
The Nitro group in organic sysnthesis - Feuer
.pdf
|
|
|
|
|
4.3 |
MICHAEL ADDITION OF NITROALKANES |
109 |
||||||||
|
OH |
|
OAc |
|
|
|
|
|
OAc |
|
O |
||||
|
|
|
|
|
KF, Bu4NBr, |
|
|
|
|
||||||
|
|
|
Ac O, py, RT |
|
|
|
|
|
|
|
|
||||
|
|
2 |
|
|
|
|
|
|
|
|
|
|
OMe |
||
|
|
|
|
|
|
|
|
|
DMSO |
|
|
NO2 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
NO2 |
|
NO2 methyl propiolate |
|
|
|
|||||||||
|
98% |
|
|
|
|
|
|
|
62% |
|
|||||
|
|
|
|
OAc |
|
O |
|
|
|
|
|
OAc |
O |
|
|
|
15% TiCl3, pH 5.3 |
|
|
OMe |
HO(CH2)2OH |
|
|
O |
O |
OMe |
|||||
|
|
|
|
|
O |
|
p-TsOH |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
60% |
|
|
|
|
|
|
|
95% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
OH |
|
O |
|
|
|
|
|
|
O |
|
|
|
KOH |
|
OMe |
|
|
|
|
|
O |
O |
|
||||
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
|
|
MeOH |
|
|
O |
95% |
O |
|
|
|
(–)-(R, R)-Pyrenophorin I |
Scheme 4.23.
Conjugate addition of nitroalkanes to allyl Baylis-Hillman acetates in the presence of NaOH (0.6 N) in THF gives 2-alkylidene-4-nitro ketones with high stereoselectivity; these are converted via the Nef reaction into the corresponding 1,4-diketones (Eq. 4.119).164
Et |
|
|
|
|
|
|
OAc |
|
NaOH |
|
|
|
|
+ |
MeCH2NO2 |
|
|
|
||
|
|
|
|
|
||
THF, 0–20 ºC |
|
|||||
O |
|
|
||||
|
|
|
|
|
|
|
Me |
|
NO2 |
|
|
O |
|
|
Et |
|
|
|||
|
|
|
NaOH |
Et |
||
|
|
Me |
|
Me (4.119) |
||
|
|
|
|
|
||
|
|
O |
H+, MeOH |
|||
|
Me |
O |
||||
|
|
|
–50 ºC |
Me |
||
|
78% |
|
|
|
61% |
|
Polyfunctionalized nitro compounds are prepared by the Michael addition using 2-alkenyl- substituted 2-siloxycyclopropanecarboxylates as Michael acceptors (Eq. 4.120).165
|
|
|
|
O |
|
Me3SiO |
CO2Me |
NO2 |
Triton B |
CO2Me (4.120) |
|
+ |
|
||||
∆ |
|||||
|
|
|
NO2 |
||
|
|
|
|
||
|
|
|
|
80% |
Newkome and coworkers have developed synthesis of dendritic molecules using the Michael addition of nitromethane to α,β-unsaturated esters as a key reaction (Scheme 4.24).166
The addition of alkyl nitronate anions to imines in the presence of a Lewis acid proceeds in high yield with up to 10:1 diastereoselection favoring the anti isomer. This reaction is used for the stereoselective synthesis of 1,2-diamines (Eq. 4.121).167 Scandium triflate catalyzes the addition of 1-trimethylsilyl nitropropanoate to imines with a similar selectivity.35
110 MICHAEL ADDITION
O
|
OH |
|
O2N |
OH |
|
|
|
|
|
O |
|
|
OH |
|
O |
|
|
+ |
DCC, HOBT |
R |
O |
DMF |
|
|
|
|
|
O |
|
3 H2N |
O |
|
|
|
|
|
O |
|
|
O |
|
O |
|
|
DCC: dicyclohexylcarbodiimide
HOBT: 1-hydroxybenzotriazole
O
|
|
|
O O |
|
|
|
O |
|
|
O |
O |
|
|
O |
HN |
|
|
|
|
|
|
O |
NH |
|
O |
O |
O |
|
OH |
||
|
O |
||
|
OH |
O |
|
O2N |
|
||
O |
|
|
|
|
|
|
|
|
OH |
|
|
|
O |
|
