
- •Preface
- •Introduction
- •Stereochemical Requirements for Cannabimimetic Activity
- •Molecular Determinants for Cannabinoid Activity: Refinement of a Molecular Reactivity Template
- •Interactions of Cannabinoids With Membranes. The Role of Cannabinoid Stereochemistry and Absolute Configuration and the Orientation of Delta-9-THC in the Membrane Bilayer
- •The High Affinity Cannabinoid Binding Site in Brain: Regulation by Guanine Nucleotides and Isolation of an Endogenous Inhibitor
- •Regulation of Adenylate Cyclase in a Cultured Neuronal Cell Line by Marijuana Constituents, Metabolites of Delta-9-tetrahydrocannabinol, and Synthetic Analogs Having Psychoactivity
- •Inhibitory and Stimulatory Effects of Cannabinoids on Eicosanoid Synthesis
Regulation of Adenylate Cyclase in a Cultured Neuronal Cell Line by Marijuana Constituents, Metabolites of Delta-9-tetrahydrocannabinol, and Synthetic Analogs Having Psychoactivity
Allyn C. Howlett, Ph.D.
The psychoactive properties of cannabinoid drugs have been described in man and investigated in a variety of animal models. However, in vitro studies have failed to elucidate the mechanism of cannabinoid action at the neuronal level. The activities of a number of enzymes have been reported to be modified by cannabinoid compounds (Martin 1986). However, in many
studies, problems arise in correlating these |
cellular events with changes in |
|||||
animal |
behavior. |
This |
may be |
because the |
drug concentrations required for |
|
alterations in |
enzyme |
activity |
are greater than would be expected at the |
|||
site of |
action |
in |
the brain. In |
other studies, |
cannabinoid drugs having little |
|
or no psychoactivity produce a cellular response similar to that of
psychoactive |
cannabinoid drugs. These |
difficulties in defining a mechanism |
of action for |
cannabimimetic drugs have |
been discussed by Martin (1986) in |
a recent review on the cellular effects of cannabinoid drugs. Studies in my laboratory have determined that cannabimimetic drugs inhibit cyclic AMP accumulation in neuronal cells (Howlett 1984; Howlett and Fleming 1984). These studies will be described in this chapter. It is proposed that the cannabimimetic inhibition of adenylate cyclase is a receptor-mediated cellular response that may be one mechanism of action for these drugs.
A neuroblastoma cell culture system is used for these studies as a model for neurons. The N18TG2 cell line was cloned from the cultured C1300 tumor (Augusti-Tocco and Sato 1969; Gilman and Minna 1973; Schubert et al. 1969). This tumor arose spontaneously in the region of the spinal cord of the A/J mouse (Augusti-Tocco and Sato 1969) and, therefore, may have been derived from sympathetic ganglial cells. N18TG2 cells have retained a typical neuronal morphology in culture (Augusti-Tocco and Sato 1969; Schubert et al. 1969), and they exhibit electrophysiological responses to neurotransmitters such as acetylcholine (Chalazonitis et al. 1977). These cells also synthesize the neuromodulator, vasoactive intestinal peptide (VIP) (Brick et al. 1985). The advantage of using cultured neuroblastoma cells rather than brain cells for biochemical studies of adenylate cyclase is that plasma membranes may be isolated from large quantities of cells that are genetically and phenotypically identical.
