
Wasserscheid P., Welton T. - Ionic Liquids in Synthesis (2002)(en)
.pdf
Ionic Liquids in Synthesis. Edited by Peter Wasserscheid, Thomas Welton Copyright © 2002 Wiley-VCH Verlag GmbH & Co. KGaA
ISBNs: 3-527-30515-7 (Hardback); 3-527-60070-1 (Electronic) XI
Preface
“We prided ourselves that the science we were doing could not, in any conceivable circumstances, have any practical use. The more firmly one could make that claim, the more superior one felt.”
The Two Cultures, C.P. Snow (1959)
A book about ionic liquids? Over three hundred pages? Why? Who needs it? Why bother? These aren’t simply rhetorical questions, but important ones of a nature that must be addressed whenever considering the publication of any new book. In the case of this one, as two other books about ionic liquids will appear in 2002, the additional question of differentiation arises – how is this distinctive from the other two? All are multi-author works, and some of the authors have contributed to all three books.
Taking the last question first, the answer is straightforward but important. The other two volumes are conference proceedings (one of a NATO Advanced Research Workshop, the other of an ACS Symposium) presenting cutting-edge snapshots of the state-of-the-art for experts; this book is structured. Peter Wasserscheid and Tom Welton have planned an integrated approach to ionic liquids; it is detailed and comprehensive. This is a book designed to take the reader from little or no knowledge of ionic liquids to an understanding reflecting our best current knowledge. It is a teaching volume, admirable for use in undergraduate and postgraduate courses, or for private learning. But it is not a dry didactic text - it is a user’s manual! Having established a historical context (with an excellent chapter by one of the fathers of ionic liquids), the volume describes the synthesis and purification of ionic liquids (the latter being crucially important), and the nature of ionic liquids and their physical properties. Central to this tome (both literally and metaphorically) is the use of ionic liquids for organic synthesis, and especially green organic synthesis, and this chapter is (appropriately) the largest, and the raison d’être for the work. The book concludes with much shorter chapters on the synthesis of inorganic materials and polymers, the study of enzyme reactions, and an overview and prospect for the area. This plan logically and completely covers the whole of our current knowledge of ionic liquids, in a manner designed to enable the tyro reader to feel confident in using them, and the expert to add to their understanding. This is the first book to
XII Preface
attempt this task, and it is remarkably successful for two reasons. Firstly, the volume has been strongly and wisely directed, and is unified despite being a multiauthor work. Secondly, the choice of authors was inspired; each one writes with authority and clarity within a strong framework. So, yes, this book is more than justified, it is a crucial and timely addition to the literature. Moreover, it is written and edited by the key people in the field.
Are ionic liquids really green? A weakly argued letter from Albrecht Salzer in Chemical and Engineering News (2002, 80 [April 29], 4-6) has raised this nevertheless valid question. Robin Rogers gave a tactful, and lucid, response, and I quote directly from this: “Salzer has not fully realized the magnitude of the number of potential ionic liquid solvents. I am sure, for example, that we can design a very toxic ionic liquid solvent. However, by letting the principles of green chemistry drive this research field, we can ensure that the ionic liquids and ionic liquid processes developed are in fact green. [. . .] The expectation that real benefits in technology will arise from ionic liquid research and the development of new processes is high, but there is a need for further work to demonstrate the credibility of ionic liquid-based processes as viable green technology. In particular, comprehensive toxicity studies, physical and chemical property collation and dissemination, and realistic comparisons to traditional systems are needed. It is clear that while the new chemistry being developed in ionic liquids is exciting, many are losing sight of the green goals and falling back on old habits in synthetic chemistry. Whereas it is true that incremental improvement is good, it is hoped that by focusing on a green agenda, new technologies can be developed that truly are not only better technologically, but are cleaner, cheaper, and safer as well.”
