|
50005 |
483 |
|
60004 |
552 |
|
70003 |
586 |
|
80002 |
655 |
|
90001 |
724 |
Automotive |
|
|
ASTM A602; SAE J158 |
M3210 |
345 |
|
M4504(a) |
448 |
|
M5003(a) |
517 |
|
M5503(b) |
517 |
|
M7002(b) |
621 |
|
M8501(b) |
724 |
|
|
|
(a)Air quenched and tempered
(b)Liquid quenched and tempered
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p171, (1984).
©2001 CRC Press LLC

Table 132. TENSILE STRENGTH OF AUSTENITIC STAINLESS STEELS
(SHEET 1 OF 5)
|
|
|
|
Tensile Strength |
|
Type |
Form |
Condition |
ASTM Specification |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Type 301(UNS S30100) |
Bar,Wire,Plate,Sheet, |
Annealed |
A167 |
515 |
|
Strip |
|||||
|
|
|
|
||
Type 302 (UNS S30200) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
|
Cold finished and annealed(b) |
A276 |
515 |
|
Type 302B (UNS S30215) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
|
Cold finished and annealed(b) |
A276 |
515 |
|
Type 302Cu(UNS S30430) |
Bar |
Annealed |
A493 |
450 to 585 |
|
Types 303 (UNS S30300) |
Bar |
Annealed |
A581 |
585 |
|
and 303Se (UNS S30323) |
|||||
|
|
|
|
||
|
Wire |
Annealed |
A581 |
585 to 860 |
|
|
|
Cold worked |
A581 |
790 to 1000 |
|
|
|
|
|
|
(a) Up to 13 mm thick (b) Over 13 mm thick.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p364-366 (1993).
©2001 CRC Press LLC

Table 132. TENSILE STRENGTH OF AUSTENITIC STAINLESS STEELS
(SHEET 2 OF 5)
|
|
|
|
Tensile Strength |
|
Type |
Form |
Condition |
ASTM Specification |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Type 304(UNS S30400) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
|
Cold finished and annealed(b) |
A276 |
515 |
|
Type 304L (UNS S30403) |
Bar |
Hot finished and annealed |
A276 |
480 |
|
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
|
Cold finished and annealed(b) |
A276 |
480 |
|
Types 304N (UNS S30451) and |
Bar |
Annealed |
A276 |
550 |
|
316N(UNS S31651) |
|||||
|
|
|
|
||
Type 304LN |
Bar |
Annealed |
— |
515 |
|
Type 305 (UNS S30500) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
|
Cold finished and annealed(a) |
A276 |
260 |
|
|
|
Cold finished and annealed(b) |
A276 |
515 |
|
|
|
|
|
|
(a) Up to 13 mm thick (b) Over 13 mm thick.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p364-366 (1993).
©2001 CRC Press LLC

Table 132. TENSILE STRENGTH OF AUSTENITIC STAINLESS STEELS
(SHEET 3 OF 5)
|
|
|
|
Tensile Strength |
|
Type |
Form |
Condition |
ASTM Specification |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Types 308 (UNS |
|
|
|
|
|
S30800),321(UNS |
Bar |
Hot finished and annealed |
A276 |
515 |
|
S32100),347(UNS34700) and |
|||||
|
|
|
|
||
348 (UNS S34800) |
|
|
|
|
|
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
|
Cold finished and annealed(b) |
A276 |
515 |
|
Type 308L |
Bar |
Annealed |
— |
550 |
|
Types 309 (UNS S30900), 309S |
|
|
|
|
|
(UNS S30908), 310 (UNS |
Bar |
Hot finished and annealed |
A276 |
515 |
|
S31000) and 310S (UNS S31008) |
|
|
|
|
|
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
|
Cold finished and annealed(b) |
A276 |
515 |
|
|
|
|
|
|
(a) Up to 13 mm thick (b) Over 13 mm thick.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p364-366 (1993).
©2001 CRC Press LLC

Table 132. TENSILE STRENGTH OF AUSTENITIC STAINLESS STEELS
(SHEET 4 OF 5)
|
|
|
|
Tensile Strength |
Type |
Form |
Condition |
ASTM Specification |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 312 Weld metal |
— |
|
MIL–E–19933 |
655 |
Type 314 (UNS S31400) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
Cold finished and annealed(b) |
A276 |
515 |
Type 316 (UNS S31600) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
Cold finished and annealed(b) |
A276 |
515 |
Type 316F (UNS S31620) |
Bar |
Annealed |
— |
585 |
Type 316L (UNS S31603) |
Bar |
Hot finished and annealed |
A276 |
480 |
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
Cold finished and annealed(b) |
A276 |
480 |
|
|
|
|
|
(a) Up to 13 mm thick (b) Over 13 mm thick.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p364-366 (1993).
©2001 CRC Press LLC

Table 132. TENSILE STRENGTH OF AUSTENITIC STAINLESS STEELS
(SHEET 5 OF 5)
|
|
|
|
Tensile Strength |
Type |
Form |
Condition |
ASTM Specification |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 316LN |
Bar |
Annealed |
— |
515 |
Type 317 (UNS S31700) |
Bar |
Hot finished and annealed |
A276 |
515 |
|
|
Cold finished and annealed(a) |
A276 |
620 |
|
|
Cold finished and annealed(b) |
A276 |
515 |
Type 317L (UNS S31703) |
Bar |
Annealed |
— |
585 |
Type 317LM |
Bar,Plate,Sheet, Strip |
Annealed |
— |
515 |
Type 329 (UNS S32900) |
Bar |
Annealed |
— |
724 |
Type 330 (UNS N08330) |
Bar |
Annealed |
B511 |
480 |
Type 330HC |
Bar,Wire,Strip |
Annealed |
— |
585 |
Types 384 (UNS S38400) |
Bar |
Annealed |
A493 |
415 to 550 |
Types 385 (UNS38500) |
Bar |
Annealed |
A493 |
415 to 550 |
|
|
|
|
|
(a) Up to 13 mm thick (b) Over 13 mm thick.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p364-366 (1993).
©2001 CRC Press LLC
Table 133. TENSILE STRENGTH OF FERRITIC STAINLESS STEELS
(SHEET 1 OF 2)
|
|
|
|
Tensile Strength |
Type |
ASTM Specification |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 405 (UNS S40500) |
A580 |
Wire |
Annealed |
480 |
|
A580 |
|
Annealed, Cold Finished |
480 |
Type 409 (UNS S40900) |
— |
Bar |
Annealed |
450(a) |
Type 429 (UNS S42900) |
— |
Bar |
Annealed |
490(a) |
Type 430 (UNS S43000) |
A276 |
Bar |
Annealed, Hot Finished |
480 |
|
A276 |
|
Annealed, Cold Finished |
480 |
Type 430F (UNS S43020) |
A581 |
Wire |
Annealed |
585 to 860 |
Type 430Ti(UNS S43036) |
— |
Bar |
Annealed |
515(a) |
Type 434 (UNS S43400) |
— |
Wire |
Annealed |
545(a) |
Type 436 (UNS S43600) |
— |
Sheet, Strip |
Annealed |
530(a) |
|
|
|
|
|
(a) Typical Values
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p368 (1993).
©2001 CRC Press LLC

Table 133. TENSILE STRENGTH OF FERRITIC STAINLESS STEELS
(SHEET 2 OF 2)
|
|
|
|
Tensile Strength |
Type |
ASTM Specification |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 442 (UNS S44200) |
— |
Bar |
Annealed |
550(a) |
Type 444 (UNS S44400) |
A176 |
Plate, Sheet, Strip |
Annealed |
415 |
Type 446 (UNS S44600) |
A276 |
Bar |
Annealed, Hot Finished |
480 |
|
A276 |
|
Annealed, Cold Finished |
480 |
|
|
|
|
|
(a) Typical Values
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p368 (1993).
©2001 CRC Press LLC

Table 134. TENSILE STRENGTH OF
PRECIPITATION -HARDENING AUSTENITIC STAINLESS STEELS
|
|
|
Tensile Strength |
Type |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
PH 13–8 Mo (UNS S13800) |
Bar, Plate, Sheet, Strip |
H950 |
1520 |
|
|
H1000 |
1380 |
15–5 PH (UNS S15500) and 17–4 PH (UNS S17400) |
Bar, Plate, Sheet, Stript |
H900 |
1310 |
|
|
H925 |
1170 |
|
|
H1025 |
1070 |
|
|
H1075 |
1000 |
|
|
H1100 |
965 |
|
|
H1150 |
930 |
|
|
H1150M |
795 |
17–7 PH (UNS S17700) |
Bar |
RH950 |
1275 |
|
|
TH1050 |
1170 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p371 (1993).
©2001 CRC Press LLC
Table 135. TENSILE STRENGTH OF
HIGH–NITROGEN AUSTENITIC STAINLESS STEELS
|
|
|
|
Tensile Strength |
Type |
ASTM Specification |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 201 (UNS S20100) |
A276 |
Bar |
Annealed |
515 |
Type 202 (UNS S20200) |
A276 |
Bar |
Annealed |
515 |
Type 205 (UNS S20500) |
— |
Plate |
Annealed* |
830 |
Type 304N (UNS S30451) |
A276 |
Bar |
Annealed |
550 |
Type 304HN (UNS S30452) |
— |
Bar |
Annealed |
620 |
Type 316N (UNS S31651) |
A276 |
Bar |
Annealed |
550 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p367 (1993).
*Typical Values.
©2001 CRC Press LLC

Table 136. TENSILE STRENGTH OF MARTENSITIC STAINLESS STEELS
(SHEET 1 OF 3)
|
|
|
|
Tensile Strength |
Type |
ASTM Specification |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 403 (UNS S40300) |
A276 |
Bar |
Annealed, hot finished |
485 |
|
A276 |
|
Annealed, cold finished |
485 |
|
A276 |
|
Intermediate temper, hot finished |
690 |
|
A276 |
|
Intermediate temper, cold finished |
690 |
|
A276 |
|
Hard temper, hot finished |
825 |
|
A276 |
|
Hard temper, cold finished |
825 |
Type 410 (UNS S41000) |
A276 |
Bar |
Annealed, hot finished |
485 |
|
A276 |
|
Annealed, cold finished |
485 |
|
A276 |
|
Intermediate temper, hot finished |
690 |
|
A276 |
|
Intermediate temper, cold finished |
690 |
|
A276 |
|
Hard temper, hot finished |
825 |
|
A276 |
|
Hard temper, cold finished |
825 |
Type 410S (UNS S41008) |
A176 |
Plate, Sheet, Strip |
Annealed |
415 |
Type 410Cb (UNS S41040) |
A276 |
Bar |
Annealed, hot finished |
485 |
|
A276 |
|
Annealed, cold finished |
485 |
|
A276 |
|
Intermediate temper, hot finished |
860 |
|
A276 |
|
Intermediate temper, cold finished |
860 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p369-370 (1993).
©2001 CRC Press LLC