R |
DCC, HOBT |
|
||
|
|
||
|
DMF |
|
|
|
|
O |
|
|
|
O |
O |
|
|
O |
|
O NHO
|
|
O O |
O |
|
|
O |
|
|
O |
|
|
|
|
|
|
O |
|
|
O |
|
|
|
O |
|
|
|
NH |
|
|
|
|
|
O |
|
|
|
H |
|
|
|
|
|
O |
|
|
|
N |
|
|
|
|
O |
|
O |
|
|
NH |
|
O |
|
O |
|
|
O O |
|
|
|
|
O |
|
O |
O |
O |
|
|
|
|||
|
|
O |
|
|
|
R = NO2 |
H2, Raney Ni |
||
|
R = NH2 |
|||
|
|
|
||
O |
O |
O |
|
|
|
|
|
|
|
O |
|
O O |
|
|
|
|
|
||
|
|
|
O |
|
HN |
|
|
O O |
|
O |
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
HN |
|
|
O |
|
|
|
NH |
|
|
|
O |
|
|
|
|
|
H |
|
|
|
H |
|
N |
|
|
N |
|
|
O |
|
O |
|
NH |
|
O |
|
|
O |
|
|
NH |
|
|
|
|
|
|
O |
O |
|
|
|
O |
|
O |
|
|
O
O
O
O
O
O
O
O
O O
O
O
|
O |
O |
NH HN |
|
|
|
|
|
|
|
|
|
|
O |
|
||
|
|
O |
|
|
|
|
||
|
|
O O |
O |
|
|
O |
|
|
|
|
|
|
|
O |
|
||
|
|
O O |
O |
|
|
|
||
|
|
O |
O |
R = NO2 |
|
|||
|
|
|
|
|
||||
|
|
|
|
|
H2, Raney Ni |
|||
|
|
|
|
|
|
R = NH2 |
||
|
|
|
|
|
|
|
||
|
|
Scheme 4.24. |
|
|
|
|
|
|
|
1) n-BuLi, THF, –78 ºC |
|
|
PhH2C |
|
|||
|
|
|
NH |
|
||||
NO2 |
2) PhCH2N=CHPh |
|
|
|
Ph |
Et |
(4.121) |
|
3) THF, AcOH, –78 to 0 ºC |
|
|
||||||
|
|
NO2 |
|
|||||
|
|
|
|
|
|
|
||
|
|
|
|
|
95% (anti/syn = 10/1) |
|
||
4.3 MICHAEL ADDITION OF NITROALKANES |
111 |
The sequence of the Michael addition of nitroalkanes and denitration provides a general method for conjugate addition of primary and secondary alkyl groups to electron deficient alkenes (Eq. 4.122).168
|
|
O |
O2N |
Me |
CO2Me |
|
CO2Me |
||
MeNO2 |
|
|
Me |
||||||
|
|
|
|
|
|
O2N |
|||
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
PhO2S |
CO2Me |
|
|
Bu3SnH |
PhO2S |
CO2Me |
|
SO2Ph |
|
|
|
|
|
|
|||
|
Me |
|
|
AIBN |
|
|
Me |
||
DBU |
|
|
|
benzene |
|
||||
O2N |
|
|
|
H |
|||||
|
|
|
|
80 ºC |
|
||||
|
|
|
O |
|
|
|
O |
||
|
|
|
|
|
|
|
|
||
(4.122)
|
|
|
|
|
NO2 |
|
TBDMSO |
CHO |
1) MeONa, MeNO2, |
|
TBDMSO |
|
|
|
|
|
|
|
||
|
Me |
MeOH, RT, 2 h |
|
|
Me |
|
|
|
2) MsCl, Et3N, CH2Cl2 |
|
|
||
|
|
|
|
|
||
Me |
|
0 ºC, 1 h |
|
Me |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
62% |
|
|
|
|
NO2 |
|
|
|
|
1) NaBH4, CHCl3/i-PrOH, |
|
amberlyst A-21, Et2O, |
||||
RT, 40 min |
RO |
|
||||
2) CH3CN/H2O, HF, |
|
Me |
RT, 2 h |
|
|
|
|
|
|
|
|||
RT, 24 h |
|
|
|
O |
OTBDMS |
|
3) (S)-2-methylbutyric |
Me |
|
|
|||
anhydride, DMAP, |
|
|
|
|
||
59% |
|
|
|
|
||
40 ºC, 20 min |
|
|
|
|
||
|
|
|
|
|
||
|
|
O |
|
|
|
|
|
O |
OTBDMS |
|
|
|
|
|
|
|
|
|
|
|
|
|
n-Bu3SnH, AIBN |
|
|
||
|
O |
NO2 |
toluene |
|
|
|
|
Me |
Me |
|
|
||
|
reflux, 30 min |
|
|
|||
|
|
|
|
|
||
|
Me |
|
|
|
|
|
|
|
70% |
|
|
|
|
|
O |
|
|
|
HO |
O |
O |
OTBDMS |
|
O |
|
O |
|
|
|
|
||||
O |
|
|
|
|
O |
|
Me |
Me |
|
|
Me |
|
Me |
Me |
|
|
|
Me |
|
|
|
55% |
|
|
(+)-dihydromevinolin |
||
Scheme 4.25.