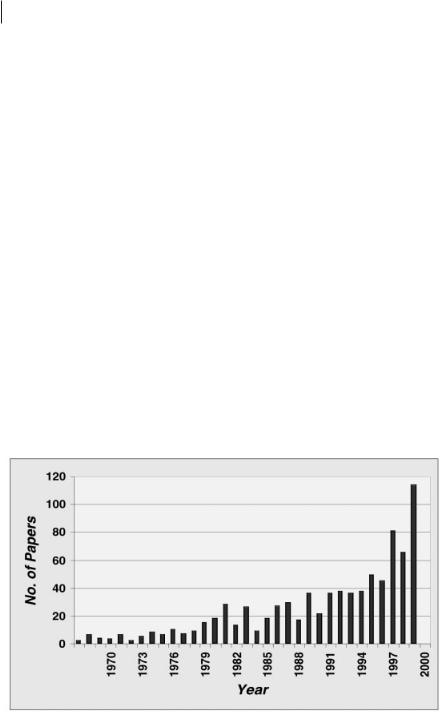
Figure 1: The rise in publications concerning ionic liquids as a function of time, as determined using SciFinder.

Preface XIII
Robin’s response is insightful. It reflects, in part, the burgeoning growth of papers in this area (see Figure 1) combined with the inevitable (and welcomed) rise in new researchers entering the area. However, with increasing activity comes the inevitable increasing “garbage” factor. In recent years we have (unfortunately) seen papers reporting physical data on ionic liquids that were demonstrably impure, liquids reported as solids and solids reported as liquids because of the impurity level, communications “rediscovering” and publishing work (without citation) already published in the patent literature, the synthesis of water-sensitive ionic liquids under conditions that inevitably result in hydrolysis, and academically weak publications appearing in commercial journals with lax refereeing standards. I truly believe that this book will help combat this; it should, and will, be referred to by all workers in the field. Indeed, if the authors citing it actually read it too, then the garbage factor should become insignificantly small!
In conclusion, this volume reflects well the excitement and rapid progress in the field of ionic liquids, whilst effectively providing an invaluable hands-on instruction manual. The lacunae are emphasised, and the directions for potential future research are clearly signposted. Unlike Snow in his renowned Two Cultures essay, many of us (Mamantov, Osteryoung, Wilkes, and Hussey, to name but a few of the founding fathers) who entered this area in its early (but not earliest!!) days prided ourselves that the science we were doing could not fail to have a practical use. Whether that use was battery applications, fuel cells, electroplating, nuclear reprocessing, or green industrial synthesis, we all believed that ionic liquids (or roomtemperature molten salts, as they were then commonly known) offered a unique chemical environment that would (must) have significant industrial application. Because of this, we suffered then (and to some extent now) from the disdain of the “pure” scientists, who failed (and still fail) to appreciate that, if selecting an example to study to illustrate a fundamental scientific principle, there is actually some merit in selecting a product manufactured at the one million ton per annum level, rather than an esoteric molecule of no use and even less interest. Unfortunately, the pride and superiority Snow refers to is still alive and well, and living in the hearts of some of the academic establishment. I believe that this book will help tackle this prejudice, and illustrate that useful practical applications and groundbreaking fundamental science are not different, opposing areas, but synergistic sides of the same coin.
K.R. Seddon
May, 2002
|
Ionic Liquids in Synthesis. Edited by Peter Wasserscheid, Thomas Welton |
|
Copyright © 2002 Wiley-VCH Verlag GmbH & Co. KGaA |
XIV |
ISBNs: 3-527-30515-7 (Hardback); 3-527-60070-1 (Electronic) |
|
|
A note from the editors
This book has been arranged in several chapters that have been prepared by different authors, and the reader can expect to find changes in style and emphasis as they go through it. We hope that, in choosing authors who are at the forefront of their particular specialism, this variety is a strength of the book. The book is intended to be didactic, with examples from the literature used to illustrate and explain. Therefore, not all chapters will give a comprehensive coverage of the literature in the area. Indeed, with the explosion of interest in some applications of ionic liquids comprehensive coverage of the literature would not be possible in a book of this length. Finally, there is a point when one has to stop and for us that was the end of 2001. We hope that no offence is caused to anyone whose work has not been included. None is intended.