A number of neuromodulators interact with receptors that regulate the synthesis of cyclic AMP in neuronal cells. Cyclic AMP diffuses into the neuron as the "second messenger" that activates cyclic AMP-dependent
148

protein kinase. Protein kinase modifies the activity of key enzymes involved in such functions as neurotransmitter synthesis or membrane excitability (Bloom 1975; Daly 1977; Greengard 1976). The regulation of adenylate cydase by neuromodulator receptors is complex and has been reviewed by Gilman (1984). According to our current understanding, stimulatory receptors interact with a regulatory protein complex, Gs, and inhibitory receptors interact with a homologous complex, Gi (figure 1). Both
regulatory proteins are heterotrimers comprising |
, , |
and |
subunits. |
|||||||
Although |
the |
and |
subunits |
of Gs and Gi |
are |
thought to |
be nearly |
|||
identical, |
the |
a subunits differ structurally and functionally. Stimulatory |
||||||||
and |
inhibitory |
neuromodulator-receptor interactions promote the binding of |
||||||||
GTP |
onto |
the |
and |
subunits, respectively. This |
causes |
dissociation |
of |
|||
t h e |
or |
f r o m t h e |
a n d |
p r o t e i n s . T h e |
subunit |
is then free |
to |
|||
activate the catalytic protein of adenylate cyclase. The free ai subunit may inhibit the catalytic protein directly. However, inhibition of adenylate
cyclase may also occur as the free |
proteins form a new equilibrium with |
|
proteins and thereby prevent the |
interaction of |
with the catalytic |
subunit. |
|
|
INHIBITION OF ADENYLATE CYCLASE BY CANNABINOID COMPOUNDS
My laboratory provided the first evidence that cannabimimetic drugs decreased cyclic AMP accumulation in neuronal cells (Howlett 1984). Tetrahydrocannabinol (THC) and
Tetrahydrocannabinol (THC) and  - THC decreased both basal and prostacydin- (prostaglandin I2 -) stimulated cyclic AMP accumulation in neuroblastoma cells. Prostanoid-stimulated adenylate cyclase in a membrane preparation from these cells was inhibited by cannabimimetic
- THC decreased both basal and prostacydin- (prostaglandin I2 -) stimulated cyclic AMP accumulation in neuroblastoma cells. Prostanoid-stimulated adenylate cyclase in a membrane preparation from these cells was inhibited by cannabimimetic
compounds, |
indicating that this enzyme |
complex was the target |
for the |
e f f e c t o f |
- T H C o n c e l l u l a r c y c l i c |
A M P c o n c e n t r a t i o n s . |
The |
cannabimimetic drugs caused a decrease in Vmax of the enzyme, with no alteration in the Km for substrate (Howlett 1985). Adenylate cyclase inhibition was apparent immediately upon addition of  -THC and the effects of the drug were readily reversible after the membranes were sedimented and resuspended (Howlett 1985). The inhibition was shown to be concentration-dependent over a nM range for both
-THC and the effects of the drug were readily reversible after the membranes were sedimented and resuspended (Howlett 1985). The inhibition was shown to be concentration-dependent over a nM range for both  -THC and
-THC and  -THC. The activity of cyclic nudeotide phosphodiesterase, responsible for the metabolic breakdown of cyclic AMP, was unaltered by these agents (Howlett 1984).
-THC. The activity of cyclic nudeotide phosphodiesterase, responsible for the metabolic breakdown of cyclic AMP, was unaltered by these agents (Howlett 1984).
The cannabimimetic inhibition of adenylate cyclase was not observed universally in all cell types. The response was not observed in either the soluble adenylate cyclase from rat sperm or the membrane-bound adenylate cyclase from C6 glioma or S49 lymphoma cells (Howlett et al. 1986). This cellular selectivity provides evidence for the existence of specific receptors for the cannabimimetic drugs. If these drugs were influencing enzyme activity by intercalating into lipid membranes and altering membrane fluidity, then one would have expected universal inhibition of adenylate cyclase in all cell types. This was not the case.