112 MICHAEL ADDITION
Hanessian and coworkers have used this strategy for a total synthesis of (+)-dihydromevi- nolin (Scheme 4.25).169
Ballini and coworkers have developed a new strategy for alkenylation of carbonyl compounds based on the Michael addition followed by elimination of HNO2 (see Section 7.3). A variety of 2-alkylidene 1,4-dioles have been conveniently prepared, in two steps, by the Michael addition of a nitroalkane to the appropriate enedione derivatives under basic conditions, followed by chemoselective reduction with LiAlH4 (Eq. 4.123).170
Me |
NO2 |
CO2Me |
|
Me |
CO2Me |
|
Me |
OH |
DBU |
|
|
LiAlH4 |
|
OH |
|||
+ |
|
|||||||
Me |
H |
MeCN Me |
CO2Me |
|
Me |
|
||
CO2Me |
|
82% |
||||||
|
|
|
|
94% |
|
|
||
(4.123)
The synthesis of 2,3,5-trialkylpyrroles can be easily achieved by conjugate addition of nitroalkanes to 2-alken-1,4-dione (prepared by oxidative cleavage of 2,5-dialkylfuran) with DBU in acetonitrile, followed by chemoselective hydrogenation (10% Pd/C as catalyst) of the C-C- double bond of the enones obtained by elimination of HNO2 from the Michael adduct. The Paal-Knorr reaction (Chapter 10) gives 2,3,5-trialkylpyrroles (Eq. 4.124).171
|
|
|
|
|
|
|
|
|
|
O |
|
|
Me |
|
Me |
|
|
DBU |
|
Me |
Me |
|
|
||
|
+ |
PhCH2CH2NO2 |
|
|
|
|
|
|||||
O |
MeCN |
|
O |
|
|
|
||||||
O |
|
|
|
CH2Ph |
|
|
||||||
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
O |
77% |
|
|
Ph |
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
Pd/C |
|
Me |
Me |
PhCH2NH2 Me |
|
Me |
|
|||
|
|
|
|
N |
(4.124) |
|||||||
|
|
H2 |
|
O |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
CH2Ph |
|
|||
|
|
|
|
H CH2Ph |
|
|
|
|
||||
|
|
|
95% |
|
|
|
|
|
92% |
|
|
|
The Michael addition of cyclic α-nitro ketones to acrolein or methyl vinyl ketone followed by reduction of the carbonyl group and treatment with base results in the ring expansion (Eq. 4.125).172 Hesse and coworkers have used this strategy for the preparation of various macrolides.173 For example, Michael addition of 2-nitrocyclodecanone methyl vinyl ketone followed by reduction with (S)-alpine-hydride gives the nitrolactone in 72% yield. Radical
denitration of the nitrolactone with Bu3SnH gives (+)-(S)-tetradecan-13-olide in 44% yield (Eq. 4.126).173c
|
O |
|
(H2C)n |
O |
|
|
||
C) |
|
|
|
|
|
|||
2 n |
CHO |
|
|
|
|
|||
|
|
|
|
|
|
|
||
|
n NO2 |
|
|
|
|
CHO |
||
|
PPh3 |
NO2 |
||||||
|
|
|
||||||
n = 0, 1, 2, 6 |
|
|
84–93% |
|
|
|||
|
|
|
|
O |
|
O |
||
|
|
|
|
|
|
|
||
|
|
|
(H2C)n |
|
|
|
O |
|
|
NaBH4 |
|
|
|
OH |
|
|
(H2C)n |