Acknowledgements
We would like to sincerely thank everyone who has been involved in the publication of this book. All of our authors have done a great job in preparing their chapters and it has been a pleasure to read their contributions as they have come in to us. When embarking on this project we were both regaled with stories of books that never saw the light of day because of missed deadlines and the general tardiness of contributors. All of our colleagues have met their commitments in the most timely and enthusiastic manner. We are truly grateful for them making our task so painless. We would also like to thank the production team at VCH-Wiley, particularly Dr. Karen Kriese.
Finally, in a project like this, someone must take responsibility for any errors that have crept in. Ultimately we are the editors and this responsibility is ours. So we apologise unreservedly for any mistakes that have found their way into the book.
P. Wasserscheid, T. Welton
August, 2002

Ionic Liquids in Synthesis. Edited by Peter Wasserscheid, Thomas Welton Copyright © 2002 Wiley-VCH Verlag GmbH & Co. KGaA
ISBNs: 3-527-30515-7 (Hardback); 3-527-60070-1 (Electronic) XV
Contributors
Prof. Dr. Joan F. Brennecke |
Dr. Charles M. Gordon |
Department of Chemical Engineering |
University of Strathclyde |
University of Notre Dame |
Department of Pure and |
Notre Dame, IN 46556 |
Applied Chemistry |
|
Thomas Graham Building |
Prof. James H. Davis, jr. |
295 Cathedral Street |
Department of Chemistry |
Glasgow G1 1XL |
University of South Alabama |
Scotland, UK |
Mobile, Alabama 36688 |
|
USA |
Prof. Dr. David M. Haddleton |
|
University of Warwick |
Dr. Martyn J. Earle |
Department of Chemistry |
School of Chemistry |
Coventry CV4 7AC |
The Queen’s University of Belfast |
U.K. |
Stranmills Road |
|
Belfast BT9 5AG, Northern Ireland |
Dr. Chris Hardacre |
|
Physical Chemistry |
Dr. Frank Endres |
School of Chemistry |
Institut für Physikalische Chemie |
The Queen’s University of Belfast |
Universität Karlsruhe |
Stranmillis Road |
D-76128 Karlsruhe |
BELFAST BT9 5AG |
|
Northern Ireland |
PD Dr. Andreas Dölle |
|
Institut für Physikalische Chemie |
Dr. Claus Hilgers |
RWTH Aachen |
Solvent Innovation GmbH |
D-52056 Aachen |
Alarichstraße 14-16 |
Germany |
D-0679 Köln |
|
Germany |

XVI Contributors
Dr. John Holbrey Department of Chemistry The University of Alabama Tuscaloosa, AL 35487 jholbrey@bama.ua.edu
Prof. Dr. Udo Kragl
Technische Chemie,
Universität Rostock
Fachbereich Chemie
Buchbinderstr. 9
D-18051 Rostock
Dr. Hugh C. de Long Department of Chemistry US Naval Academy
572 Holloway RD Annapolis, MD 21402-5026 USA
Dr. Hélène Olivier-Bourbigou Division cinetique a Catalyse Institut Francais du Petrole 129 Av. DeBois-Preau
92852 Rueil-Malmaison, France
Prof. Dr. Robin D. Rogers
Professor of Chemistry
Director, Center for
Green Manufacturing
Department of Chemistry
The University of Alabama
Tuscaloosa, AL 35487