The inhibition of adenylate cyclase by -THC in neuroblastoma plasma membranes was not due to an effect on stimulatory receptor regulation of the enzyme (Howlett and Fleming 1984). Inhibition by
-THC in neuroblastoma plasma membranes was not due to an effect on stimulatory receptor regulation of the enzyme (Howlett and Fleming 1984). Inhibition by  -THC was not competitive with prostaglandin E1 or prostacyclin. Furthermore, noncompetitive inhibition was also observed when the peptide hormones
-THC was not competitive with prostaglandin E1 or prostacyclin. Furthermore, noncompetitive inhibition was also observed when the peptide hormones
149

FIGURE 1. Hypothetical model of the regulation of adenylate cyclase by stimulatory and inhibitory neuromodulators. Rs and Ri represent one of several pharmacologically distinct receptors for stimulatoy and inhibitory neuromodulators, respectively, and Hs and Hi indicate occupancy by their respective agonist ligands. The G-protein complexes, Gs and Gi are
denoted as their subunit constituents, |
and |
respectively. The catalytic protein is designated C. Details |
concerning the interaction of these protein |
subunits are |
found in the text. |

secretin and VIP stimulated the enzyme. Additional evidence that the THC was not acting at the level of the receptors that stimulate adenylate cyclase was provided by the observation that inhibition also occurred when forskolin was used to activate the enzyme. Forskolin is believed to act directly at the catalytic subunit (Seamon and Daly 1981).
THC was not acting at the level of the receptors that stimulate adenylate cyclase was provided by the observation that inhibition also occurred when forskolin was used to activate the enzyme. Forskolin is believed to act directly at the catalytic subunit (Seamon and Daly 1981).
The cannabimimetic inhibition of adenylate cyclase appeared to resemble the regulation resulting from inhibitory neuromodulator receptors that act via Gi (Howlett 1985). The effects of cannabimimetic drugs were related to the ability of the enzyme to be regulated by divalent cations and guanine nucleotides. The inhibition was greatest at micromolar Mg2 + or Mn2 + concentrations and was attenuated by millimolar concentrations of Mn2 +.
Half-maximal inhibition of adenylate cyclase was observeo at 800 nM |
GTP |
for both cannabimimetic and muscarinic cholinergic agents. |
This |
concentration is higher than that required for stimulation of the enzyme by hormones and is typical of the high GTP concentrations required by inhibitory hormones and neuromodulators in other systems (Cooper 1982). These observations illustrate the similarities between the enzyme inhibition by cannabimimetic drugs and by muscarinic cholinergic drugs. It is inferred that the cannabimimetic drugs must act via regulatory mechanisms similar to those operating for hormonal inhibition of adenylate cyclase.
Receptor-mediated inhibition of adenylate cyclase in most eukaryotic systems requires the presence of the guanine nucleotide-binding protein complex, Gi. Further studies confirmed that cannabimimetic inhibition of adenylate cyclase also required the presence of Gi (Howlett et al. 1986). Ci can be functionally inactivated as the result of an ADP-ribosylation modification catalyzed by pertussis toxin. Therefore, pertussis toxin can be used as a tool to identify Gi-mediated events in the cell. Pertussis toxin treatment of intact N18TG2 cells abolished the cannabimimetic regulation of cellular cyclic AMP content. The adenylate cyclase response to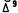 -THC was also attenuated in membranes isolated from cells that had been treated with pertussis toxin. The action of pertussis toxin for in vitro modification of neuroblastoma Gi requires NAD as substrate for the ADP-ribosylation. The toxin was able to catalyze the labeling of a neuroblastoma membrane protein in vitro using (32P)NAD under conditions similar to those by which attenuation of the cannabimimetic inhibition of adenylate cyclase could be
-THC was also attenuated in membranes isolated from cells that had been treated with pertussis toxin. The action of pertussis toxin for in vitro modification of neuroblastoma Gi requires NAD as substrate for the ADP-ribosylation. The toxin was able to catalyze the labeling of a neuroblastoma membrane protein in vitro using (32P)NAD under conditions similar to those by which attenuation of the cannabimimetic inhibition of adenylate cyclase could be
demonstrated. Protein labeling |
and the loss of the response |
to |
-THC |
|
followed the same dose-response |
curve for |
pertussis toxin. This |
strengthens |
|
the argument that cannabimimetic drugs |
act via receptors that interact |
|||
with the inhibitory GTP-binding |
protein complex. |
|
|
|
THE PHARMACOLOGY OF CANNABIMIMETIC INHIBITION OF ADENYLATE CYCLASE
Inhibition of adenylate cyclase in N18TG2 cells is known to be regulated by
muscarinic |
cholinergic, -adrenergic, and |
-opioid receptors. Thus, it was |
|
possible that |
-THC may be acting via one of these pharmacological |
||
receptor types. When this was tested, it was found that inhibition by |
|||
THC was |
neither |
synergistic nor additive |
with muscarinic cholinergic or |
a d r e n e r g i c a g e n t s w h e n e a c h w a s p r e s e n t a t m a x i m a l l y i n h i b i t o r y concentrations (Howlett and Fleming 1984). Furthermore, the response to  -THC was not blocked by the antagonists to these receptors, atropine and yohim bine, respectively.