|
|
|
NaH |
|
||||
|
|
|
|
NO2 |
|
|
DME |
|
|
|
|
|
90–95% |
|
|
|
NO2 |
|
|
|
|
|
|
|
75–81% (4.125) |
|
|
|
|
|
|
|
|
|
|
|
|
4.3 MICHAEL ADDITION OF NITROALKANES 113 |
||
O |
O |
O |
O |
|
|
|
|||
|
NO2 |
|
|
|
|
PPh3 |
|
NO2 |
|
|
O |
Me |
O |
Me |
|
(S)-alpine |
|
Bu3SnH |
|
|
hydride |
|
|
|
|
|
NO2 |
AIBN |
|
|
|
|
|
(4.126) |
|
|
72% |
|
44% |
The Michael addition of α-nitro ketones to α,β-unsaturated ketones followed by radical denitration provides a useful strategy for the preparation of 1,4-diketones.134b 1-Phenylheptane-
1,5-dione, isolated from the decayed heart wood of aspens, is prepared by this strategy (Eq. 4.127).174
|
|
|
|
|
|
|
O |
O |
|
O |
|
+ |
|
PPh3 |
|
Ph |
|
|
|
NO2 |
|
|
|
|||
|
|
|
|
|
|
|
||
Ph |
|
O |
|
|
|
|
NO2 |
|
|
|
|
|
|
|
|||
|
|
|
|
O |
O |
71% |
||
|
|
|
|
|
||||
|
|
|
Bu3SnH |
Ph |
|
|
(4.127) |
|
|
|
|
AIBN |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
70% |
|
|
|
|
The Michael addition of nitro compounds to α,β-unsaturated ketones or esters followed by reduction of the nitro to amino group is useful for the preparation of various heterocycles. This is presented in Chapter 10 (Synthesis of Heterocycles).
4.3.2 Intramolecular Addition
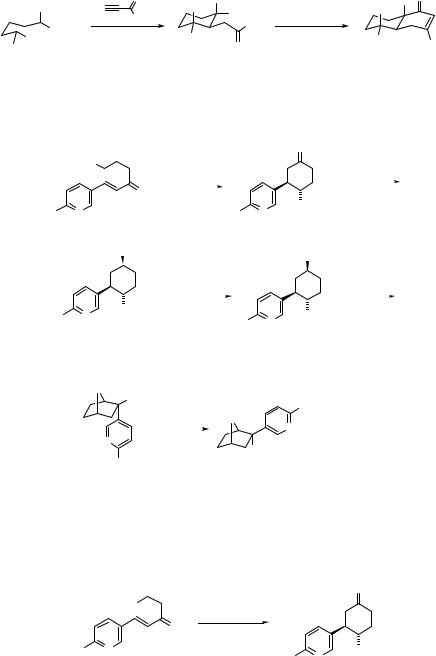
Intramolecular Michael addition of nitro compounds proceeds in a stereoselective way to give various types of cyclic nitro compounds with high stereoselectivity. The Michael addition of 1-acetylcyclohexene to nitrostyrene followed by treatment with MeONa in MeOH gives 4-nitro-3-phenyldecalone with high stereoselectivity (Eq. 4.128).175
O |
|
|
O Ph |
|
|
|
|
NO2 |
|
|
1) LDA |
|
|
|
|
2) Ph |
NO2 |
|
|
|
|
75% |
|
|
|
|
|
|
|
|
|
|
O |
O |
|
|
|
H |
H |
|
MeONa-MeOH |
+ |
(4.128) |
|
|
|
|
||
|
RT |
|
Ph |
Ph |
|
|
H |
H |
|
|
|
|
NO2 |
NO2 |
|
|
|
45% (7:93) |
|
114 MICHAEL ADDITION
Double Michael additions of nitro compounds bearing tethered acidic carbons to 3-butyn- 2-one under NaH catalysis give nitrocyclohexanes with high stereoselectivity. The products are transformed into trans-fused bicyclic compounds via the Dickmann reaction on treatment with base. (Eq. 4.129).176
|
O |
CN |
|
|
Me |
CNO |
CN |
Me |
CO2Et |
NaOEt |
|
||
|
Me |
|
|
|
||
|
CO2Et NaH, THF |
|
Me |
|
|
|
NO |
O2N |
|
O |
N |
|
|
Me |
2 |
|
O |
2 |
|
OEt |
|
|
58% |
|
|
|
80% |
(4.129)
The intramolecular Michael addition is used as a key step for synthesis of epibatidine (Scheme 4.26).177 Epibatidine is an analgesic, operating by a nonopioid mechanism, it is several hundred times more potent than morphine.