Prof. K. R. Seddon
Chair of Inorganic Chemistry
School of Chemistry
The Queen’s University of Belfast
Stranmillis Road
BELFAST BT9 5AG
Northern Ireland
Dr. Paul C. Trulove
AFOSR/NL
801 North Randolph Street
Arlington, VA 22203-1977
USA
Dr. Peter Wasserscheid
Institut für Technische Chemie und Makromolekulare Chemie der RWTH Aachen
Worringer Weg 1
D-52074 Aachen Germany
Dr. Tom Welton
Department of Chemistry
Imperial College
London SW7 2AY UK
Prof. John S. Wilkes
Department of Chemistry
2355 Fairchild Drive, Suite 2N255
USAF, Colorado 80840-6230
USA
|
Ionic Liquids in Synthesis. Edited by Peter Wasserscheid, Thomas Welton |
|
Copyright © 2002 Wiley-VCH Verlag GmbH & Co. KGaA |
356 |
ISBNs: 3-527-30515-7 (Hardback); 3-527-60070-1 (Electronic) |
|
|
Index
a
ab initio method |
153 ff. |
||
acetate |
5, 64 |
|
|
acetylation |
208 |
|
|
acetylation reaction 204 ff. |
|||
acidity |
43 |
|
|
activation energy |
164 |
||
acylation |
291, 343 ff. |
||
addition reaction |
191, 193 |
||
alkanesulfonate |
99 |
||
alkylammonium |
2, 94, 96, 137 |
||
– chloride |
2, 137 |
||
– nitrate |
2, 96 |
|
|
– sulfonate 96 |
|||
– thiocyanate |
96 |
||
alkylation 9, 24 f., 29, 77, 89, 100, 185, 193, 196, 198 ff., 201 ff., 264,
270, 275, 277 |
|
|
|
1-alkylimidazole |
9, 11, 327 |
||
alkylimidazolium |
59, 111, 123, 189 |
||
1-alkylimidazolium |
9 |
||
– chloride |
9 |
|
|
– nitrate |
9 |
|
|
– tetrafluoroborate |
9 |
||
1-alkyl-3-methylimidazolium [RMIM] 9, 11 f., 16 f., 24, 43 ff., 50 ff., 73 ff., 128, 136 ff., 146 ff., 149, 328
–bis(trifluoromethylsulfonyl)imide 51, 52, 73
– bromide |
52 |
– chloride |
52 |
– halide |
11, 16 |
–hexafluorophosphate 52, 74, 146 ff., 328
– tetrachloroaluminate |
52 |
|
– tetrachloropalladate |
146 |
|
– tetrafluoroborate |
51 ff., 146, 149 |
|
– triflate 52 |
|
|
alkylphosphonium |
137 |
|
alkylpyridinium 4 |
|
|
1-alkylpyridinium |
|
9, 12, 135, 138, |
|||
145 |
|
|
|
|
|
– alkylsulphate |
|
138 |
|||
– chloride |
145 |
|
|||
allylation |
187 |
|
|
||
allylic alkylation |
252 |
||||
allylic substitution |
252 |
||||
aluminium |
|
295, 297 ff., 302, 306 ff., |
|||
309 ff., 312 |
|
|
|||
aluminium chloride |
3, 12, 17, 33, |
||||
43, 47, 52, 70, 108 ff., 117 ff., 131, |
|||||
135, 143 ff., 177 ff., 180 ff., 192, |
|||||
194, 196 ff., 201 ff., 210, 215, |
|||||
224 ff., 228, 245 ff., 266, 273, |
|||||
275 ff., 278, 290 ff., 299, 306 ff., |
|||||
309 ff., 319 ff., 322 ff., 327 ff., 331 f. |
|||||
amidation |
|
340 |
|
|
|
amine |
36 |
|
|
|
|
1-(3-aminopropyl)imidazole 35 ff. |
|||||
ammonium |
34 ff., 39, 42, 46, 100, |
||||
107, 136, 200 |
|
|
|||
– halide |
|
100 |
|
|
|
anion exchange |
8 |
|
|||
antimony |
298, 300 ff., 304 ff. |
||||
Arrhenius equation |
164, 170 |
||||
arsenic |
298, 304 |
|
|
||
aza-Diels-Alder reaction 183 |
|||||
b
battery |
2, 5, 45, 70, 103, 298, 350, |
|||
353 |
|
|
|
|
Beckmann rearrangement |
189 |
|||
Beer-Lambert relationship |
