
PAGE PROOF: 2ND PASS
Growth and Reproduction of Individuals 163
Percentage of fruits dispersed
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Artificial fruits |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Morphology of |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
Mass |
|
|
|
|
|
|
Surface area |
|
|
|
|
|
|
Shape |
|
|
|
|
natural fruit |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
20 |
|
|
|
|
|
|
|
|
30 |
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Least) |
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
(Lightest) |
20 |
10 |
|
|
|
|
|
|
|
|
|
|
10 |
20 |
40 |
60 |
80 |
|
|
|
|
|
20 |
|
20 |
|
|
|
10 |
30 |
10 |
|
||
|
|
20 |
fpo |
FPO |
|
||
10 |
|
|
20 |
|
20 |
10 |
10 |
|
|
|
20 |
|
|
|
20 |
40 |
60 |
80 |
20 |
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
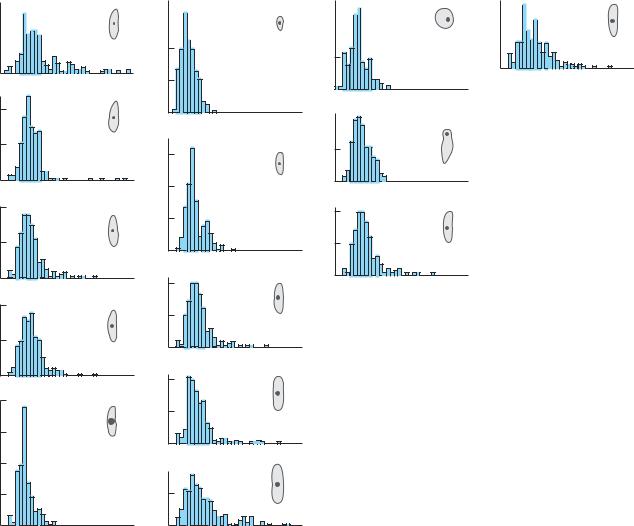
Figure 8.14 |
|||
|
|
|
|
|
|
|
|
|||
10 |
|
|
|
|
|
|
|
Carol Augspurger and Susan Franson (1987) |
||
|
|
|
|
|
|
|
studied the effects of mass and area on the |
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
dispersal of the fruit of the tropical tree Tachi- |
||
|
|
|
20 |
|
|
|
|
galia versicolor (Fabaceae). They created 3000 |
||
|
|
|
|
|
|
|
|
artificial fruits, 200 each of 15 different types, |
||
40 |
|
|
10 |
|
|
|
|
from cardboard of various colors. Each artifi- |
||
|
|
|
|
|
|
|
cial fruit was dropped from the top of a 40- |
|||
|
|
|
|
|
|
|
|
|||
30 |
|
|
|
|
|
|
|
meter tower located in the forest on Barro Col- |
||
|
|
(Heaviest) |
|
|
|
|
orado Island, a large island in the Panama |
|||
20 |
|
|
|
|
|
|
|
Canal. The landing point of each fruit was |
||
|
|
|
|
|
|
|
determined, and then the experiment was |
|||
|
|
|
|
|
|
|
|
|||
10 |
|
|
10 |
|
|
|
|
repeated four more times. The figures show, |
||
|
|
|
|
|
|
for each type of fruit, the proportion traveling |
||||
|
|
|
|
|
|
(Greatest) |
a given distance. Those artificial fruits that |
|||
20 |
40 |
60 |
80 |
20 |
40 |
60 |
80 |
traveled farther were lighter (column 1), had a |
||
greater surface area (column 2), and were |
||||||||||
|
|
|
|
|
|
Distance dispersed (m) |
shaped more like the real seeds (column 3). |
|||
ing to eat seeds often drop them accidentally. Animals that bury seeds for later use also act as dispersers if they fail to return. Both birds and mammals cache seeds in this fashion. For example, Pinus albicaulis (whitebark pine, Pinaceae) in the mountains of the northwestern United States and southwestern Canada is mainly dispersed by granivorous birds, especially Nucifraga columbiana, Clark’s nutcracker. The seeds of this tree are not released from the cones when they are mature, and the tree depends on birds to remove and disperse them. The nutcrackers pry the seeds loose from the cones, remove them, and cache them, storing from 1 to 15 seeds together in each of many caches just under the soil surface. They later retrieve the seeds and eat them. Although Clark’s
nutcrackers have excellent memories, they occasionally forget where some of their caches are, or they may be killed or driven away, leaving their uneaten seeds to germinate and grow into trees (Lanner 1996).