-THC was not blocked by the antagonists to these receptors, atropine and yohim bine, respectively.
151

The opioid drugs are another pharmacological class of neuromodulatory compounds that inhibit adenylate cyclase in N18TG2 cells. Because of the interaction of cannabimimetic and opioid drugs in animal models of tolerance, the possible involvement of opioid receptors was examined (see
Devane |
et al. |
1986 |
and |
references |
contained |
therein). |
-Opioid |
receptors |
were |
found |
on neuroblastoma membranes using ( 3 H) - D - ala 2 - D - ieu 5 - |
||||||
enkephalin. |
N o µ - |
o r |
-opioid |
receptors |
were detected. The |
-opioid |
||
receptor binding affinity and capacity were unaltered by cannabimimetic
drugs. Opioid drugs as well as |
-THC and the nantradol series of |
analgesic |
|
cannabimimetic |
analogs decreased |
cyclic AMP accumulation in intact cells |
|
and inhibited |
adenylate cyclase in membrane preparations. |
Opioid |
|
antagonists blocked the response to the opioid agonists but not to the cannabimimetic drugs. The interaction between cannabimimetic drugs and the opioid agonist etorphine showed no antagonistic, synergistic, or additive effects at maximal concentrations. These results suggest that the opioid and cannabimimetic drugs operate via distinct, noninteractive receptors that can be coupled to the same effector, adenylate cyclase.
Other neuronal cell lines having a  -opioid response were also examined (Devane et al. 1986). The cannabimimetic inhibition of cyclic AMP accumulation in NC108-15 neuroblastoma x glioma hybrid cells was not as great as the response in N18TG2 cells. N4TG1 neuroblastoma cells, which exhibit a prominent inhibition of adenylate cyclase in response to opioid drugs, did not respond to cannabimimetic drugs under any conditions tested. Thus, the cannabimimetic response does not correlate with the efficacy of the
-opioid response were also examined (Devane et al. 1986). The cannabimimetic inhibition of cyclic AMP accumulation in NC108-15 neuroblastoma x glioma hybrid cells was not as great as the response in N18TG2 cells. N4TG1 neuroblastoma cells, which exhibit a prominent inhibition of adenylate cyclase in response to opioid drugs, did not respond to cannabimimetic drugs under any conditions tested. Thus, the cannabimimetic response does not correlate with the efficacy of the  -opioid response.
-opioid response.