140 |
|||
benzensulfonate |
240 |
|
||
benzimidazolium |
138 |
|
||
benzoylation |
208 |
|
|
|
Berson’s W scale |
100 |
|
||
Biginelli reaction |
190 |
|
||
binary mixture |
47 |
|
||

Index 357
biocatalysis |
336 |
ff., 339, 342, 345, |
97, 99 f., 113, 144 ff., 149, 154, |
353 |
|
|
163 ff., 166 f., 171 ff., 181, 185 ff., |
biocatalyst |
338, 343, 346 |
190 ff., 194, 201, 215, 218, 231 ff., |
|
biotransformation |
336 ff., 340, 345 |
235 ff., 242, 249 ff., 261 ff., 264, |
|
biphasic reaction |
190 |
266, 270 ff., 281 ff., 304, 312 ff., |
|
biphasic system |
69 ff., 72, 77 |
325 ff., 329, 331 ff., 337, 339 ff., |
|
bis(trifluoromethylsulfonyl)imide |
343 ff. |
|
|
|
|
||
15, 30, 33, 35, 44, 47, 53, 64, 66, 76, |
– hydrogensulfate |
201 |
|
||||
107, 114, 137, 221 |
|
– lactate |
183 |
|
|
|
|
Born-Landé equation |
45 |
– methylsulphate |
340 |
|
|||
Bragg’s law |
134 |
|
– nitrate |
18, 88, 261 |
|
||
bromide 5, 104, 107, 137, 242 |
– octylsulfate |
216 |
|
|
|||
bromoaluminate 66, 131 |
– perchlorate |
97 |
|
|
|||
Brønsted acid |
191, 196 |
– tetracarbonylcobaltate |
225 |
||||
Brønsted superacid |
18 |
– tetrachloropalladate |
146 |
||||
buffered ionic liquid |
109 |
– tetrafluoroborate |
16, 65 ff., |
||||
butylation |
198 |
|
|
89 ff., 97, 104, 106, 149, 187 ff., |
||||||
1-butyl-3-ethyl-imidazolium [BEIM] |
190 ff., 194 ff., 215, 223, 225, 229, |
|||||||||
61, 64, 66, 113 |
|
|
231, 234, 242 ff., 251 ff., 261 ff., |
|||||||
– bis(trifluoromethylsulfonyl)imide |
266, 270 ff., 282, 291, 337, 340, |
|||||||||
61, 113 |
|
|
|
|
342 ff. |
|
|
|
|
|
– mesylate |
61, 113 |
|
– triflate |
61, 97, 113, 195, 251, |
||||||
– triflate |
|
61, 113 |
|
261, 271 |
|
|
|
|
||
– trifluoroacetate |
61, 113 |
– trifluoroacetate |
61, 113, 261, |
|||||||
1-butylimidazole 35 |
|
343 ff. |
|
|
|
|
||||
1-butyl-3-methylimidazolium [BMIM] |
1-butyl-4-methylpyridinium |
275 |
||||||||
16, 18 ff., 25 ff., 29, 37, 53, 59, 61 f., |
– chloride |
275 |
|
|
|
|||||
64 ff., 73 ff., 77 ff., 84, 86 ff., 96 ff., |
1-butylpyridinium [BP] |
12, 41, 62 f., |
||||||||
99 f., 104, 106, 113, 115, 144 ff., |
88, 106, 108, 115 f., 120, 122, 135, |
|||||||||
149, 154, 163 ff., 166 f., 171 ff., 181, |
145, 181, 189, 324, 331 ff. |
|
||||||||
183, 185 ff., 190 ff., 194 ff., 201 ff., |
– bis(trifluoromethylsulfonyl)imide |
|||||||||
207 ff., 215 ff., 218, 223, 225 ff., |
63, 116, 120, 122 |
|
|
|
||||||
229 ff., 234 ff., 242 ff., 245 ff., |
– chloride |
108, 135, 181, 331 |
||||||||
249 ff., 261 ff., 264, 266, 268, |
– chloroaluminate |
62, 115 |
||||||||
270 ff., 273, 277, 281 ff., 290 ff., |
– hexafluorophosphate |
145 |
||||||||
304, 306 ff., 309 ff., 312 ff., |
– tetrafluoroborate |
63, 88, 106, |
||||||||
319, 325 ff., 329, 331 ff., 337, |
116, 120, 122, 145, 189, 324, 332 |
|||||||||
339 ff. |
|
|
|
|
butyltriphenylphosphonium |
240 |
||||
– bis(trifluoromethylsulfonyl)imide |
– tosylate |
240 |
|
|
|