|
|
|
|
|
|
|
|
|
O |
|||||
|
O2N |
|
|
|
|
|
|
|
1) NaBH4/EtOH |
|||||
|
|
|
|
|
KF/Al2O3 |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
O |
|
|
|
2) MeSO2Cl |
||||||||
|
|
|
THF, RT |
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
CH2Cl2, py |
|||||
Cl |
N |
|
|
|
|
Cl |
NO2 |
|||||||
|
|
|
|
N |
||||||||||
|
|
|
|
|
|
|
|
|
59% |
|
|
|
|
|
|
OR |
|
|
|
|
|
OMs |
|||||||
|
|
|
|
|
SnCl2•2H2O |
|
||||||||
|
|
|
|
|
|
|
|
|
toluene |
|
||||
|
|
|
|
|
|
|
|
|
||||||
|
NO2 |
|
EtOH |
|
NH2 |
|||||||||
Cl |
|
|
|
|
|
|||||||||
N |
|
|
|
|
Cl |
|||||||||
R = H (67%) |
|
|
|
|
N |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
||||
R = Ms (91%) |
|
|
|
|
|
80% |
|
|
|
|
|
|||
R = CO-menthyl |
|
|
|
|
|
|
|
|
|
|
|
|||
|
H |
|
|
|
|
|
|
|
|
|
|
|
||
|
N |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
H |
|
|
|
|
|
Cl |
||||||
|
|
|
|
|
|
|
|
H |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t-BuOK |
|
N |
N |
|||||||
|
|
|
|
|
||||||||||
|
N |
t-BuOH |
|
|||||||||||
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
H |
||||||||
|
|
|
|
|
|
|
|
|
||||||
|
Cl |
|
|
|
|
|
|
|
|
|
|
|
||
|
50% |
|
|
|
|
|
|
|
60% |
|
|
|
|
|
Scheme 4.26.
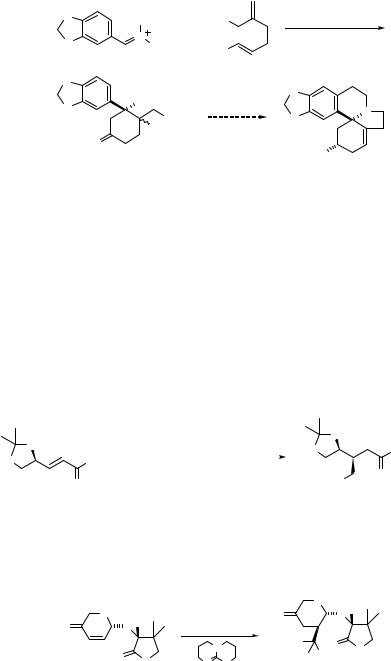
Barco and coworkers have reported a more elegant synthesis of the nitrocyclohexanone via the double Michael addition of nitromethane with enones. (Eq. 4.130).178
|
|
O |
TolSO2 |
MeNO2 |
|
O |
(4.130) |
|
KF |
|
|
|
|
|
Cl N |
Cl |
NO2 |
|
N |
4.4 ASYMMETRIC MICHAEL ADDITION |
115 |
The sequential process consisting of palladium-catalyzed alkylation and the intramolecular Michael addition of nitro compound provides a nitrocyclohaxane derivative, which is a good precursor for synthesis of Erythrina alkaloids (Eq. 4.131).179
O |
O- Li+ |
AcO |
Pd(PPh3)4, PPh3 |
|
||
|
N |
|
+ |
THF |
|
|
O |
O- |
|
|
|
||
|
MeO2C |
|
|
|
||
|
|
|
|
|
|
|
O |
|
|
|
O |
|
|
|
NO2 |
|
|
|
||
O |
|
D |
C |
|
||
|
|
CO2Me |
O |
N |
(4.131) |
|
|
1 |
H |
B |
|||
|
|
|
|
A 6 |
|
|
|
H2C |
|
|
3 |
|
|
|
|
|
MeO |
|
|
|
|
57% |
|
|
|
|
|
|
|
|
|
|
|
|
4.4 ASYMMETRIC MICHAEL ADDITION
4.4.1 Chiral Alkenes and Chiral Nitro Compounds
Diastereoselective conjugate addition of nucleophiles to enones, enals, and enoates occurs with high stereocontrol and constitutes a powerful method in stereoselective synthesis.185