STRUCTURE-ACTIVITY RELATIONSHIPS AMONG THE CANNABINOID DRUGS
Given that |
the |
cannabimimetic response in neuroblastoma cells is mediated |
|
by Gi and does |
not result from drug |
interaction with -opioid, muscarinic |
|
cholinergic, |
or |
-adrenergic receptors |
that inhibit adenylate cyclase, the |
hypothesis can be proposed that these drugs act via a "cannabinoid" receptor. The classical means to describe a receptor is to define its pharmacological spectrum of activity. T h i s w a s p e r f o r m e d u s i n g constituents of marijuana extracts and metabolites of these compounds
(Howlett |
1987). The concentration of |
-THC |
exhibiting |
half-maximal |
inhibition |
was less than 500 nM (figure |
2A). |
-THC was |
less active, |
cannabinol was only partially active, and cannabidiol was inactive. Other constituents of marijuana that have no psychoactivity (cannabigerol, cannabichromene, olivetol) and compounds having a reduced length of the
C3 |
alkyl side chain were not active as inhibitors of adenylate cyclase (table |
1). |
These inactive compounds did not behave as antagonists to the effects |
of |
-THC. |
The nantradol class of cannabinoid analogs were potent inhibitors of adenylate cyclase (figure 2B). Desacetyllevonantradol was more potent than nantradol. The nonpsychoactive isomer dextronantradol was a poor inhibitor of adenylate cyclase.
The |
metabolities |
of |
-THC and |
-THC hydroxylated at the C11-position |
were |
more potent |
than |
the parent drugs (table 1). However, hydroxylation |
|
at the C8-position |
of |
the terpenoid |
ring resulted in loss of activity of |
|
152

THC. Compounds hydroxylated along the C3 alkyl side chain were equally efficacious but less potent than  -THC.
-THC.
These structure-activity relationships are consistent with cannabinoid pharmacology reported for psychological effects in humans and for behavioral effects reported in a variety of animal models. Potency
estimates |
have |
suggested |
that |
the |
psychological high |
experience in humans |
is greater |
for |
- T H C |
t h a n |
f o r |
-THC (Hollister |
1974; Hollister and |
Gillespie 1973). Neither cannabinol nor cannabidiol produced responses in human subjects at the oral dosages tested (Hollister 1973, 1974). However, when infused intravenously, cannabinol (but not cannabidiol) produced a
psychological |
high |
and |
cardioacceleration at |
ten-fold the dose of |
-THC |
|||
(Hollister 1973; Perez-Reyes et |
al. |
1973a). |
11-OH-metabolites of |
-THC |
||||
a n d |
-THC |
were |
up |
to twice |
as |
active |
as the parent compounds in |
|
producing a psychological high (Hollister 1974; Lemberger et al. 1973). The ability of these compounds to inhibit adenylate cyclase parallel these responses.
FIGURE 2. Inhibition of adenylate cyclase by cannabinoid and nantradol compounds. Forskolin was present at 1.0 µM to activate adenylate cyclase. Other compounds present at the indicated concentmtfons were cannabinol (CBN), cannabidiol (CBD), levonantradol ( -Nan), dextronantradol (d-Nan), and desacetyllevonantradol (Da-
-Nan), dextronantradol (d-Nan), and desacetyllevonantradol (Da- -Nan).
-Nan).
In animal studies, -THC was less potent than
-THC was less potent than  -THC in such tests as dog ataxia (Martin et al. 1981; Wilson et al. 1976), monkey behavior (Edery and Crunfeld 1971), mouse hypothermia (Martin et al. 1981), and hot-plate analgesia (Uliss et al. 1975; Wilson and May 1975) and in genetically THCseizure prone rabbits (Consroe and Fish 1981). Cannabidiol (Consroe and Fish 1981; Edery and Crunfeld 1971; Pertwee 1972; Uliss et al. 1975), cannabinol (Edery and Grunfeld 1971), and cannabichromene (Edery and Grunfeld 1971) were inactive in a number of these models. The THC-seizure
-THC in such tests as dog ataxia (Martin et al. 1981; Wilson et al. 1976), monkey behavior (Edery and Crunfeld 1971), mouse hypothermia (Martin et al. 1981), and hot-plate analgesia (Uliss et al. 1975; Wilson and May 1975) and in genetically THCseizure prone rabbits (Consroe and Fish 1981). Cannabidiol (Consroe and Fish 1981; Edery and Crunfeld 1971; Pertwee 1972; Uliss et al. 1975), cannabinol (Edery and Grunfeld 1971), and cannabichromene (Edery and Grunfeld 1971) were inactive in a number of these models. The THC-seizure
prone rabbits responded to cannabinol at |
high doses (Consroe |
and Fish 1981). |
|
The 11-OH metabolites of |
-THC and |
-THC were more |
potent than the |
153

parent compounds in the dog ataxia (Wilson et al. 1976), mouse immobility (Gill et al. 1973), hot-plate analgesia (Wilson and May 1975), and THCseizure prone rabbit models (Consroe and Fish 1981) and in the drug discrimination studies using rats or pigeons (Jarbe and McMillan 1980). Animal studies of the hydroxylated metabolites at the 8-position are inconsistent; although in each study, 8-OH metabolites are either inactive or less potent than  -THC (Ben-Zvi et al. 1971; Wilson and May 1975; PerezReyes et al. 1973b; Jarbe and McMillan 1980). The data reported here for inhibition of adenylate cyclase are consistent with this pharmacological pattern.