Table 136. TENSILE STRENGTH OF MARTENSITIC STAINLESS STEELS
(SHEET 2 OF 3)
|
|
|
|
Tensile Strength |
|
Type |
ASTM Specification |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Type 414 (UNS S41400) |
A276 |
Bar |
Intermediate temper, hot finished |
795 |
|
|
A276 |
|
Intermediate temper, cold finished |
795 |
|
Type 414L |
— |
Bar |
Annealed |
795 |
|
Types 416 (UNS S41600) and 416Se (UNS |
A581 |
Wire |
Annealed |
585 to 860 |
|
S41623) |
|||||
|
|
|
|
||
|
A581 |
|
Intermediate temper |
795 to 1000 |
|
|
A581 |
|
Hard temper |
965 to 1210 |
|
Type 420 (UNS S42000) |
— |
Bar |
Tempered 205 °C |
1720 |
|
|
A580 |
Wire |
Annealed, cold finished |
860 max |
|
Type 422 (UNS S42200) |
A565 |
Bar |
Intermediate and hard tempers* |
965 |
|
Type 431 (UNS S43100) |
— |
Bar |
Tempered 260 °C |
1370 |
|
|
— |
|
Tempered 595 °C |
965 |
|
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p369-370 (1993).
©2001 CRC Press LLC
Table 136. TENSILE STRENGTH OF MARTENSITIC STAINLESS STEELS
(SHEET 3 OF 3)
|
|
|
|
Tensile Strength |
Type |
ASTM Specification |
Form |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Type 440A (UNS S44002) |
— |
Bar |
Annealed |
725 |
|
— |
|
Tempered 315 °C |
1790 |
Type 440B (UNS S44003) |
— |
Bar |
Annealed |
740 |
|
— |
|
Tempered 315 °C |
1930 |
Type 440C (UNS S44004) |
— |
Bar |
Annealed |
760 |
|
— |
|
Tempered 315 °C |
1970 |
Type 501 (UNS S50100) |
— |
Bar, Plate |
Annealed |
485 |
|
— |
|
Tempered 540 °C |
1210 |
Type 502 (UNS S50200) |
— |
Bar, Plate |
Annealed |
485 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p369-370 (1993).
*Heat treated for high-temperature service
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 1 OF 11)
|
|
|
Tensile |
|
|
Nominal |
Commercial |
Strength |
|
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
C10100 Oxygen-free electronic |
99.99 Cu |
F, R, W, T, P, S |
221-455 |
|
C10200 Oxygen-free copper |
99.95 Cu |
F, R, W, T, P, S |
221-455 |
|
C10300 Oxygen-free extra-low phosporus |
99.95 Cu, 0.003 P |
F, R, T, P, S |
221-379 |
|
C10400, C10500, C10700 Oxygen-free, silver-bearing |
99.95 Cu(e) |
F, R, W, S |
221-455 |
|
C10800 Oxygen-free, low phosporus |
99.95 Cu, 0.009 P |
F, R, T, P |
221-379 |
|
CS11000 Electrolytic tough pitch copper |
99.90 Cu, 0.04 O |
F, R, W, T, P, S |
221-455 |
|
C11100 Electrolytic tough pitch, anneal resistant |
99.90 Cu, 0.04 O, 0.01 Cd |
W |
455 |
|
C11300, C11400, C11500, C11600 Silver-bearing tough pitch copper |
99.90 Cu, 0.04 O, Ag(f) |
F, R, W, T, S |
221-455 |
|
C12000, C12100 |
99.9 Cu(g) |
F, T, P |
221-393 |
|
C12200 Phosphorus deoxidized copper, high residual phosphorus |
99.90 Cu, 0.02 P |
F, R, T, P |
221-379 |
|
C12500, C12700, C12800, C12900, C13000 Fire-refined tough pitch |
99.88 Cu(h) |
F, R, W, S |
221-462 |
|
with silver |
||||
|
|
|
||
C14200 Phosphorus deoxidized, arsenical |
99.68 Cu, 0.3 As, 0.02 P |
F, R, T |
221-379 |
|
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC
Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 2 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C19200 |
98.97 Cu, 1.0 Fe, 0.03 P |
F, T |
255-531 |
C14300 |
99.9 Cu, 0.1 Cd |
F |
221-400 |
C14310 |
99.8 Cu, 0.2 Cd |
F |
221-400 |
C14500 Phosphorus deoxidized, tellurium bearing |
99.5 Cu, 0.50 Te, 0.008 P |
F, R, W, T |
221-386 |
C14700 Sulfur bearing |
99.6 Cu, 0.40 S |
R, W |
221-393 |
C15000 Zirconium copper |
99.8 Cu, 0.15 Zr |
R, W |
200-524 |
C15500 |
99.75 Cu, 0.06 P, 0.11 Mg, Ag(i) |
F |
276-552 |
C15710 |
99.8 Cu, 0.2 Al2O3 |
R, W |
324-724 |
C15720 |
99.6 Cu, 0.4 Al2O3 |
F, R |
462-614 |
C15735 |
99.3 Cu, 0.7 Al2O3 |
R |
483-586 |
C15760 |
98.9 Cu, 1.1 Al2O3 |
F, R |
483-648 |
C16200 Cadmium copper |
99.0 Cu, 1.0 Cd |
F, R, W |
241-689 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 3 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C16500 |
98.6 Cu, 0.8 Cd, 0.6 Sn |
F, R, W |
276-655 |
C17000 Beryllium copper |
99.5 Cu, 1.7 Be, 0.20 Co |
F, R |
483-1310 |
C17200 Beryllium copper |
99.5 Cu, 1.9 Be , 0.20 Co |
F, R, W, T, P, S |
469-1462 |
C17300 Beryllium copper |
99.5 Cu, 1.9 Be, 0.40 Pb |
R |
469-1479 |
C17500 Copper-cobalt-beryllium alloy |
99.5 Cu, 2.5 Co, 0.6 Be |
F, R |
310-793 |
C18200, C18400, C18500 Chromium copper |
99.5 Cu(j) |
F, W, R, S, T |
234-593 |
C18700 leaded copper |
99.0 Cu, 1.0 Pb |
R |
221-379 |
C18900 |
98.75 Cu, 0.75 Sn, 0.3 Si, 0.20 Mn |
R, W |
262-655 |
C19000 Copper-nickel-phosphorus alloy |
98.7 Cu, 1.1 Ni, 0.25 P |
F, R, W |
262-793 |
C19100 Copper-nickel-phosphorus-tellurium alloy |
98.15 Cu, 1.1 Ni, 0.50 Te, 0.25 P |
R, F |
248-717 |
C19400 |
97.5 Cu, 2.4 Fe, 0.13 Zn, 0.03 P |
F |
310-524 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 4 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C19500 |
97.0 Cu, 1.5 Fe, 0.6 Sn, 0.10 P, 0.80 Co |
F |
552-669 |
C21000 Gilding, 95% |
95.0 Cu, 5.0 Zn |
F, W |
234-441 |
C22000 Commercial bronze, 90% |
90.0 Cu, 10.0 Zn |
F, R, W, T |
255-496 |
C22600 Jewelry bronze, 87.5% |
87.5 Cu, 12.5 Zn |
F, W |
269-669 |
C23000 Red brass, 85% |
85.0 Cu, 15.0 Zn |
F, W, T, P |
269-724 |
C24000 Low brass, 80% |
80.0 Cu, 20.0 Zn |
F, W |
290-862 |
C26000 Cartridge brass, 70% |
70.0 Cu, 30.0 Zn |
F, R, W, T |
303-896 |
C26800, C27000 Yellow brass |
65.0 Cu, 35.0 Zn |
F, R, W |
317-883 |
C28000 Muntz metal |
60.0 Cu, 40.0 Zn |
F, R, T |
372-510 |
C31400 Leaded commercial bronze |
89.0 Cu, 1.75 Pb, 9.25 Zn |
F, R |
255-414 |
C31600 Leaded commercial bronze, nickel-bearing |
89.0 Cu, 1.9 Pb, 1.0 Ni, 8.1 Zn |
F, R |
255-462 |
C33000 Low-leaded brass tube |
66.0 Cu, 0.5 Pb, 33.5 Zn |
T |
324-517 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 5 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C33200 High-leaded brass tube |
66.0 Cu, 1.6 Pb, 32.4 Zn |
T |
359-517 |
C33500 Low-leaded brass |
65.0 Cu, 0.5 Pb, 34.5 Zn |
F |
317-510 |
C34000 Medium-leaded brass |
65.0 Cu, 1.0 Pb, 34.0 Zn |
F, R, W, S |
324-607 |
C34200 High-leaded brass |
64.5 Cu, 2.0 Pb, 33.5 Zn |
F, R |
338-586 |
C34900 |
62.2 Cu, 0.35 Pb, 37.45 Zn |
R, W |
365-469 |
C35000 Medium-leaded brass |
62.5 Cu, 1.1 Pb, 36.4 Zn |
F, R |
310-655 |
C35300 High-leaded brass |
62.0 Cu, 1.8 Pb, 36.2 Zn |
F, R |
338-586 |
C35600 Extra-high-leaded brass |
63.0 Cu, 2.5 Pb, 34.5 Zn |
F |
338-510 |
C36000 Free-cutting brass |
61.5 Cu, 3.0 Pb, 35.5 Zn |
F, R, S |
339-469 |
C36500 to C36800 Leaded Muntz metal |
60.0 Cu(k), 0.6 Pb, 39.4 Zn |
F |
372 (As hot rolled) |
C37000 Free-cutting Muntz metal |
60.0 Cu, 1.0 Pb, 39.0 Zn |
T |
372-552 |
C37700 Forging brass |
59.0 Cu, 2.0 Pb, 39.0 Zn |
R, S |
359 (as extruded) |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 6 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C38500 Architectural bronze |
57.0 Cu, 3.0 Pb, 40.0 Zn |
R, S |
414 (as extruded) |
C40500 |
95 Cu, 1 Sn, 4 Zn |
F |
269-538 |
C40800 |
95 Cu, 2 Sn, 3 Zn |
F |
290-545 |
C41100 |
91 Cu, 0.5 Sn, 8.5 Zn |
F, W |
269-731 |
C41300 |
90.0 Cu, 1.0 Sn, 9.0 Zn |
F, R, W |
283-724 |
C41500 |
91 Cu, 1.8 Sn, 7.2 Zn |
F |
317-558 |
C42200 |
87.5 Cu, 1.1 Sn, 11.4 Zn |
F |
296-607 |
C42500 |
88.5 Cu, 2.0 Sn, 9.5 Zn |
F |
310-634 |
C43000 |
87.0 Cu, 2.2 Sn, 10.8 Zn |
F |
317-648 |
C43400 |
85.0 Cu, 0.7 Sn, 14.3 Zn |
F |
310-607 |
C43500 |
81.0 Cu, 0.9 Sn, 18.1 Zn |
F, T |
317-552 |
C44300, C44400, C44500 Inhibited admiralty |
71.0 Cu, 28.0 Zn, 1.0 Sn |
F, W, T |
331-379 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 7 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C46400 to C46700 Naval brass |
60.0 Cu, 39.25 Zn, 0.75 Sn |
F, R, T, S |
379-607 |
C48200 Naval brass, medium-leaded |
60.5 Cu, 0.7 Pb, 0.8 Sn, 38.0 Zn |
F, R, S |
386-517 |
C48500 Leaded naval brass |
60.0 Cu, 1.75 Pb, 37.5 Zn, 0.75 Sn |
F, R, S |
379-531 |
C50500 Phosphor bronze, 1.25% E |
98.75 Cu, 1.25 Sn, trace P |
F, W |
276-545 |
C51000 Phosphor bronze, 5% A |
95.0 Cu, 5.0 Sn, trace P |
F, R, W, T |
324-965 |
C51100 |
95.6 Cu, 4.2 Sn, 0.2 P |
F |
317-710 |
C52100 Phosphor bronze, 8% C |
92.0 Cu, 8.0 Sn, trace P |
F, R, W |
379-965 |
C52400 Phosphor bronze, 10% D |
90.0 Cu, 10.0 Sn, trace P |
F, R, W |
455-1014 |
C54400 Free-cutting phosphor bronze |
88.0 Cu, 4.0 Pb, 4.0 Zn, 4.0 Sn |
F, R |
303-517 |
C60800 Aluminum bronze, 5% |
95.0 Cu, 5.0 Al |
T |
414 |
C61000 |
92.0 Cu, 8.0 Al |
R, W |
483-552 |
C61300 |
92.65 Cu, 0.35 Sn, 7.0 Al |
F, R, T, P, S |
483-586 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 8 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C61400 Aluminum bronze, D |
91.0 Cu, 7.0 Al, 2.0 Fe |
F, R, W, T, P, S |
524-614 |
C61500 |
90.0 Cu, 8.0 Al, 2.0 Ni |
F |
483-1000 |
C61800 |
89.0 Cu, 1.0 Fe, 10.0 Al |
R |
552-586 |
C61900 |
86.5 Cu, 4.0 Fe, 9.5 Al |
F |
634-1048 |
C62300 |
87.0 Cu, 10.0 Al, 3.0 Fe |
F, R |
517-676 |
C62400 |
86.0 Cu, 3.0 Fe, 11.0 Al |
F, R |
621-724 |
C62500 |
82.7 Cu, 4.3 Fe, 13.0 Al |
F, R |
689 |
C63000 |
82.0 Cu, 3.0 Fe, 10.0 Al, 5.0 Ni |
F, R |
621-814 |
C63200 |
82.0 Cu, 4.0 Fe, 9.0 Al, 5.0 Ni |
F, R |
621-724 |
C63600 |
95.5 Cu, 3.5 Al, 1.0 Si |
R, W |
414-579 |
C63800 |
99.5 Cu, 2.8 Al, 1.8 Si, 0.40 Co |
F |
565-896 |
C64200 |
91.2 Cu, 7.0 Al |
F, R |
517-703 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 9 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C65100 Low-silicon bronze, B |
98.5 Cu, 1.5 Si |
R, W, T |
276-655 |
C65500 High-silicon bronze, A |
97.0 Cu, 3.0 Si |
F, R, W, T |
386-1000 |
C66700 Manganese brass |
70.0 Cu, 28.8 Zn, 1.2 Mn |
F, W |
315-689 |
C67400 |
58.5 Cu, 36.5 Zn, 1.2 Al, 2.8 Mn, 1.0 Sn |
F, R |
483-634 |
C67500 Manganese bronze, A |
58.5 Cu, 1.4 Fe, 39.0 Zn, 1.0 Sn, 0.1 Mn |
R, S |
448-579 |
C68700 Aluninum brass, arsenical |
77.5 Cu, 20.5 Zn, 2.0 Al, 0.1 As |
T |
414 |
C68800 |
73.5 Cu, 22.7 Zn, 3.4 Al, 0.40 Co |
F |
565-889 |
C69000 |
73.3 Cu, 3.4 Al, 0.6 Ni, 22.7 Zn |
F |
496-896 |
C69400 Silicon red brass |
81.5 Cu, 14.5 Zn, 4.0 Si |
R |
552-689 |
C70400 |
92.4 Cu, 1.5 Fe, 5.5 Ni, 0.6 Mn |
F, T |
262-531 |
C70600 Copper nickel, 10% |
88.7 Cu, 1.3 Fe, 10.0 Ni |
F, T |
303-414 |
C71000 Copper nickel, 20% |
79.00 Cu, 21.0 Ni |
F, W, T |
338-655 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 10 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C71500 Copper nickel, 30% |
70.0 Cu, 30.0 Ni |
F, R, T |
372-517 |
C71700 |
67.8 Cu, 0.7 Fe, 31.0 Ni, 0.5 Be |
F, R, W |
483-1379 |
C72500 |
88.20 Cu, 9.5 Ni, 2.3 Sn |
F, R, W, T |
379-827 |
C73500 |
72.0 Cu, 18.0 Ni , 10.0 Zn |
F, R, W, T |
345-758 |
C74500 Nickel silver, 65-10 |
65.0 Cu, 25.0 Zn, 10.0 Ni |
F, W |
338-896 |
C75200 Nickel silver, 65-18 |
65.0 Cu, 17.0 Zn, 18.0 Ni |
F, R, W |
386-710 |
C75400 Nickel silver, 65-15 |
65.0 Cu, 20.0 Zn, 15.0 Ni |
F |
365-634 |
C75700 Nickel silver, 65-12 |
65.0 Cu, 23.0 Zn, 12.0 Ni |
F, W |
359-641 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
©2001 CRC Press LLC