The conjugate addition of nucleophiles to optically pure γ-oxygenated substrates is of special interest in the enantioselective synthesis of natural products. Highly syn-selectivity is observed in the conjugate addition of ammonia,186 alkoxides,187 and alkyllithium derivatives.188 High anti-selective addition is observed in the addition of metal enolates and cuprates.189 The Michael addition of nitromethane to enoates derived from glyceraldehyde acetonide leads to syn-adducts with high diastereoselectivity (de 76–90%) (Eq. 4.132).190 Primary and secondary nitroalkanes afford syn-adducts diastereoselectively in the Bu4NFor DBU-catalyzed Michael addition to the same enoates of Eq. 4.132.191
|
O |
|
|
|
O |
||
|
|
Bu4NF·3H2O |
O |
|
|
OEt |
|
|
|
+ MeNO2 |
|
|
|||
O |
OEt |
|
|
|
|
(4.132) |
|
THF |
|
|
|||||
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
O2N |
||
70% (syn/anti = 95/5)
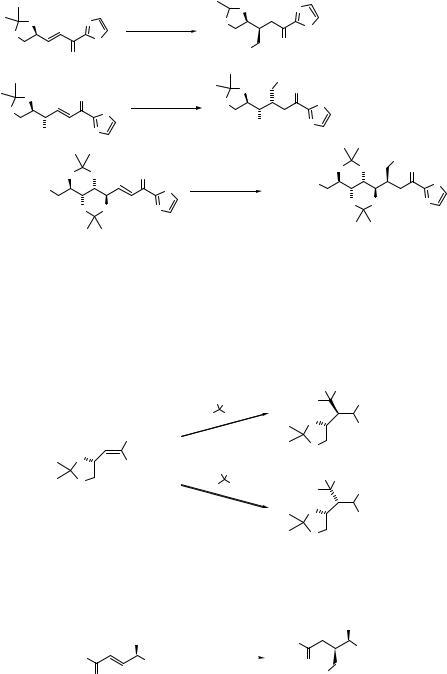
Syn-selectivity of the DBU-catalyzed Michael addition of nitromethane to chiral γ-oxygen- ated enones is quite general, as shown in Scheme 4.27.192
Feringa and coworkers have used the optical active furanone or pyranone as an acceptor for the diastereoselective Michael reactions (Eq. 4.133).193
|
|
|
|
|
|
|
|
O |
|
|
O |
O H |
|
|
|
|
O |
O H |
|
O |
|
Me |
CHNO |
2 |
|
|
(4.133) |
||
|
|
|
2 |
|
|
O2N |
|
||
|
O |
|
|
|
N |
|
O |
O |
|
|
O |
N |
N |
|
Me |
Me |
|
||
|
|
|
76% (90% de) |
|
|||||
|
|
|
|
|
H |
|
|
||
116 MICHAEL ADDITION |
|
|
|
|
|
|
|||
|
|
|
|
|
O |
N |
|
|
|
|
O |
N |
|
MeNO2 |
O |
|
S |
|
|
O |
S |
|
DBU |
|
O |
|
|
|
|
|
|
|
O2N |
|
|
|
|||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
92% (88% de) |
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
NO2 |
|
|
|
|
O |
O |
|
|
O |
O |
|
|
|
|
|
MeNO2 |
O |
|
S |
|
|
||
O |
|
|
S |
|
|
|
|||
|
|
DBU |
|
|
|
|
|
||
|
|
|
|
|
OBn |
N |
|
|
|
|
OBn |
N |
|
|
|
|
|
||
|
|
|
94% (83% de) |
|
|
|
|||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
O |
O |
NO2 |
|
O |
O |
|
O |
|
|
O |
||
|
|
MeNO2 |
ButMe2SiO |
|
S |
||||
t |
Me2SiO |
|
|
S |
|
||||
Bu |
|
|
DBU |
|
|
|
|
||
|
|
|
|
|
|
O |
O |
N |
|
|
|
O |
O |
N |
|
|
|||
|
|
|
|
|
|
|
|||
92% (86% de)
Scheme 4.27.