-THC (Ben-Zvi et al. 1971; Wilson and May 1975; PerezReyes et al. 1973b; Jarbe and McMillan 1980). The data reported here for inhibition of adenylate cyclase are consistent with this pharmacological pattern.
TABLE 1
Pharmacological Parameters of Marijuana Constituents and Metabolites
|
Compound |
|
|
Efficacya |
Potency |
||
|
|
|
|
|
|
K inh |
|
|
|
|
|
|
|
|
|
|
|
-THC |
|
|
1.0 |
430 |
|
|
|
-THC |
|
|
0.9 |
560 |
|
|
Cannabinol |
|
0 . 5 |
1400 |
|
||
|
Cannabidiol |
|
Ob |
- - |
|
||
|
Cannabichromene |
0 |
- - |
|
|||
|
Cannabigerol |
|
0 |
- - |
|
||
|
Olivetol |
|
|
0 |
- - |
|
|
|
|
-THC |
|
0 . 6 |
290 |
|
|
|
11-OH- |
-THC |
1.0 |
100 |
|
||
|
11-OH- |
-THC |
1.0 |
260 |
|
||
|
11-OH-cannabinol |
1.0 |
320 |
|
|||
8 |
OH- |
-THC |
0 |
- - |
|
||
8 |
OH- |
-THC |
0 |
- - |
|
||
|
8 ,11-diOH- |
-THC |
0 |
- - |
|
||
|
2´-OH- |
-THC |
1.0 |
2800 |
|
||
|
3´-OH- |
-THC |
1.0 |
840 |
|
||
|
4´-OH- |
-THC |
1.0 |
1800 |
|
||
|
5´-OH- -THC |
|
1580 |
|
|||
|
Tri Nor- |
-THC- |
0 |
- - |
|
||
|
|
carboxylic |
acid |
|
|||
|
Penta Nor- |
-THC- |
0 |
- - |
|
||
|
|
carboxylic |
acid |
|
|
|
|
|
|
|
|
|
|
|
|
a Relative |
to |
-THC equal to 1. |
|
|
|
||
b An efficacy of 0 was assigned to all compounds exhibiting a lack of inhibition at concentrations up to 50 µM .
The parallels in cannabinoid structure-activity relationships exhibited by the
154

neuronal cells compared to the human and animal behaviors is intriguing. These findings suggest that certain behavioral effects of cannabimimetic d r u g s m a y b e t h e r e s u l t o f a n i n t e r a c t i o n o f t h e s e d r u g s w i t h a pharmacologically distinct receptor associated with neuronal adenylate cyclase. At least one possible mechanism of action for the cannabimimetic compounds may involve regulation of cyclic AMP synthesis in populations of neurons associated with modification of such behaviors.
REFERENCES
Augusti-Tocco, G., and Sato, G. Establishment of functional donal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci USA 64:311315, 1969.