Table 137. TENSILE STRENGTH OF WROUGHT COPPERS AND COPPER ALLOYS
(SHEET 11 OF 11)
|
|
|
Tensile |
|
Nominal |
Commercial |
Strength |
UNS Number and Name |
Composition (%) |
Forms(a) |
(MPa) |
|
|
|
|
|
|
|
|
C76200 |
59.0 Cu, 29.0 Zn, 12.0 Ni |
F, T |
393-841 |
C77000 Nickel silver, 55-18 |
55.0 Cu, 27.0 Zn, 18.0 Ni |
F, R, W |
414-1000 |
C72200 |
82.0 Cu, 16.0 Ni, 0.5 Cr, 0.8 Fe, 0.5 Mn |
F, T |
317-483 |
C78200 Leaded nickel silver, 65-8-2 |
65.0 Cu, 2.0 Pb, 25.0 Zn, 8.0 Ni |
F |
365-627 |
|
|
|
|
(a) F, flat products; R, rod; W, wire; T, tube; P, pipe; S, shapes.
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p442–454, (1993).
(d)Based on 100% for C360000.
(e)C10400, 8 oz/ton Ag; C10500, 10 oz/ton; C10700, 25 oz/ton .
(f)C11300, 8 oz/ton Ag; C11400,10 oz/ton; C11500, 16 oz/ton; C11600, 25 oz/ton
(g)C12000, 0.008 P; C12100, 0.008 P and 4 oz/ton Ag;
(h)C12700, 8 oz/ton Ag; C12800,10 oz/ton; C12900,16 oz/ton; C13000, 25 oz/ton.
(i)8.30 oz/ton Ag.
(j)C18200, 0.9 Cr; C18400, 0.8 Cr; C18500, 0.7 Cr
(k)Rod, 61.0 Cu min.
©2001 CRC Press LLC

Table 138. TENSILE STRENGTH OF
ALUMINUM CASTING ALLOYS (SHEET 1 OF 3)
Alloy |
|
Tensile Strength |
AA No. |
Temper |
(MPa ) |
|
|
|
|
|
|
201.0 |
T4 |
365 |
|
T6 |
485 |
|
T7 |
460 |
206.0, A206.0 |
T7 |
435 |
208.0 |
F |
145 |
242.0 |
T21 |
185 |
|
T571 |
220 |
|
T77 |
205 |
|
T571 |
275 |
|
T61 |
325 |
295.0 |
T4 |
220 |
|
T6 |
250 |
|
T62 |
285 |
296.0 |
T4 |
255 |
|
T6 |
275 |
|
T7 |
270 |
308.0 |
F |
195 |
319.0 |
F |
185 |
|
T6 |
250 |
|
F |
235 |
|
T6 |
280 |
336.0 |
T551 |
250 |
|
T65 |
325 |
354.0 |
T61 |
380 |
355.0 |
T51 |
195 |
|
T6 |
240 |
|
T61 |
270 |
|
T7 |
265 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, (1984).
©2001 CRC Press LLC

Table 138. TENSILE STRENGTH OF
ALUMINUM CASTING ALLOYS (SHEET 2 OF 3)
Alloy |
|
Tensile Strength |
AA No. |
Temper |
(MPa ) |
|
|
|
|
|
|
355.0 (Con’t) |
T71 |
175 |
|
T51 |
210 |
|
T6 |
290 |
|
T62 |
310 |
|
T7 |
280 |
|
T71 |
250 |
356.0 |
T51 |
175 |
|
T6 |
230 |
|
T7 |
235 |
|
T71 |
195 |
|
T6 |
265 |
|
T7 |
220 |
357.0, A357.0 |
T62 |
360 |
359.0 |
T61 |
330 |
|
T62 |
345 |
360.0 |
F |
325 |
A360.0 |
F |
320 |
380.0 |
F |
330 |
383.0 |
F |
310 |
384.0, A384.0 |
F |
330 |
390.0 |
F |
280 |
|
T5 |
300 |
A390.0 |
F,T5 |
180 |
|
T6 |
280 |
|
T7 |
250 |
|
F,T5 |
200 |
|
T6 |
310 |
|
T7 |
260 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, (1984).
©2001 CRC Press LLC