Krief and coworkers have found that 2-lithio-2-sulfonylpropane and 2-lithio-2-nitropropane behave differently in the addition to dimethyl alkylidenemalonate. Thus, 2-lithio-2-sulfonyl- propane reacts with it almost exclusively on the (Si)-face and leads to the anti-adduct, whereas 2-lithio-2-nitropropane reacts under similar reaction conditions, exclusively on the (Re)-face, providing the syn-product (Eq. 4.134).194
|
|
Me SO2Ph |
Me |
SO2Ph |
|
|
|
|
Me |
CO2Me |
|
||
|
|
Me |
Li |
O |
|
|
|
|
|
|
CO2Me |
|
|
|
CO2Me |
THF |
|
|
||
|
O |
|
|
|||
O |
|
|
|
|
|
|
CO2Me |
Me NO2 |
72% (92% de) |
(4.134) |
|||
|
Me |
NO2 |
|
|||
O |
|
|
||||
|
Me |
Li |
|
|||
|
Me |
CO2Me |
|
|||
|
|
|
|
|
||
|
|
THF |
O |
CO2Me |
|
|
|
|
|
|
|
|
|
O
71% (100% de)
The Michael addition of nitromethane to vinylogous esters of N-protected amino acids proceeds with good yields and with good diastereoselectivity (Eq. 4.135).195
|
|
|
|
NHBoc |
|||
NHBoc |
MeNO2 |
EtO |
|
Me |
|||
|
|||||||
EtO |
|
|
|
|
|||
Me |
|
O |
(4.135) |
||||
DBU |
|||||||
|
|
||||||
O |
|
O2N |
|||||
|
|
|
95% (60% de) |
||||
Levoglucosenone, a cellulose-derived α,β-unsaturated ketone, is an interesting material because it is both chiral and contains an activated double bond. This compound has been used
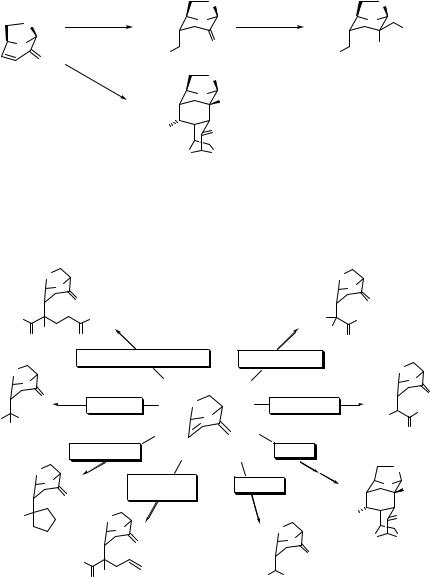
4.4 ASYMMETRIC MICHAEL ADDITION |
117 |
as a chiral starting material for synthesis of a variety of natural compounds such as alkaloids or antibiotics.196 The Michael addition of nitro compounds to levoglucosenone provides a useful tool for the synthesis of various chiral compounds. Nitromethane undergoes TMG-catalyzed addition to levoglucosenone, affording 2:1 and 1:2 adducts in high yield (95%); the products result from initial Michel addition exclusively at the exo-face of the alkene anti to the 1,6-anhydro bridge (Eq. 4.136).197 If nitromethane is used as a solvent, the 2:1 adduct is obtained. A reaction is carried out by stirring a mixture of nitromethane and levoglucosenone (2:1 ratio) in CH2Cl2 in the presence of catalytic amounts of TMG to give a 1:2 adduct.