Ben-Zvi, Z.; Mechoulam, R.; Edery, H.; and Porath, G. 6 Hydroxy-
Hydroxy-  tetrahydrocannabinol synthesis and biological activity. Science 174:951952, 1971.
tetrahydrocannabinol synthesis and biological activity. Science 174:951952, 1971.
Bloom, F. The role of cyclic nucleotides in central synaptic function. Rev Physiol Biochem Pharmacol 74:1-104, 1975.
Brick, P.L.; Howlett, A.C.; and Beinfeld, M.C. Synthesis and release of vasoactive intestinal polypeptide (VIP) by mouse neuroblastoma cells: Modulation by cyclic nucleotides and ascorbic acid. Peptides 6:10751078, 1985.
Chalazonitis, A.; Minna, J.D.; and Nirenberg, M. Expression and properties of acetylcholine receptors in several clones of mouse neuroblastoma x L cell somatic hybrids. Exp Cell Res 105:269-280, 1977.
Consroe, P., and Fish, B.S. Rabbit behavioral model of marijuana psychoactivity in humans. Med Hypothesis 7:1079-1090, 1981.
Cooper, D.M.F. Bimodal regulation of adenylate cyclase. FEBS Lett 138:157-163, 1982.
Daly, J. Cyclic Nucleotides in the Nervous System. New York: Plenum
Press, 1977. |
|
|
Devane, W.A.; Spain, J.W.; Coscia, C.J.; |
and Howlett, A.C. An assessment |
|
of the role of opioid receptors in the |
response to cannabimimetic drugs. |
|
J Neurochem 46:1929-1935, 1986. |
|
|
Edery, H., |
and Grunfeld, Y. Structural requirements for cannabinoid |
|
activity. |
Ann NY Acad Sci 191:40-53, |
1971. |
Gill, E.W.; Jones, G.; and Lawrence, D.K. Contribution of the metabolite 7-
hydroxy- |
-tetrahydrocannabinol |
toward |
the |
pharmacological activity |
|
o f |
-tetrahydrocannabinol in |
mice. |
Biochem |
Pharmacol 22:175-184, |
|
1973. |
|
|
|
|
|
Gilman, A.G. G proteins and dual control of adenylate cyclase. Cell 36:577-579, 1984.
Gilman, A.G., and Minna, J.D. Expression of genes for metabolism of cyclic adenosine 3':5'-monophosphate in somatic cells I. responses to catecholamines in parental and hybrid cells. J Biol Chem 248:6610-6617, 1973.
Greengard, P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature 260:101-108, 1976.
Hollister, L.E. Cannabidiol and cannabinol in man. Experientia 29:825-826,
1973. |
|
|
Hollister, L . E . |
S t r u c t u r e - a c t i v i t y r e l a t i o n s h i p s |
i n m a n o f c a n n a b i s |
constituents, |
and homologs and metabolites of |
-tetrahydrocannabinol. |
Pharmacology |
11:3-11, 1974. |
|
155

H o l l i s t e r , L . E . , |
and |
G i l l e s p i e , H . K . |
Delta-8- |
and |
delta-9- |
tetrahydrocannabinol. |
Comparison in man by oral and intravenous |
||||
administration. Clin Pharmacol Ther 14:353-357, 1973. |
|
|
|||
Howlett, A.C. Inhibition of |
neuroblastoma adenylate cyclase by cannabinoid |
||||
and nantradol compounds. Life Sci 35:1803-1810, 1984. |
|
|
|||
Howlett, A.C. Cannabinoid |
inhibition of adenylate cyclase. |
Biochemistry of |
|||
the response in neuroblastoma cell membranes. Mol Pharmacol 27:429436, 1985.
Howlett, A.C. Cannabinoid inhibition of adenylate cyclase: Relative activities of marihuana constituents and metabolites of marihuana. Neuropharmacology 26:507-512, 1987.
Howlett, A.C., and Fleming, R.M. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol 26:532-538, 1984.