Table 138. TENSILE STRENGTH OF
ALUMINUM CASTING ALLOYS (SHEET 3 OF 3)
Alloy |
|
Tensile Strength |
AA No. |
Temper |
(MPa ) |
|
|
|
|
|
|
413.0 |
F |
300 |
A413.0 |
F |
290 |
443.0 |
F |
130 |
B443.0 |
F |
159 |
C443.0 |
F |
228 |
514.0 |
F |
170 |
518.0 |
F |
310 |
520.0 |
T4 |
330 |
535.0 |
F |
275 |
712.0 |
F |
240 |
713.0 |
T5 |
210 |
|
T5 |
220 |
771.0 |
T6 |
345 |
850.0 |
T5 |
160 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 1 OF 7)
|
|
Tensile Strength |
Alloy |
Temper |
(MPa) |
|
|
|
|
|
|
1050 |
0 |
76 |
|
H14 |
110 |
|
H16 |
130 |
|
H18 |
160 |
1060 |
0 |
69 |
|
H12 |
83 |
|
H14 |
97 |
|
H16 |
110 |
|
H18 |
130 |
1100 |
0 |
90 |
|
H12 |
110 |
|
H14 |
125 |
|
H16 |
145 |
|
H18 |
165 |
1350 |
0 |
83 |
|
H12 |
97 |
|
H14 |
110 |
|
H16 |
125 |
|
H19 |
185 |
2011 |
T3 |
380 |
|
T8 |
405 |
2014 |
0 |
185 |
|
T4 |
425 |
|
T6 |
485 |
Alclad 2014 |
0 |
170 |
|
T3 |
435 |
|
T4 |
420 |
|
T6 |
470 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 2 OF 7)
|
|
Tensile Strength |
Alloy |
Temper |
(MPa) |
|
|
|
|
|
|
2024 |
0 |
185 |
|
T3 |
485 |
|
T4, T351 |
470 |
|
T361 |
495 |
Alclad 2024 |
0 |
180 |
|
T |
450 |
|
T4, T351 |
440 |
|
T361 |
460 |
|
T81, T851 |
450 |
|
T861 |
485 |
2036 |
T4 |
340 |
2048 |
|
455 |
2124 |
T851 |
490 |
2218 |
T61 |
405 |
|
T71 |
345 |
|
T72 |
330 |
2219 |
0 |
170 |
|
T42 |
360 |
|
T31, T351 |
360 |
|
T37 |
395 |
|
T62 |
415 |
|
T81, T851 |
455 |
|
T87 |
475 |
2618 |
All |
440 |
3003 |
0 |
110 |
Alclad |
H12 |
130 |
3003 |
H14 |
150 |
|
H16 |
180 |
|
H18 |
200 |
3004 |
0 |
180 |
Alclad |
H32 |
215 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 3 OF 7)
|
|
Tensile Strength |
Alloy |
Temper |
(MPa) |
|
|
|
|
|
|
3004 |
H34 |
240 |
|
H36 |
260 |
|
H38 |
285 |
3105 |
0 |
115 |
|
H12 |
150 |
|
H14 |
170 |
|
H16 |
195 |
|
H18 |
215 |
|
H25 |
180 |
4032 |
T6 |
380 |
4043 |
0 |
145 |
|
H18 |
285 |
5005 |
0 |
125 |
|
H12 |
140 |
|
H14 |
160 |
|
H16 |
180 |
|
H18 |
200 |
|
H32 |
140 |
|
H34 |
160 |
|
H36 |
180 |
|
H38 |
200 |
5050 |
0 |
145 |
|
H32 |
170 |
|
H34 |
195 |
|
H36 |
205 |
|
H38 |
220 |
5052 |
0 |
195 |
|
H32 |
230 |
|
H34 |
260 |
|
H36 |
275 |
|
H38 |
290 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 4 OF 7)
|
|
|
|
Tensile Strength |
Alloy |
|
Temper |
|
(MPa) |
|
|
|
|
|
|
|
|
|
|
5056 |
|
0 |
|
290 |
|
|
H18 |
|
435 |
|
|
H38 |
|
415 |
5083 |
|
0 |
|
290 |
|
|
H112 |
|
305 |
|
|
H113 |
|
315 |
|
|
H321 |
|
315 |
|
H323, H32 |
325 |
||
|
H343, H34 |
345 |
||
5086 |
|
0 |
|
260 |
|
H32, |
H116, |
H117 |
290 |
|
|
H34 |
|
325 |
|
|
H112 |
|
270 |
5154 |
|
0 |
|
240 |
|
|
H32 |
|
270 |
|
|
H34 |
|
290 |
|
|
H36 |
|
310 |
|
|
H38 |
|
330 |
|
|
H112 |
|
240 |
5182 |
|
0 |
|
275 |
|
|
H32 |
|
315 |
|
|
H34 |
|
340 |
|
|
H19(n) |
|
420 |
5252 |
|
H25 |
|
235 |
|
|
H28, H38 |
285 |
|
5254 |
|
0 |
|
240 |
5254 |
|
H32 |
|
270 |
|
|
H34 |
|
290 |
|
|
H36 |
|
310 |
|
|
H38 |
|
330 |
|
|
H112 |
|
240 |
|
|
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 5 OF 7)
|
|
Tensile Strength |
Alloy |
Temper |
(MPa) |
|
|
|
|
|
|
5454 |
0 |
250 |
|
H32 |
275 |
|
H34 |
305 |
|
H36 |
340 |
|
H38 |
370 |
|
H111 |
260 |
|
H112 |
250 |
|
H311 |
260 |
5456 |
0 |
310 |
|
H111 |
325 |
|
H112 |
310 |
|
H321, H116 |
350 |
5457 |
0 |
130 |
|
H25 |
180 |
|
H28, H38 |
205 |
5652 |
0 |
195 |
|
H32 |
230 |
|
H34 |
260 |
|
H36 |
275 |
|
H38 |
290 |
5657 |
H25 |
160 |
|
H28, H38 |
195 |
6005 |
T1 |
170 |
|
T5 |
260 |
6009 |
T4 |
235 |
|
T6 |
345 |
6010 |
T4 |
255 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 6 OF 7)
|
|
Tensile Strength |
Alloy |
Temper |
(MPa) |
|
|
|
|
|
|
6061 |
0 |
125 |
|
T4, T451 |
240 |
|
T6, T651 |
310 |
Alclad 6061 |
0 |
115 |
|
T4, T451 |
230 |
|
T6, T651 |
290 |
6063 |
0 |
90 |
|
T1 |
150 |
|
T4 |
170 |
|
T5 |
185 |
|
T6 |
240 |
|
T83 |
255 |
|
T831 |
205 |
|
T832 |
290 |
6066 |
0 |
150 |
|
T4, T451 |
360 |
|
T6, T651 |
395 |
6070 |
0 |
145 |
|
T4 |
315 |
|
T6 |
380 |
6101 |
Hlll |
97 |
6151 |
T6 |
220 |
6201 |
T6 |
330 |
|
T81 |
330 |
6205 |
Tl |
260 |
|
T5 |
310 |
6262 |
T9 |
400 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 139. TENSILE STRENGTH OF
WROUGHT ALUMINUM ALLOYS (SHEET 7 OF 7)
|
|
Tensile Strength |
Alloy |
Temper |
(MPa) |
|
|
|
|
|
|
6351 |
T4 |
250 |
|
T6 |
310 |
6463 |
Tl |
150 |
|
T5 |
185 |
|
T6 |
240 |
7005 |
0 |
193 |
|
T53 |
393 |
|
T6,T63,T6351 |
372 |
7050 |
T736 |
515 |
7075 |
0 |
230 |
|
T6,T651 |
570 |
|
T73 |
505 |
Alclad 7075 |
0 |
220 |
|
T6,T651 |
525 |
7175 |
T66 |
595 |
|
T736 |
525 |
7475 |
T61 |
525 |
|
|
|
Source: data from ASM Metals Reference Book, Second Edition, American Society for Metals, Metals Park, Ohio 44073, p.299—302, (1984).
©2001 CRC Press LLC

Table 140. TENSILE STRENGTH OF
COBALT-BASE SUPERALLOYS
|
Temperature |
Tensile Strength |
Alloy |
(°C) |
(MPa) |
|
|
|
|
|
|
Haynes 25 (L–605) sheet |
21 |
1010 |
|
540 |
800 |
|
650 |
710 |
|
760 |
455 |
|
870 |
325 |
Haynes 188, sheet |
21 |
960 |
|
540 |
740 |
|
650 |
710 |
|
760 |
635 |
|
870 |
420 |
S-816, bar |
21 |
965 |
|
540 |
840 |
|
650 |
765 |
|
760 |
650 |
|
870 |
360 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p387, (1993).
©2001 CRC Press LLC


Table 141. TENSILE STRENGTH OF
NICKEL-BASE SUPERALLOYS (SHEET 2 OF 5)
|
Temperature |
Tensile Strength |
Alloy |
(°C) |
(MPa) |
|
|
|
|
|
|
Inconel 625, bar |
21 |
855 |
|
540 |
745 |
|
650 |
710 |
|
760 |
505 |
|
870 |
285 |
Inconel 706, bar |
21 |
1300 |
|
540 |
1120 |
|
650 |
1010 |
|
760 |
690 |
Inconel 718, bar |
21 |
1430 |
|
540 |
1280 |
|
650 |
1230 |
|
760 |
950 |
|
870 |
340 |
Inconel 718, sheet |
21 |
1280 |
|
540 |
1140 |
|
650 |
1030 |
|
760 |
675 |
Inconel X-750, bar |
21 |
1120 |
|
540 |
965 |
|
650 |
825 |
|
760 |
485 |
|
870 |
235 |
M-252, bar |
21 |
1240 |
|
540 |
1230 |
|
650 |
1160 |
|
760 |
945 |
|
870 |
510 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p387-389, (1993).
©2001 CRC Press LLC

Table 141. TENSILE STRENGTH OF
NICKEL-BASE SUPERALLOYS (SHEET 3 OF 5)
|
Temperature |
Tensile Strength |
Alloy |
(°C) |
(MPa) |
|
|
|
|
|
|
Nimonic 75, bar |
21 |
750 |
|
540 |
635 |
|
650 |
538 |
|
760 |
290 |
|
870 |
145 |
Nimonic 80A, bar |
21 |
1240 |
|
540 |
1100 |
|
650 |
1000 |
|
760 |
760 |
|
870 |
400 |
Nimonic 90, bar |
21 |
1240 |
|
540 |
1100 |
|
650 |
1030 |
|
760 |
825 |
|
870 |
430 |
Nimonic 105, bar |
21 |
1140 |
|
540 |
1100 |
|
650 |
1080 |
|
760 |
965 |
|
870 |
605 |
Nimonic 115, bar |
21 |
1240 |
|
540 |
1090 |
|
650 |
1120 |
|
760 |
1080 |
|
870 |
825 |
Pyromet 860, bar |
21 |
1300 |
|
540 |
1250 |
|
650 |
1110 |
|
760 |
910 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p387-389, (1993).
©2001 CRC Press LLC

Table 141. TENSILE STRENGTH OF
NICKEL-BASE SUPERALLOYS (SHEET 4 OF 5)
|
Temperature |
Tensile Strength |
Alloy |
(°C) |
(MPa) |
|
|
|
|
|
|
René 41, bar |
21 |
1420 |
|
540 |
1400 |
|
650 |
1340 |
|
760 |
1100 |
|
870 |
620 |
René 95, bar |
21 |
1620 |
|
540 |
1540 |
|
650 |
1460 |
|
760 |
1170 |
Udimet 500, bar |
21 |
1310 |
|
540 |
1240 |
|
650 |
1210 |
|
760 |
1040 |
|
870 |
640 |
Udimet 520, bar |
21 |
1310 |
|
540 |
1240 |
|
650 |
1170 |
|
760 |
725 |
|
870 |
515 |
Udimet 700, bar |
21 |
1410 |
|
540 |
1280 |
|
650 |
1240 |
|
760 |
1030 |
|
870 |
690 |
Udimet 710, bar |
21 |
1190 |
|
540 |
1150 |
|
650 |
1290 |
|
760 |
1020 |
|
870 |
705 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p387-389, (1993).
©2001 CRC Press LLC
Table 141. TENSILE STRENGTH OF
NICKEL-BASE SUPERALLOYS (SHEET 5 OF 5)
|
Temperature |
Tensile Strength |
Alloy |
(°C) |
(MPa) |
|
|
|
|
|
|
Unitemp AF2–1DA, bar |
21 |
1290 |
|
540 |
1340 |
|
650 |
1360 |
|
760 |
1150 |
|
870 |
830 |
Waspaloy, bar |
21 |
1280 |
|
540 |
1170 |
|
650 |
1120 |
|
760 |
795 |
|
870 |
525 |
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p387-389, (1993).
©2001 CRC Press LLC