|||||
25, 27, 61, 77, 97, 106, 113, 261, |
|
|
|
|
|
|
||||
270, 337, 339, 343 ff. |
|
c |
|
|
|
|
|
|||
– bromide |
223, 242, 268, 290 |
cadmium |
301 |
|
|
|
||||
– chloride |
|
18, 29, 77, 97, 201 ff., |
capacitor |
103 |
|
|
|
|||
215, 225 ff., 245 ff., 273, 277, |
carbene |
34, 145, 223 ff., 242, 267 ff., |
||||||||
292 ff., 306 ff., 309 ff., 319 |
290, 329 |
|
|
|
|
|||||
– chloroaluminate 62, 115 |
carbonylation |
252 |
|
|
|
|||||
– chloroferrate |
207 ff. |
|
Carius tube |
10 |
|
|
|
|||
– chlorostannate |
234 |
|
catalysis |
25 f., 39, 145, 174, 210, |
||||||
– chlorozincate |
264 |
|
213 ff., 218 ff., 221 ff., 225, 230, |
|||||||
– halide |
261 |
|
|
234, 244, 253, 258 ff., 263, 266 ff., |
||||||
– hexafluoroantimonate |
195, 201, |
269 ff., 288, 293, 302, 327, 338, 353 |
||||||||
229 ff., 261, 343 |
|
– biphasic |
71, 214, 218 ff., 230, |
|||||||
– hexafluorophosphate |
19, 26, 37, |
234, 244, 258 ff., 266 ff., 269 ff., |
||||||||
53, 59, 61, 73 ff., 77 ff., 84, 86 ff., |
293 |
|
|
|
|
|
||||
358 |
Index |
|
|
– heterogeneous |
218 ff., 222, 253, |
|
||
258, 288 |
|
|
|
– homogeneous |
70, 213 ff., |
|
218 ff., 222, 253, 258 ff., 263, 288, |
|
353 |
|
|
|
– multiphasic 220, 259 ff., 263 |
|
|
– phase-transfer |
258 |
–transition metal, see transition metal catalysis
catalyst |
33, 69 ff., 77 ff., 193, 201 ff., |
|||
207, 214, 218, 222, 225 ff., 229 ff., |
||||
234 ff., 241, 243 ff., 246, 248, |
||||
250 ff., 258 ff., 263, 265 ff., 269 ff., |
||||
275 ff., 278, 281, 283 ff., 287, 300, |
||||
319, 326, 328 ff., 332, 336, 338, 348, |
||||
352 f. |
|
|
|
|
– activity |
319 |
|
||
– complex |
213 ff., 218 ff. |
|||
– concentration |
271, 281 |
|||
– consumption |
241, 272, 281 |
|||
– deactivation |
244 |
|||
– decomposition |
72, 283 |
|||
– heterogeneous |
338 |
|||
– layer |
218, 243, 251 f. |
|||
– leaching |
70 ff., 235 |
|||
– lifetime |
220, 235, 250 |
|||
– phase 219, 229, 252, 259 ff., |
||||
269, 287 |
|
|
|
|
– poison |
270 |
|
||
– precursor |
225, 285 |
|||
– recovery |
277 f. |
|||
– recycling |
70, 234, 243, 248, 260, |
|||
276, 278, 284, 328f. |
||||
– removal |
329 |
|
||
– separation |
234, 248, 258, 263, |
|||
276, 281 |
|
|
|
|
– solubility |
69, 213, 221 |
|||
– solution |
237, 251, 283 |
|||
– solvent |
219, 252, 266, 272 |
|||
– stability |
336 |
|
||
– structure |
319 |
|
||
– support |
78 |
|
||
– system |
77, 244 |
|||
C-C-coupling 217 |
|
|||
cerium |
297, 300 |
|
||
charge density |
87 |
|
||
chemical stability |
43 |
|||
chloride |
44, 46, 59, 69 ff., 84, 99, |
|||
104, 107, 109, 120 ff., 131, 137, 142 ff., 159 ff., 185, 224, 227, 278, 291, 299, 332
chlorination 192 ff. chloroaluminate 3 ff., 18, 22 f., 27,
33 ff., 41, 43, 64 ff., 104 ff., 107 ff.,
120 ff., 131, 144, 156, 174 ff., 177,
179 ff., 191 ff., 198, 200 f., 203, 205,
207 ff., 214 ff., 221, 225 ff., 245 ff., 266, 270, 275 f., 278, 289, 292, 297 ff., 309, 320 ff., 326 ff., 332 f.