|
|
|
O |
O |
O |
MeNO2 |
O |
MeNO2 |
O |
O |
TMG |
|
TMG |
NO2 |
|
O2N |
O |
OH |
|
O |
MeNO2 |
|
O2N |
|
|
TMG |
|
O |
|
|
|
|
|
|
|
|
O |
OH |
(4.136) |
|
|
|
||
|
|
|
|
O2N O
O O
A variety of important synthetic reactions can be promoted by electrogenerated bases (EGB). The Michael addition and alkylation of compounds with activated hydrogen atoms are two examples in which using EGB has been very successful. The electrochemical method is very successful for functionalization of levoglucosenone as shown in Scheme 4.28.198
|
O |
|
|
O |
|
|
|
O |
|
|
O |
|
|
|
|
O |
|
O |
|
|
EtO |
|
OEt |
Me |
OEt |
|
|
|
NO2 |
O |
O2N |
O |
|
|
|
O |
|
|
|
||
O |
|
EtO2CCH(NO2)CH2CH2CO2Et |
MeCH(NO2)CO2Et |
|
|
O |
|
|
|
|
|
||
|
|
|
|
|
O |
|
O |
|
|
|
|
||
|
|
|
|
|
||
|
O |
O |
O2NCH2CO2Et |
|
|
O |
|
|
|
|
|||
|
|
|
|
OEt |
||
|
|
Me2CHNO2 |
O2N |
|||
Me NO |
O |
|
|
|||
Me |
2 |
|
|
|
|
O |
|
|
O |
|
|
||
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
cyclo-C5H9NO2 |
CH3NO2 |
|
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
O2NCH2CO2Et |
CH2(NO2)2 |
|
O |
OH |
|
O |
CH2=CHCH2Br |
|
|
|
|
|
|
|
|
|
||
O2N |
|
O |
O |
O2N |
|
O |
|
|
O |
O |
|
O |
|
|
|
O |
O |
|
||
|
|
|
|
|
||
|
|
O |
|
|
|
|
|
EtO |
|
|
|
||
|
|
|
|
|
||
|
|
NO2 |
O2N NO2 |
|
|
|
|
|
O |
|
|
|
|
Scheme 4.28.
118 MICHAEL ADDITION
4.4.2 Chiral Catalysts
Catalytic enantioselective nucleophilic addition of nitroalkanes to electron-deficient alkenes is a challenging area in organic synthesis. The use of cinchona alkaloids as chiral catalysts has been studied for many years. Asymmetric induction in the Michael addition of nitroalkanes to enones has been carried out with various chiral bases. Wynberg and coworkers have used various alkaloids and their derivatives, but the enantiomeric excess (ee) is generally low (up to 20%).199 The Michael addition of methyl vinyl ketone to
2-nitrocycloalkanes catalyzed by the cinchona alkaloid cinchonine affords adducts in high yields in up to 60% ee (Eq. 4.137).200
|
|
|
N |
|
|
|
HO |
|
|
|
|
H |
H |
O |
O |
|
|
O |
|
|
O |
|
|
|
|
NO2 |
N |
|
|
|
+ |
(4.137) |
||
|
|
NO2 |
||
|
|
CCl4, –20 ºC, 60 h |
|
|
84% (54.6% ee)
Matsumoto and coworkers have introduced a new strategy for asymmetric induction under high pressure. The Michael addition of nitromethane to chalcone is performed at 10 kbar in the presence of a catalytic amount of chiral alkaloids. The extent of asymmetric induction reaches up to 50% ee with quinidine in toluene.201
Chiral monoaza-crown ethers containing glucose units have been applied as phase-transfer
catalysts in the Michael addition of 2-nitropropane to a chalcone to give the corresponding adduct in up to 90% ee. (Eq. 4.138).202
O |
|
|
|
|
|
|
O |
Me |
Me |
NaOtBu, toluene |
|
||||
+ |
|
* |
|||||
NO2 |
|
|
|
|
|
||
H |
MeO |
H |
|
O |
|
||
|
|
|
|
O |
O2N Me |
||
|
|
O |
|
|
|
||
|
|
|
|
|
N R |
||
|
|
|
|
|
|
Me |
|
|
|
|
|
H O |
|
||
|
1 |
1 |
O |
O |
82% (90% ee) |
||
|
R |
O R |
|
|
|
|
|
(4.138)
Yamaguchi and coworkers have found that proline rubidium salts catalyze the asymmetric Michael addition of nitroalkanes to prochiral acceptors. When (2S)-L-prolines are used, acyclic (E)-enones give (S)-adducts. Cyclic (Z)-enones give (R)-adducts predominantly (Eq. 4.139).203 Recently, Hanessian has reported that L-proline (3 ~ 7% mol equiv) and 2,5-dimethylpiperazine are more effective to induce catalytic asymmetric conjugate addition of nitroalkanes to cycloalkanones.204
O |
NO2 |
N CO2Rb |
O |
|
|||
H |
|
||
|
+ |
(5–10 mol%) |
(4.139) |
|
20 h |
||
|
|
O2N |
|
|
|
|
|
|
|
|
84% (84% ee) |