Table 142. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT ROOM TEMPERATURE (SHEET 1 OF 3)
|
|
|
Tensile Strength |
Class |
Alloy |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
Commercially Pure |
99.5 Ti |
Annealed |
331 |
|
99.2 Ti |
Annealed |
434 |
|
99.1 Ti |
Annealed |
517 |
|
99.0 Ti |
Annealed |
662 |
|
99.2Ti-0.2Pd |
Annealed |
434 |
|
Ti-0.8Ni-0.3Mo |
Annealed |
517 |
Alpha Alloys |
Ti-5Al-2.5Sn |
Annealed |
862 |
|
Ti-5Al-2.5Sn (low O2) |
Annealed |
807 |
Near Alpha Alloys |
Ti-8Al-1Mo-1V |
Duplex Annealed |
1000 |
|
Ti-11Sn-1Mo-2.25Al-5.0Zr-1Mo-0.2Si |
Duplex Annealed |
1103 |
|
Ti-6Al-2Sn-4Zr-2Mo |
Duplex Annealed |
979 |
|
Ti-5Al-2Sn-2Zr-2Mo-0.25Si |
975 ˚C (1/2h), AC + 595˚C (2h), AC |
1048 |
|
Ti-6Al-2Nb-1Ta-1Mo |
As rolled 2.5 cm (1 in.) plate |
855 |
|
Ti-6Al-2Sn-1.5Zr-1Mo- 0.35Bi-0.1Si |
Beta forge + duplex anneal |
1014 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 142. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT ROOM TEMPERATURE (SHEET 2 OF 3)
|
|
|
Tensile Strength |
Class |
Alloy |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
Alpha-Beta Alloys |
Ti-8Mn |
Annealed |
945 |
|
Ti-3Al-2.5V |
Annealed |
689 |
|
Ti-6Al-4V |
Annealed |
993 |
|
|
Solution + age |
1172 |
|
Ti-6Al-4V(low O2) |
Annealed |
896 |
|
Ti-6Al-6V-2Sn |
Annealed |
1069 |
|
|
Solution + age |
1276 |
|
Ti-7Al-4Mo |
Solution + age |
1103 |
|
Ti-6Al-2Sn-4Zr-6Mo |
Solution + age |
1269 |
|
Ti-6Al-2Sn-2Zr-2Mo- 2Cr-0.25Si |
Solution + age |
1276 |
|
Ti-10V-2Fe-3Al |
Solution + age |
1276 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 142. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT ROOM TEMPERATURE (SHEET 3 OF 3)
|
|
|
Tensile Strength |
Class |
Alloy |
Condition |
(MPa) |
|
|
|
|
|
|
|
|
Beta Alloys |
Ti-13V-1Cr-3Al |
Solution + age |
1220 |
|
|
|
1276 |
|
Ti-8Mo-8V-2Fe-3Al |
Solution + age |
1310 |
|
Ti-3Al-8V-6Cr-4Mo-4Zr |
Solution + age |
1448 |
|
|
Annealed |
883 |
|
Ti-11.5Mo-6Zr-4.5Sn |
Solution + age |
1386 |
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 143. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT HIGH TEMPERATURE (SHEET 1 OF 4)
|
|
|
Test |
|
|
|
|
Temperature |
Tensile Strength |
Class |
Alloy |
Condition |
(°C) |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Commercially Pure |
99.5 Ti |
Annealed |
315 |
152 |
|
99.2 Ti |
Annealed |
315 |
193 |
|
99.1 Ti |
Annealed |
315 |
234 |
|
99.0 Ti |
Annealed |
315 |
310 |
|
99.2Ti-0.2Pd |
Annealed |
315 |
186 |
|
Ti-0.8Ni-0.3Mo |
Annealed |
205 |
345 |
|
Ti-0.8Ni-0.3Mo |
Annealed |
315 |
324 |
Alpha Alloys |
Ti-5Al-2.5Sn |
Annealed |
315 |
565 |
|
Ti-5Al-2.5Sn (low O2) |
Annealed |
-195 |
1241 |
|
|
|
-255 |
1579 |
Near Alpha Alloys |
Ti-8Al-1Mo-1V |
Duplex Annealed |
315 |
793 |
|
|
|
425 |
738 |
|
|
|
540 |
621 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 143. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT HIGH TEMPERATURE (SHEET 2 OF 4)
|
|
|
Test |
|
|
|
|
Temperature |
Tensile Strength |
Class |
Alloy |
Condition |
(°C) |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
Ti-11Sn-1Mo-2.25Al-5.0Zr-1Mo-0.2Si |
Duplex Annealed |
315 |
896 |
|
|
|
425 |
827 |
|
|
|
540 |
758 |
|
Ti-6Al-2Sn-4Zr-2Mo |
Duplex Annealed |
315 |
772 |
|
|
|
425 |
703 |
|
|
|
540 |
648 |
|
Ti-5Al-2Sn-2Zr-2Mo-0.25Si |
975 ˚C (1/2h), AC + 595 ˚C (2h), AC |
315 |
793 |
|
|
|
425 |
779 |
|
|
|
540 |
689 |
|
Ti-6Al-2Nb-1Ta-1Mo |
As rolled 2.5 cm (1 in.) plate |
315 |
586 |
|
|
|
425 |
517 |
|
|
|
540 |
483 |
|
Ti-6Al-2Sn-1.5Zr-1Mo- 0.35Bi-0.1Si |
Beta forge + duplex anneal |
480 |
724 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 143. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT HIGH TEMPERATURE (SHEET 3 OF 4)
|
|
|
Test |
|
|
|
|
Temperature |
Tensile Strength |
Class |
Alloy |
Condition |
(°C) |
(MPa) |
|
|
|
|
|
|
|
|
|
|
Alpha-Beta Alloys |
Ti-8Mn |
Annealed |
315 |
717 |
|
Ti-3Al-2.5V |
Annealed |
315 |
483 |
|
Ti-6Al-4V |
Annealed |
315 |
724 |
|
|
Annealed |
425 |
669 |
|
|
Annealed |
540 |
531 |
|
|
Solution + age |
315 |
862 |
|
|
Solution + age |
425 |
800 |
|
|
Solution + age |
540 |
655 |
|
Ti-6Al-4V(low O2) |
Annealed |
160 |
1517 |
|
Ti-6Al-6V-2Sn |
Annealed |
315 |
931 |
|
|
Solution + age |
315 |
979 |
|
Ti-7Al-4Mo |
Solution + age |
315 |
976 |
|
|
|
425 |
848 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 143. TENSILE STRENGTH OF WROUGHT TITANIUM ALLOYS
AT HIGH TEMPERATURE (SHEET 4 OF 4)
|
|
|
Test |
|
|
|
|
Temperature |
Tensile Strength |
Class |
Alloy |
Condition |
(°C) |
(MPa) |
|
|
|
|
|
|
|
|
|
|
|
Ti-6Al-2Sn-4Zr-6Mo |
Solution + age |
315 |
1020 |
|
|
|
425 |
951 |
|
|
|
540 |
848 |
|
Ti-6Al-2Sn-2Zr-2Mo- 2Cr-0.25Si |
Solution + age |
315 |
979 |
|
Ti-10V-2Fe-3Al |
Solution + age |
205 |
1117 |
|
|
|
315 |
1103 |
Beta Alloys |
Ti-13V-1Cr-3Al |
Solution + age |
315 |
883 |
|
|
|
425 |
1103 |
|
Ti-8Mo-8V-2Fe-3Al |
Solution + age |
315 |
1131 |
|
Ti-3Al-8V-6Cr-4Mo-4Zr |
Solution + age |
315 |
1034 |
|
|
|
425 |
938 |
|
|
Annealed |
315 |
724 |
|
Ti-11.5Mo-6Zr-4.5Sn |
Solution + age |
315 |
903 |
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p512, (1993).
©2001 CRC Press LLC

Table 144. TENSILE STRENGTH OF REFRACTORY METAL ALLOYS
(SHEET 1 OF 3)
|
|
|
|
|
|
Tensile |
|
|
|
|
|
|
Temperature |
Strength |
|
Class |
Alloy |
Alloying Additions (%) |
Form |
Condition |
(°F) |
(ksi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Niobium and |
Pure Niobium |
— |
All |
Recrystallized |
2000 |
10 |
|
Niobium Alloys |
|||||||
|
|
|
|
|
|
||
|
Nb–1Zr |
1 Zr |
All |
Recrystallized |
2000 |
23 |
|
|
C103(KbI–3) |
10 Hf, 1 Ti 0.7 Zr |
All |
Recrystallized |
2000 |
27 |
|
|
SCb291 |
10 Ta, 10 W |
Bar, Sheet |
Recrystallized |
2000 |
32 |
|
|
C129 |
10 W, 10 Hf, 0.1 Y |
Sheet |
Recrystallized |
2400 |
26 |
|
|
FS85 |
28 Ta, 11 W, 0.8 Zr |
Sheet |
Recrystallized |
2400 |
23 |
|
|
SU31 |
17 W, 3.5 Hf, 0.12 C, 0.03 Si |
Bar, Sheet |
Special Thermal Processing |
2400 |
40 |
|
Molybdenum and |
Pure Molybdenum |
— |
All |
Stress-relieved Annealed |
1800 |
52 |
|
Molybdenum Alloys |
|||||||
|
|
|
|
|
|
||
|
Doped Mo |
K, Si; ppm levels |
Wire, Sheet |
Cold Worked |
3000 |
30 |
|
|
Low C Mo |
None |
All |
Stress-relieved Annealed |
1800 |
50 |
|
|
TZM |
0.5 Ti, 0.08 Zr, 0.015 C |
All |
Stress-relieved Annealed |
2400 |
45 |
|
|
TZC |
1.0 Ti, 0.14 Zr, 0.02 to 0.08 C |
All |
Stress-relieved Annealed |
2400 |
55 |
|
|
Mo–5Re |
5 Re |
All |
Stress-relieved Annealed |
3000 |
2 |
|
|
Mo–30W |
30 W |
All |
Stress-relieved Annealed |
2000 |
50 |
|
|
|
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p390, (1993).
©2001 CRC Press LLC

Table 144. TENSILE STRENGTH OF REFRACTORY METAL ALLOYS
(SHEET 2 OF 3)
|
|
|
|
|
|
Tensile |
|
|
|
|
|
|
Temperature |
Strength |
|
Class |
Alloy |
Alloying Additions (%) |
Form |
Condition |
(°F) |
(ksi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tantalum Alloys |
Unalloyed |
None |
All |
Recrystallized |
2400 |
8.5 |
|
|
FS61 |
7.5 W(P/M) |
Wire, Sheet |
Cold Worked |
75 |
165 |
|
|
FS63 |
2.5 W, 0.15 Nb |
All |
Recrystallized |
200 |
46 |
|
|
TA–10W |
10 W |
All |
Recrystallized |
2400 |
50 |
|
|
KBI–40 |
40 Nb |
All |
Recrystallized |
500 |
42 |
|
Tungsten Alloys |
Unalloyed |
None |
Bar, Sheet, |
Stress-relieved Annealed |
3000 |
25 |
|
Wire |
|||||||
|
|
|
|
|
|
||
|
Doped |
K, Si, Al; ppm levels |
Wire |
Cold Worked |
3000 |
94 |
|
|
W–1 ThO2 |
1ThO2 |
Bar, Sheet, |
Stress-relieved Annealed |
3000 |
37 |
|
|
Wire |
||||||
|
W–2 ThO2 |
2 ThO2 |
Bar, Sheet, |
Stress-relieved Annealed |
3000 |
30 |
|
|
Wire |
||||||
|
W–3 ThO2 |
3 ThO2 |
Bar, Wire |
Stress-relieved Annealed |
3000 |
30 |
|
|
W–4 ThO2 |
4 ThO2 |
Bar |
Stress-relieved Annealed |
3000 |
30 |
|
|
|
|
|
|
|
|
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p390, (1993).
©2001 CRC Press LLC