chloroferrate |
225 |
||
chlorogallate |
|
198, 200 |
|
chlorostannate |
207, 221, 227 |
||
chromium |
246, 300 |
||
Claisen rearrangement 191, 194 |
|||
cluster |
145 |
|
|
coal 193 |
|
|
|
cobalt |
214, 230, 235 ff., 299, 302, |
||
338 |
|
|
|
cobaltocinium |
235, 267 |
||
co-catalyst |
221, 352 |
||
co-dimerization 251, 275, 285 |
|||
color |
17, 23 ff., 28 |
||
co-miscibility |
69 |
||
compressed carbondioxide |
251, 265, |
||
281, 283 ff. |
|
|
|
compressibility |
81 |
|
|
computational chemistry |
152 |
||
conductivity |
3, 59 ff., 103 ff., 109 ff., |
||
112 ff., 115 ff., 121, 123, 164, 166, |
|||
262, 295, 297, 324, 331, 348, 350, |
|||
352 |
|
|
|
coordination |
70 |
|
|
copolymerization |
326 ff. |
|
|
copper 210, 297, 299, 301 ff., 309 ff.,
329 ff. |
|
|
|
|
corrosion |
216, 294, 299, 303 |
|||
co-solvent |
64 ff., 117 ff., 218, 259, |
|||
262, 263 f., 270, 304, 336 ff., 339, |
||||
342 |
|
|
|
|
Coulombic attraction |
45 ff., 53 |
|||
Coulombic interaction |
51, 95, 98, |
|||
158 |
|
|
|
|
cracking |
208 ff. |
|
|
|
crown ether |
73 |
|
|
|
crystallization |
164 |
|
||
crystal structure 75 |
|
|||
cyclic voltammetry |
104, 296 ff., |
|||
306 ff., 309 ff., 313 |
|
|||
cyclization reaction |
175, 178 ff., 194, |
|||
196 f., 203 ff., 328 |
|
|||
cycloaddition |
35, 100 |
|
||
cyclodehydration |
196, 198 |
|||
cyclodimerization |
251 |
|||
cyclovoltammetry |
166 |
|
||
d
deactivation 220, 230, 235, 243, 264, 286, 338

Index 359
dealkylation |
193, 223 |
||||
dealkylation reaction |
175 |
||||
debromination |
|
225 |
|||
decomposition |
|
261 |
|||
decomposition temperature 44 |
|||||
dehydrogenase |
|
342 |
|||
denaturation |
|
339 |
|
||
density |
3, 18, 49, 52, 56 ff., 60 ff., |
||||
65 ff., 81, 112 ff., 115 ff., 164, 174, |
|||||
272 |
|
|
|
|
|
Density Functional Theory (DFT) |
|||||
153 ff. |
|
|
|
|
|
deoxochlorination 290 |
|||||
detergent |
320 |
|
|
||
deuteration |
128, 191, 226 |
||||
diacetylation |
|
205 |
|
||
dialkylammonium |
96 ff. |
||||
– thiocyanate |
97 |
|
|||
dialkylimidazolium |
5, 30, 269, 272 |
||||
– chloroaluminate |
272 |
||||
1,3-dialkylimidazolium 189, 261 |
|||||
diastereoselectivity |
187 |
||||
dibutylammonium |
324 |
||||
– chloride |
|
324 |
|
||
dielectric constant |
76, 94 |
||||
Diels-Alder reaction |
100 ff., 181 ff., |
||||
319 |
|
|
|
|
|
Difasol process |
246, 274 ff., 278 |
||||
differential scanning calorimetry |
|||||
(DSC) |
43 |
|
|
|
|
1,3-dimethylimidazolium [MMIM]
60, 62, 112, 115, 133 ff., 158 ff., 224, 261, 337, 340, 342