Howlett, A.C.; Qualy, J.M.; and Khachatrian, L.L. Involvement of G; in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 29:307-313, 1986.
Jarbe, T.U.C., and McMillan, D.E. -THC as a discriminative stimulus in rats and pigeons: Generalization to THC metabolites and SP-111. Psychopharmacology 71:281-289, 1980.
-THC as a discriminative stimulus in rats and pigeons: Generalization to THC metabolites and SP-111. Psychopharmacology 71:281-289, 1980.
L e m b e r g e r , L . ; M a r t z , R . ; R o d d a , B . ; F o r n e y , R . ; a n d R o w e , H .
C o m p a r a t i v e p h a r m a c o l o g y |
o f |
-tetrahydrocannabinol |
and its |
|||
metabolite, 11-OH- |
-tetrahydrocannabinol. |
J Clin |
Invest |
52:2411- |
||
2417, 1973. |
|
|
|
|
|
|
Martin, B.R. Cellular |
effects of |
cannabinoids. Pharmacol |
Rev |
38:45-74, |
||
1986. |
|
|
|
|
|
|
Martin, B.R.; Balster, R.L.; Razdan, R.K.; Harris, L.S.; and Dewey, W.L. Behavioral comparisons of the stereoisomers of tetrahydrocannabinols.
Life Sci 29:565-574, 1981. |
|
|
|
Perez-Reyes, M.; Timmons, M.C.; Davis, |
K.H.; and Wall, M.E. A |
comparison |
|
of the pharmacological |
activity in |
man of intravenously administered |
|
-tetrahydrocannabinol, cannabinol, and cannabidiol. |
Experientia |
||
29:1368-1369, 1973a. |
|
|
|
Perez-Reyes, M.; Timmons, M.C.; Lipton, M.A.; Christensen, H.D.; Davis, |
|||
K.H.; and Wall, M.E. A comparison |
of the pharmacological |
activity of |
|
-tetrahydrocannabinol and its monohydroxylated metabolites in man. |
|||
Experientia 29:1009-1010, |
1973b. |
|
|
Pertwee, R.G. The ring test: A quantitative method for assessing the "cataleptic" effect of cannabis in mice. Br J Pharmacol 46:753-763, 1972.
S c h u b e r t , D . ; H u m p h r e y s , S . ; B a r o n i , C . ; a n d C o h n , M . I n v i t r o differentiation of a mouse neuroblastoma. Proc Natl Acad Sci USA 64:316-323, 1969.
Seamon, K.B., and Daly, J.W. Forskolin: A unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res 7:201-224, 1981.
Uliss, D.B.; Dalzell, H.C.; Handrick, C.R.; Howes, J.F.; and Razdan, R.K.
Hashish. |
I m p o r t a n c e |
o f |
the phenolic |
|
hydroxyl |
group |
in |
tetrahydrocannabinols. J Med Chem 18:213-215, |
1975. |
|
|
||||
Wilson, R.S., |
and M a y , |
E . L . |
Analgesic |
p r o p e r t i e s o f |
the |
||
tetrahydrocannabinols, their metabolites, and analogs. J Med Chem 18:700-703, 1975.
Wilson, R.S.;’ May, E.L.; Martin, B.R.; and Dewey, W.L. 9-Nor-9- hydroxyhexahydrocannabinols. Synthesis, some behavioral and analgesic
156
properties, and comparison with the tetrahydrocannabinols. J Med Chem 19:1165-1167, 1976.
ACKNOWLEDGMENTS
This work was supported by National Institute on Drug Abuse grant DA03690. Cannabinoid compounds were supplied by the National Institute on Drug Abuse and nantradol compounds were a gift from Pfizer Inc.
AUTHOR
Allyn C. Howlett, Ph.D.
Department of Pharmacology
St. Louis University School of Medicine
1402 S. Grand Boulevard
St. Louis, Missouri 63104
157