Table 144. TENSILE STRENGTH OF REFRACTORY METAL ALLOYS
(SHEET 3 OF 3)
|
|
|
|
|
|
Tensile |
|
|
|
|
|
Temperature |
Strength |
Class |
Alloy |
Alloying Additions (%) |
Form |
Condition |
(°F) |
(ksi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
W–15 Mo |
15 Mo |
Bar, Wire |
Stress-relieved Annealed |
3000 |
36 |
|
W–50 Mo |
50 Mo |
Bar, Wire |
Stress-relieved Annealed |
3000 |
20 |
|
W–3 Re |
3 Re |
Wire |
Cold Worked |
— |
— |
|
W–25 Re |
25 Re |
Bar, Sheet, |
Stress-relieved Annealed |
3000 |
33 |
|
Wire |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Data from ASM Metals Reference Book, Third Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p390, (1993). |
|
|||||
|
|
|
|
|
|
|
©2001 CRC Press LLC

Table 145. TENSILE STRENGTH OF CERAMICS
(SHEET 1 OF 4)
|
|
Tensile Strength |
|
Type |
Ceramic |
(psi) |
Temperature |
|
|
|
|
|
|
|
|
Borides |
Chromium Diboride (CrB2) |
10.6x104 |
|
|
Titanium Diboride (TiB2) |
18.4x103 |
|
|
Zirconium Diboride (ZrB2) |
28.7x103 |
|
Carbides |
Boron Carbide (B4C) |
22.5x103 |
980˚C |
|
Silicon Carbide (SiC) |
5-20x103 psi |
25˚C |
|
(hot pressed) |
29x103 psi |
20˚C |
|
(hot pressed) |
5.75-21.75 x103 psi |
1400˚C |
|
(reaction bonded) |
11.17x103 psi |
20˚C |
|
Tantalum Monocarbide (TaC) |
2-42x103 psi |
|
|
Titanium Monocarbide (TiC) |
17.2x103 |
1000˚C |
|
Tungsten Monocarbide (WC) |
50x103 psi |
|
|
Zirconium Monocarbide (ZrC) |
16.0x103 |
room temp. |
|
|
11.7-14.45x103 |
980˚C |
|
|
12.95-15.85x103 |
1250˚C |
Nitrides |
Boron Nitride (BN) |
0.35x103 |
1000˚C |
|
|
0.35x103 |
1500˚C |
|
|
1.15x103 |
1800˚C |
|
|
2.25x103 |
2000˚C |
|
|
6.80x103 |
2400˚C |
|
Trisilicon tetranitride (Si3N4) |
|
|
|
(hot pressed) |
54.4 x103 |
20˚C |
|
(hot pressed) |
21.8 x103 |
1400˚C |
|
(reaction bonded) |
24.7 x103 |
20˚C |
|
(reaction bonded) |
20.3 x103 |
1400˚C |
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991).
©2001 CRC Press LLC

Table 145. TENSILE STRENGTH OF CERAMICS
(SHEET 2 OF 4)
|
|
Tensile Strength |
|
Type |
Ceramic |
(psi) |
Temperature |
|
|
|
|
|
|
|
|
Oxides |
Aluminum Oxide (Al2O3) |
37-37.8 x103 |
room temp. |
|
|
33.6 x103 |
300˚C |
|
|
40 x103 |
500˚C |
|
|
34.6 x103 |
800˚C |
|
|
35 x103 |
1000˚C |
|
|
33.9 x103 |
1050˚C |
|
|
31.4 x103 |
1140˚C |
|
|
18.5-20 x103 |
1200˚C |
|
|
6.4 x103 |
1300˚C |
|
|
4.3 x103 |
1400˚C |
|
|
1.5 x103 |
1460˚C |
|
Beryllium Oxide (BeO) |
13.5-20 x103 |
room temp. |
|
|
11.1 x103 |
500˚C |
|
|
7.0 x103 |
900˚C |
|
|
5.0 x103 |
1000˚C |
|
|
2.0 x103 |
1140˚C |
|
|
0.6 x103 |
1300˚C |
|
Magnesium Oxide (MgO) |
14 x103 |
room temp. |
|
|
14 x103 |
200˚C |
|
|
15.2 x103 |
400˚C |
|
|
16 x103 |
800˚C |
|
|
11.5 x103 |
1000˚C |
|
|
10 x103 |
1100˚C |
|
|
8 x103 |
1200˚C |
|
|
6 x103 |
1300˚C |
|
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991).
©2001 CRC Press LLC

Table 145. TENSILE STRENGTH OF CERAMICS
(SHEET 3 OF 4)
|
|
Tensile Strength |
|
|
Type |
Ceramic |
(psi) |
Temperature |
|
|
|
|
|
|
|
|
|
|
|
Oxides |
Thorium Dioxide (ThO2) |
14x103 |
room temp. |
|
(Con’t) |
||||
|
|
|
||
|
Zircoium Oxide (ZrO2) |
17.9-20x103 |
room temp. |
|
|
|
16.8x103 |
200˚C |
|
|
|
17.5x103 |
400˚C |
|
|
|
20.0x103 |
500˚C |
|
|
|
17.6x103 |
600˚C |
|
|
|
16.0x103 |
800˚C |
|
|
|
6.75-17.0x103 |
1000˚C |
|
|
|
13.0-13.5x103 |
1100˚C |
|
|
|
12.1x103 |
1200˚C |
|
|
|
10.2x103 |
1300˚C |
|
|
(MgO stabilized) |
21x106 psi |
room temp. |
|
|
Cordierite (2MgO 2Al2O3 5SiO2) |
|
|
|
|
(ρ=2.51g/cm3) |
7.8x103 |
25˚C |
|
|
(ρ=2.1g/cm3) |
3.5x103 |
800˚C |
|
|
(ρ=1.8g/cm3) |
2.5x103 |
1200˚C |
|
|
Mullite (3Al2O3 2SiO2) |
16x103 |
25˚C |
|
|
Spinel (Al2O3 MgO) |
19.2x103 |
room temp. |
|
|
|
13.7x103 |
550˚C |
|
|
|
110.8x103 |
900˚C |
|
|
|
6.1x103 |
1150˚C |
|
|
|
1.1x103 |
1300˚C |
|
|
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991).
©2001 CRC Press LLC
Table 145. TENSILE STRENGTH OF CERAMICS
(SHEET 4 OF 4)
|
|
Tensile Strength |
|
|
Type |
Ceramic |
(psi) |
Temperature |
|
|
|
|
|
|
|
|
|
|
|
Oxides |
Zircon (SiO2 ZrO2) |
12.7x103 |
room temp. |
|
(Con’t) |
||||
|
|
|
||
|
|
8.7x103 |
1050˚C |
|
|
|
3.6x103 |
1200˚C |
|
Silicide |
Molybdenum Disilicide (MoSi2) |
40x103 |
980˚C |
|
|
|
42.16x103 |
1090˚C |
|
|
|
42.8x103 |
1200˚C |
|
|
|
41.07x103 |
1300˚C |
|
|
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from No. 1 Materials Index, Peter T.B. Shaffer, Plenum Press, New York, (1964); Smithells Metals Reference Book, Eric A. Brandes, ed., in association with Fulmer Research Institute Ltd. 6th ed. London, Butterworths, Boston, (1983); and Ceramic Source, American Ceramic Society (1986-1991).
©2001 CRC Press LLC