–bis(trifluoromethylsulfonyl)imide 60, 112
– chloride |
133 ff., 160 |
||||
– chloroaluminate |
62, 115 |
||||
– hexafluorophosphate |
261 |
||||
– methylsulphate |
|
337, 340, 342 |
|||
– tetrafluoroborate |
224 |
||||
diffusion |
89, 119, 123, 160, 164, 261 |
||||
diffusion coefficient |
|
118 ff. |
|||
diffusion, mutual |
162 ff. |
||||
diffusion, translational |
162 |
||||
digital image holography |
165, 167 |
||||
dimerization |
89, 179, 210, 214, 217, |
||||
244 ff., 251 ff., 266, 270, 274 ff., |
|||||
278, 319 ff. |
|
|
|
|
|
Dimersol process |
245, 270 ff., 275, |
||||
319 |
|
|
|
|
|
discoloration |
10, 13, 18 |
|
|||
disposal |
278 |
|
|
|
|
distribution of charge |
45 |
||||
dysprosium |
195 |
|
|
|
|
e
electrocatalysis |
331 |
|
|||
electrochemical analysis |
25 |
||||
electrochemical behavior |
3 |
||||
electrochemical window |
103 ff., |
||||
106 ff., 294 ff., 300, 303, 305, |
|||||
313, 349, 354 |
|
|
|
||
electrochemistry |
42, 104 f., 294, 299 |
||||
electrodeposition |
|
43, 294 ff., 297 ff., |
|||
305 ff., 309, 312, 314 ff., 350, 353 |
|||||
electrodissolution |
315 ff. |
||||
electrolysis |
294, 297 |
|
|||
electrolyte |
|
42, 45, 68, 70, 103, 118, |
|||
121, 324, 349 f. |
|
|
|||
electrooxidation |
315 |
|
|||
electrophilicity |
222, 227, 244, 249, |
||||
266 |
|
|
|
|
|
electrophilic substitution |
191 |
||||
electroplating 70 |
|
|
|||
electroreduction |
298, 306 |
||||
electrosynthesis |
68 |
|
|||
electrowinning |
70 |
|
|||
enantioselectivity |
|
182, 230 ff., 270, |
|||
285, 287, 342, 344 ff. |
|
||||
enthalpy of absorption |
87 |
||||
enthalpy of adsorption |
89 |
||||
enthalpy of melting |
44 |
|
|||
enzyme |
337 ff. |
|
|
|
|
epoxidation |
233, 319 |
|
|||
esterase |
344 |
|
|
|
|
esterification reaction 181, 344 |
|||||
ethylammonium |
9, 182, 339 ff. |
||||
– nitrate |
9, 182, 339 ff. |
||||
ethyldiisopropylammonium 192 |
|||||
– trifluoroacetate |
192 |
||||
ethylimidazolium |
158 |
|
|||
1-ethyl-3-methylimidazolium [EMIM] 5, 10, 13 ff., 18, 25 ff., 41, 43, 46 ff., 52, 60, 62, 64 ff., 70, 77, 79, 88,
99 f., 105 ff., 111 f., 115, 117 ff.,
120 ff., 133, 143 ff., 147, 180 ff.,
188 ff., 191 ff., 198 ff., 201, 205 ff., 210, 215 ff., 224, 228, 233, 278, 285 ff., 290 ff., 298, 301, 304,
320 ff., 324, 327 ff., 332, 340, 343
– acetate 18, 60, 106, 112
–bis(trifluoromethylsulfonyl)imide 60, 99, 106, 111 f., 120, 122, 216, 285 ff., 292, 340, 343
– bromide |
100 |
– bromoaluminate 62, 115 |
|
– chloride |
10, 13 ff., 17 f., 25, 43, |
47 ff., 64 ff., 70, 77, 105, 108,
117 ff., 133, 143 ff., 180 ff.,