Table 146. TENSILE STRENGTH OF GLASS
(SHEET 1 OF 3)
|
|
Tensile Strength |
|
Type |
Glass |
(Kg • mm–2) |
Temperature |
|
|
|
|
|
|
|
|
SiO2 glass |
(48 μm diameter fiber) |
49.6 |
|
|
(56 μm diameter fiber) |
44.3 |
|
|
(60 μm diameter fiber) |
42.3 |
|
|
(65 μm diameter fiber) |
39.7 |
|
|
(74 μm diameter fiber) |
36.5 |
|
|
(78 μm diameter fiber) |
35.8 |
|
|
(108 μm diameter fiber) |
28.8 |
|
|
(112 μm diameter fiber) |
28.3 |
|
|
(1.5 mm diameter rod, 0.5 g/mm2•s stress rate) |
5.84–7.08 |
|
|
(1.5 mm diameter rod, 50 g/mm2•s stress rate) |
9.73±2.13 |
|
|
(1.5 mm diameter rod, 54 g/mm2•s stress rate) |
8.52±2.52 |
|
|
(Corning 7940 silica glass) |
5.6 |
100˚C |
|
(Corning 7940 silica glass) |
6.2 |
300˚C |
|
(Corning 7940 silica glass) |
6.6 |
500˚C |
|
|
|
|
Source: data compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 146. TENSILE STRENGTH OF GLASS
(SHEET 2 OF 3)
|
|
Tensile Strength |
|
Type |
Glass |
(Kg • mm–2) |
Temperature |
|
|
|
|
|
|
|
|
SiO2 glass (Con’t) |
(Corning 7940 silica glass) |
7.1 |
700˚C |
|
(Corning 7940 silica glass) |
7.6 |
900˚C |
SiO2–Na2O glass |
(6.0μm diameter fiber, 19.5% mol Na2O) |
173±1.36 |
|
|
(8.6μm diameter fiber, 19.5% mol Na2O) |
134±1.34 |
|
|
(25.7μm diameter fiber, 19.5% mol Na2O) |
92.5±10.08 |
|
|
(5 mm diameter rod, 20% mol Na2O) |
15 |
|
|
(3.6μm diameter fiber, 25.5% mol Na2O) |
142±0.189 |
|
|
(6.3μm diameter fiber, 25.5% mol Na2O) |
127±0.259 |
|
|
(12.8μm diameter fiber, 25.5% mol Na2O) |
103±1.020 |
|
|
(5.4μm diameter fiber, 36.3% mol Na2O) |
107.6±0.308 |
|
|
(8.6μm diameter fiber, 36.3% mol Na2O) |
98.0±0.344 |
|
|
(11.4μm diameter fiber, 36.3% mol Na2O) |
91.2±1.480 |
|
|
|
|
|
Source: data compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 146. TENSILE STRENGTH OF GLASS
(SHEET 3 OF 3)
|
|
Tensile Strength |
|
Type |
Glass |
(Kg • mm–2) |
Temperature |
|
|
|
|
|
|
|
|
SiO2–PbO glass |
(3.0 μm diameter fiber, 50% mol PbO) |
70.8 |
|
|
(4.3 μm diameter fiber, 50% mol PbO) |
64 |
|
|
(5.7 μm diameter fiber, 50% mol PbO) |
66–67.2 |
|
|
(7.1 μm diameter fiber, 50% mol PbO) |
62–71.3 |
|
|
(8.0 μm diameter fiber, 50% mol PbO) |
64.5 |
|
|
(11.4 μm diameter fiber, 50% mol PbO) |
51.9–56 |
|
|
(17.2 μm diameter fiber, 50% mol PbO) |
43–51.6 |
|
B2O3 glass |
(10–30 μm diameter fiber) |
60 |
|
B2O3–Na2O glass |
(10–30 μm diameter fiber, 10% mol Na2O) |
102 |
|
|
(10–30 μm diameter fiber, 20% mol Na2O) |
137 |
|
|
(10–30 μm diameter fiber, 30% mol Na2O) |
152 |
|
|
|
|
|
Source: data compiled by J.S. Park from O. V. Mazurin, M. V. Streltsina and T. P. Shvaiko–Shvaikovskaya, Handbook of Glass Data, Part A and Part B, Elsevier, New York, 1983
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 1 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
ABS Resins; Molded, Extruded |
Medium impact |
6.3—8.0 |
|
High impact |
5.0—6.0 |
|
Very high impact |
4.5—6.0 |
|
Low temperature impact |
4—6 |
|
Heat resistant |
7.0—8.0 |
Acrylics; Cast, Molded, Extruded |
Cast Resin Sheets, Rods: |
|
|
General purpose, type I |
6—9 |
|
General purpose, type II |
8—10 |
|
Moldings: |
|
|
Grades 5, 6, 8 |
8.8—10.5 |
|
High impact grade |
5.5—8.0 |
Thermoset Carbonate |
Allyl diglycol carbonate |
5—6 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 2 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Alkyds; Molded |
Putty (encapsulating) |
4—5 |
|
Rope (general purpose) |
7—8 |
|
Granular (high speed molding) |
3—4 |
|
Glass reinforced (heavy duty parts) |
5—9 |
Cellulose Acetate; Molded, Extruded |
ASTM Grade: |
(Tensile Strength at Fracture) |
|
H4—1 |
7—8 |
|
H2—1 |
5.8—7.2 |
|
MH—1, MH—2 |
4.8—6.3 |
|
MS—1, MS—2 |
3.9—5.3 |
|
S2—1 |
3.0—4.4 |
Cellulose Acetate Butyrate; |
Molded, Extruded |
(Tensile Strength at Fracture) |
|
ASTM Grade: |
|
|
H4 |
6.9 |
|
MH |
5.0—6.0 |
|
S2 |
3.0—4.0 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 3 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Cellusose Acetate Propionate; Molded, Extruded |
ASTM Grade: |
|
|
1 |
5.9—6.5 |
|
3 |
5.1—5.9 |
|
6 |
4 |
Chlorinated Polymers |
Chlorinated polyether |
6 |
|
Chlorinated polyvinyl chloride |
7.3 |
Polycarbonates |
Polycarbonate |
9.5 |
|
Polycarbonate (40% glass fiber reinforced) |
18 |
Diallyl Phthalates; Molded |
Orlon filled |
4.5—6 |
|
Dacron filled |
4.6—6.2 |
|
Asbestos filled |
4—6.5 |
|
Glass fiber filled |
5.5—11 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 4 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Fluorocarbons; Molded,Extruded |
Polytrifluoro chloroethylene (PTFCE) |
4.6—5.7 |
|
Polytetrafluoroethylene (PTFE) |
2.5—6.5 |
|
Ceramic reinforced (PTFE) |
0.75—2.5 |
|
Fluorinated ethylene propylene(FEP) |
2.5—4.0 |
|
Polyvinylidene— fluoride (PVDF) |
5.2—8.6 |
Epoxies; Cast, Molded, Reinforced |
Standard epoxies (diglycidyl ethers of bisphenol A) |
|
|
Cast rigid |
9.5-11.5 |
|
Cast flexible |
1.4—7.6 |
|
Molded |
8—11 |
|
General purpose glass cloth laminate |
50-58 |
|
High strength laminate |
160 |
|
Filament wound composite |
230-240 (hoop) |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 5 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Epoxies—Molded, Extruded |
High performance resins |
|
|
(cycloaliphatic diepoxides) |
|
|
Cast, rigid |
8—12 |
|
Molded |
5.2—5.3 |
|
Glass cloth laminate |
50—52 |
|
Epoxy novolacs |
|
|
Cast, rigid |
9.6—12.0 |
|
Glass cloth laminate |
59.2 |
Melamines; Molded |
Filler & type |
|
|
Cellulose electrical |
5—9 |
|
Glass fiber |
6—9 |
|
Alpha cellulose and mineral |
5—8 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 6 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Nylons; Molded, Extruded |
Type 6 |
|
|
General purpose |
9.5—12.5 |
|
Glass fiber (30%) reinforced |
21—24 |
|
Cast |
12.8 |
|
Flexible copolymers |
7.5—10.0 |
|
Type 12 |
7.1—8.5 |
|
6/6 Nylon |
|
|
General purpose molding |
11.2—11.8 |
|
Glass fiber reinforced |
25—30 |
|
Glass fiber Molybdenum disulfide filled |
19—22 |
|
General purpose extrusion |
1.26–8.6 |
|
6/10 Nylon |
|
|
General purpose |
7.1—8.5 |
|
Glass fiber (30%) reinforced |
19 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 7 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Phenolics; Molded |
Phenolics; Molded |
(ASTM D651) |
|
Type and filler |
|
|
General: woodflour and flock |
5.0—8.5 |
|
Shock: paper, flock, or pulp |
5.0—8.5 |
|
High shock: chopped fabric or cord |
5—9 |
|
Very high shock: glass fiber |
5—10 |
|
Arc resistant—mineral |
6 |
|
Rubber phenolic—woodflour or flock |
4.5—9 |
|
Rubber phenolic—chopped fabric |
3—5 |
|
Rubber phenolic—asbestos |
4 |
|
ABS–Polycarbonate Alloy |
8.2 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 8 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Polyacetals |
Homopolymer: |
|
|
Standard |
10 |
|
20% glass reinforced |
8.5 |
|
22% TFE reinforced |
6.9 |
|
Copolymer: |
|
|
Standard |
8.8 |
|
25% glass reinforced |
18.5 |
|
High flow |
8.8 |
Polyesters: Thermosets |
Cast polyyester |
|
|
Rigid |
5—15 |
|
Flexible |
1—8 |
Reinforced polyester moldings |
High strength (glass fibers) |
5—10 |
|
Heat and chemical resistant (asbestos) |
4—6 |
|
Sheet molding compounds, general purpose |
15—17 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 9 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Polyarylsulfone |
Polyarylsulfone |
13 |
Polypropylene: |
General purpose |
4.5—6.0 |
Polyethylenes; Molded, Extruded |
Type I—lower density (0.910—0.925) |
(ASTM D412) |
|
Melt index 0.3—3.6 |
1.4—2.5 |
|
Melt index 6—26 |
1.4—2.0 |
|
Melt index 200 |
0.9—1.1 |
|
Type II—medium density (0.926—0.940) |
|
|
Melt index 20 |
2 |
|
Melt index l.0—1.9 |
2.3—2.4 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC
Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 10 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Polyethylenes; Molded, Extruded (Con’t) |
Type III—higher density (0.941—0.965) |
|
|
Melt index 0.2—0.9 |
4.4 |
|
Melt Melt index 0.l—12.0 |
2.9—4.0 |
|
Melt index 1.5—15 |
4.4 |
|
High molecular weight |
5.4 |
Olefin Copolymers; Molded |
EEA (ethylene ethyl acrylate) |
0.2 |
|
EVA (ethylene vinyl acetate) |
0.36 |
|
Ethylene butene |
0.35 |
Propylene—ethylene |
Propylene—ethylene |
0.4 |
|
Ionomer |
0.4 |
|
Polyallomer |
3—4.3 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 11 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Polystyrenes |
Polystyrenes; Molded |
|
|
General purpose |
5.0—10 |
|
Medium impact |
4.0—6.0 |
|
High impact |
3.3—5.1 |
|
Glass fiber -30% reinforced |
14 |
|
Styrene acrylonitrile (SAN) |
8.3—12.0 |
|
Glass fiber (30%) reinforced SAN |
18 |
Polyvinyl Chloride And Copolymers; |
Polyvinyl Chloride And Copolymers; Molded, Extruded |
D412 |
|
Nonrigid—general |
1—3.5 |
|
Nonrigid—electrical |
2—3.2 |
|
Rigid—normal impact |
5.5—8 |
|
Vinylidene chloride |
4—40 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 147. TENSILE STRENGTH OF POLYMERS
(SHEET 12 OF 12)
|
|
Tensile Strength, (ASTM D638) |
Class |
Polymer |
(103 psi) |
|
|
|
|
|
|
Silicones |
Silicones; Molded, Laminated |
(ASTM D651) |
|
Fibrous (glass) reinforced silicones |
6.5 |
|
Granular (silica) reinforced silicones |
4—6 |
|
Woven glass fabric/ silicone laminate |
30—35 |
Ureas; Molded |
Alpha—cellulose filled (ASTM Type l) |
5—10 |
|
|
|
To convert psi to MPa, multiply by 145.
Source: data compiled by J.S. Park from Charles T. Lynch, CRC Handbook of Materials Science, Vol. 3, CRC Press, Boca Raton, Florida, 1975 and Engineered Materials Handbook, Vol.2, Engineering Plastics, ASM International, Metals Park, Ohio, 1988.
©2001 CRC Press LLC

Table 148. TENSILE STRENGTH OF
FIBERGLASS REINFORCED PLASTICS
|
|
Glass |
Tensile |
|
|
|
fiber content |
strength at yield |
|
Class |
Material |
(wt%) |
(ksi) |
|
|
|
|
|
|
|
|
|
|
|
Glass fiber reinforced |
Sheet molding compound (SMC) |
15 to 30 |
8 to 20 |
|
thermosets |
||||
|
|
|
||
|
Bulk molding compound(BMC) |
15 to 35 |
4 to 10 |
|
|
Preform/mat(compression molded) |
25 to 50 |
25 to 30 |
|
|
Cold press molding–polyester |
20 to 30 |
12 to 20 |
|
|
Spray–up–polyester |
30 to 50 |
9 to 18 |
|
|
Filament wound–epoxy |
30 to 80 |
80 to 250 |
|
|
Rod stock–polyester |
40 to 80 |
60 to 180 |
|
|
Molding compound–phenolic |
5 to 25 |
7 to 17 |
|
Glass–fiber–reinforced |
Acetal |
20 to 40 |
9 to 18 |
|
thermoplastics |
||||
|
|
|
||
|
Nylon |
6 to 60 |
13 to 33 |
|
|
Polycarbonate |
20 to 40 |
12 to 25 |
|
|
Polyethylene |
10 to 40 |
6.5 to 11 |
|
|
Polypropylene |
20 to 40 |
5.5 to 10.5 |
|
|
Polystyrene |
20 to 35 |
10 to 15 |
|
|
Polysulfone |
20 to 40 |
13 to 20 |
|
|
ABS(acrylonitrile butadiene styrene) |
20 to 40 |
11 to 16 |
|
|
PVC (polyvinyl chloride) |
15 to 35 |
14 to 18 |
|
|
Polyphenylene oxide(modified) |
20 to 40 |
15 to 22 |
|
|
SAN (styrene acrylonitrile) |
20 to 40 |
13 to 18 |
|
|
Thermoplastic polyester |
20 to 35 |
14 to 19 |
|
|
|
|
|
To convert (ksi) to (MPa), multiply by 6.89
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p106, (1994).
©2001 CRC Press LLC

Table 149. TENSILE STRENGTH OF CARBON- AND GLASS-
REINFORCED ENGINEERING THERMOPLASTICS (SHEET 1 OF 2)
|
|
|
Tensile Strength |
Class |
Resin Type |
Composition |
(MPa) |
|
|
|
|
|
|
|
|
Amorphous |
Acrylonitrile-butadiene-styrene(ABS) |
30% glass fiber |
100 |
|
|
30% carbon fiber |
130 |
|
Nylon |
30% glass fiber |
148 |
|
|
30% carbon fiber |
207 |
|
Polycarbonate |
30% glass fiber |
128 |
|
|
30% carbon fiber |
165 |
|
Polyetherimide |
30% glass fiber |
197 |
|
|
30% carbon fiber |
234 |
|
Polyphenylene oxide (PPO) |
30% glass fiber |
145 |
|
|
30% carbon fiber |
159 |
|
Polysulfone |
30% glass fiber |
124 |
|
|
30% carbon fiber |
159 |
|
Styrene-maleic-anhydride (SMA) |
30% glass fiber |
103 |
|
Thermoplastic polyurethane |
30% glass fiber |
57 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p111–112, (1994).
©2001 CRC Press LLC

Table 149. TENSILE STRENGTH OF CARBON- AND GLASS-
REINFORCED ENGINEERING THERMOPLASTICS (SHEET 2 OF 2)
|
|
|
Tensile Strength |
Class |
Resin Type |
Composition |
(MPa) |
|
|
|
|
|
|
|
|
Crystalline |
Acetal |
30% glass fiber |
134 |
|
|
20% carbon fiber |
81 |
|
Nylon 66 |
30% glass fiber |
179 |
|
|
30% carbon fiber |
241 |
|
Polybutylene telphthalate (PBT) |
30% glass fiber |
134 |
|
|
30% carbon fiber |
152 |
|
Polythylene terephthalate (PET) |
30% glass fiber |
159 |
|
Polyphenylene sulfide (PPS) |
30% glass fiber |
138 |
|
|
30% carbon fiber |
186 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p111–112, (1994).
©2001 CRC Press LLC
Table 150. STRENGTH OF
GRAPHITE FIBER REINFORCED METALS
|
Fiber content |
Strength |
Composite |
(vol%) |
(ksi) |
|
|
|
|
|
|
Graphite(a)/lead |
41 |
104 |
Graphite(b)/lead |
35 |
72 |
Graphite(a)/zinc |
35 |
110.9 |
Graphite(a)/magnesium |
42 |
65 |
|
|
|
(a) Thornel 75 fiber (b) Courtaulds HM fiber
To convert psi to MPa, multiply by 145.
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p148,(1994).
©2001 CRC Press LLC
Table 151. TENSILE STRENGTH OF
GRAPHITE/MAGNESIUM CASTINGS*
|
|
|
|
|
Tensile |
Tensile |
|
|
|
|
Fiber Preform |
Strength |
Strength,90° |
Fiber Type |
Fiber content |
Fiber orientation |
Casting |
Method |
(GPa) |
(GPa) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P75 |
40% |
±16° |
Hollow cylinder |
Filament wound |
0.45 |
0.061 |
|
plus 9% |
90° |
Hollow cylinder |
Filament wound |
0.45 |
0.061 |
P100 |
40% |
± 16° |
Hollow cylinder |
Filament wound |
0.56 |
0.38 |
P55 |
40% |
0° |
Plate |
Prepreg |
0.48 |
0.02 |
|
30% |
0° plus |
Plate |
Prepreg |
0.28 |
0.010 |
|
10% |
90° |
Plate |
Prepreg |
0.28 |
0.010 |
|
20% |
0° plus |
Plate |
Prepreg |
8.45 |
0.24 |
|
20% |
90° |
Plate |
Prepreg |
8.45 |
0.24 |
|
|
|
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p148,(1994).
*Pitch-base fibers
©2001 CRC Press LLC
Table 152. TENSILE STRENGTH OF
GRAPHITE/ALUMINUM COMPOSITES
|
Fiber loading |
Wire diameter |
Tensile Strength |
Composite |
(vol %) |
(mm) |
(MPa) |
|
|
|
|
|
|
|
|
VS0054/201 Al |
48 to 52 |
0.64 (2-strand) |
1035 to 1070 |
GY70SE/201 Al |
37 to 38 |
0.71(8-strand) |
793 to 827 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p148,(1994).
Table 153. TENSILE STRENGTH OF
GRAPHITE/ALUMINUM COMPOSITES
|
Longitudinal |
Transverse |
|
Tensile Strength |
Tensile Strength |
Thornel Fiber |
(MPa) |
(MPa) |
|
|
|
|
|
|
P55 |
517 to 621 |
28 to 48 |
P75 |
621 to 724 |
28 to 48 |
P100 |
552 to 834 |
28 to ~48 |
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p148,(1994).
©2001 CRC Press LLC

Table 154. TENSILE STRENGTH OF
SILICON CARBIDE SCS–2–AL
|
|
Tensile Strength |
Fiber orientation |
No. of plies |
(MPa) |
|
|
|
|
|
|
0° |
6, 8, 12 |
1462 |
90° |
6, 12,40 |
86.2 |
[0°/90°/0°/90°]s |
8 |
673 |
[02 °90°20°]s |
8 |
1144 |
[902/0°/90°]s |
8 |
341.3 |
± 45° |
8, 12, 40 |
309.5 |
[0°±45°/0°]s+2s |
8, 16 |
800.0 |
[0°±45°/90°]s |
8 |
572.3 |
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p149,(1994).
Table 155. ULTIMATE TENSILE STRENGTH OF INVESTMENT
CAST SILICON CARBIDE SCS–AL
|
|
Ultimate Tensile |
|
|
|
Strength |
Range of Measurement |
Fiber orientation |
Fiber vol (%) |
(MPa) |
(%) |
|
|
|
|
|
|
|
|
0°3/90°6/0°3 |
33 |
458.5 |
75 |
90°3/0°6/90°3 |
33 |
584.0 |
95 |
0° |
34 |
1034.2 |
85 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p149,(1994).
©2001 CRC Press LLC
Table 156. ULTIMATE TENSILE STRENGTH OF SILICON
CARBIDE–ALUMINUM ALLOY COMPOSITES *
|
|
Ultimate Tensile Strength |
|
|
|
|
(MPa) |
|
|
|
|
Material |
Fiber (vol %) |
Base |
Reinforced |
|
|
|
|
|
|
|
|
Pure Aluminum |
11 |
59 |
235 |
6061–T6 |
16 |
300 |
441 |
2024–T4 |
20 |
470 |
565 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p149,(1994).
*Room Temperature
Table 157. TENSILE STRENGTH OF
SIC-WHISKER–REINFORCED ALUMINUM ALLOY
|
|
Tensile Strength |
|
Fiber |
|
|
|
|
|
|
|
Content |
|
Standard |
Range of |
(vol %) |
( MPa) |
Deviation |
Measurement |
|
|
|
|
|
|
|
|
0 |
297 |
1.8 |
3.5 |
12 |
359 |
33.6 |
85.6 |
16 |
374 |
8.0 |
23.0 |
20 |
383.6 |
15.2 |
38.8 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p150,(1994).
©2001 CRC Press LLC

Table 158. ULTIMATE TENSILE STRENGTH OF
ALUMINUM ALLOY REINFORCED WITH SIC WHISKERS
VS. TEMPERATURE
|
|
Ultimate Tensile Strength |
|
||
|
|
|
(MPa) |
|
|
|
|
|
|
|
|
Fiber |
|
|
|
|
|
(Vol%) |
350 °C |
|
300 °C |
|
250 °C |
|
|
|
|
|
|
|
|
|
|
|
|
Polycrystalline alumina |
|
|
|
|
|
0 |
55 |
|
70 |
|
115 |
0.05 |
63 |
|
88 |
|
134 |
0.12 |
74 |
|
— |
|
— |
0.20 |
112 |
|
155 |
|
198 |
SiC whiskers |
|
|
|
|
|
0 |
55 |
|
70 |
|
115 |
0.12 |
124 |
|
180 |
|
226 |
0.16 |
147 |
|
— |
|
— |
0.20 |
184 |
|
235 |
|
284 |
|
|
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p150,(1994).
©2001 CRC Press LLC

Table 159. ULTIMATE TENSILE STRENGTH OF
REINFORCED ALUMINUM ALLOY
VS. TEMPERATURE
|
|
|
Ultimate Tensile Strength |
|
||
|
|
|
|
(MPa) |
|
|
|
Vol |
|
|
|
|
|
|
|
|
|
|
|
|
Fiber |
% |
350°C |
|
300°C |
|
250°C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Polycrystalline alumina |
0 |
55 |
|
70 |
|
115 |
|
5 |
63 |
|
88 |
|
134 |
|
12 |
74 |
|
— |
|
— |
|
20 |
112 |
|
155 |
|
198 |
SiC whiskers |
0 |
55 |
|
70 |
|
115 |
|
12 |
124 |
|
180 |
|
226 |
|
16 |
147 |
|
— |
|
— |
|
20 |
184 |
|
235 |
|
284 |
|
|
|
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154,(1994).
©2001 CRC Press LLC
Table 160. TENSILE STRENGTH OF
POLYCRYSTALLINE –ALUMINA–REINFORCED
ALUMINUM ALLOY
|
|
Tensile Strength |
|
|
|
|
|
Fiber Content |
|
Standard |
Range of |
(vol %) |
(MPa) |
Deviation |
Measurement |
|
|
|
|
|
|
|
|
0 |
297 |
1.8 |
3.5 |
5 |
282 |
6.5 |
15.1 |
12 |
273 |
19.6 |
49.6 |
20 |
312 |
16.0 |
42.3 |
|
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p154,(1994).
Table 161. TENSILE STRENGTH OF
BORON/ALUMINUM COMPOSITES*
|
|
Tensile Strength |
Matrix |
Fiber Orientation |
( MPa) |
|
|
|
|
|
|
Al-6061 |
0° |
1515 |
|
90° |
138 |
Al-2024 |
0° |
1550 |
|
90° |
214 |
|
|
|
Data from ASM Engineering Materials Reference Book, Second Edition, Michael Bauccio, Ed., ASM International, Materials Park, OH, p157,(1994).
*These samples contain 48% Avco (142 µm) boron. Longitudinal tensile specimens are 152 mm by 7.9 mm by 6 ply. Transverse tensile bars are 152 mm by 12.7 mm by 6 ply.
©2001 CRC Press LLC