Sometimes the mode of animal dispersal can be subtle. Many species of ants collect seeds for food. In some plant species, however, the seeds are attached to a lipid body called an elaiosome. Instead of eating these seeds, ants carry the seeds to their nest, remove and eat the elaiosomes, and toss the seeds outside the nest. Thus seed dispersal by animals may be highly clumped, with many seeds ending up in a refuse heap near an ant nest or in the dung pile below the feeding tree of a monkey troop. Other seeds disperse by getting stuck in the hair
164 Chapter 8
or feathers of an animal (Figure 8.13C), which can be a very effective mode of long-distance dispersal.
While most seeds end up relatively close to the parent plant, a small percentage of the seeds may be dispersed long distances, and this long-distance dispersal can have important demographic consequences. Note, however, that a long distance for one species could be a short distance for another. When we speak of “longdistance dispersal,” we mean that individuals are moving far enough from their parent population that they can no longer interact with individuals in that population. It also helps to have a rough idea of the actual distances involved; for many plants, dispersing more than a few hundred meters can move them out of their parent population.
In Chapter 21, we discuss the migration of trees northward in North America following the retreat of the glaciers. Some tree species are estimated to have migrated as fast as 400 meters per year. Since it takes several years for a tree to become large enough to produce seeds, migration at this rate must have depended on very long distance dispersal events. The tail of the distribution of dispersal distributions is therefore quite important, but estimating the shape of the tail presents some important statistical problems (Portnoy and Willson 1993). Interestingly, wind-dispersed species migrated faster than animal-dispersed species (see Table 21.2); one might expect the reverse, since animal-dis- persed species often appear to have more long-distance dispersal events.
Colonization of newly disturbed habitats or new islands must also rely on long-distance dispersal. Once a plant becomes established in such a habitat, short-dis- tance dispersal is responsible for local population growth. For example, the seeds of Cocos nucifera (coconut, Arecaceae) disperse from island to island by floating in seawater—one of the few species whose seeds can survive this experience. On a given island, however, most seeds travel only very short distances, as you might imagine would be so for such large seeds (although they travel from tree to ground very fast!).
Seed Banks
Dispersal in time, as well as in space, can be important for some plant species. Once a seed is buried in the ground, it can remain there for years or decades. The seed bank is the collection of seeds in the soil; this term is sometimes used to refer to the seeds of a single species and sometimes to the seeds of an entire community. There is a tendency for short-lived plants (such as annuals) to have long-lived seeds, while long-lived plants (such as trees) and plants with large seeds tend to have short-lived seeds, although this is not a hard-and-fast rule. As a result, the abundances of species in the seed bank may have little relationship to those of the plants
PAGE PROOF: 2ND PASS
growing in the same spot. Many species growing above ground have few seeds in the soil. Other species may exist only in the seed bank at a given time. Species that specialize in colonizing disturbed places by wind dispersal, for example, may exist above ground for only a few years following a disturbance.
Seed banks also occur above ground in populations that have serotinous fruits or cones. Serotinous fruits or cone scales are bonded shut with resins and open only when temperatures are sufficiently high (see Figure 13.4). Normally this occurs only in response to fire. The seeds are thus dispersed only under conditions suitable for germination (bare mineral soil and increased nutrient availability) and are protected from granivorous animals at other times (Groom and Lamont 1997; Hanley and Lamont 2000; Lamont and Enright 2000). Serotiny is especially important in many conifers and in a number of species of Australian Proteaceae such as Banksia and Hakea.
We actually know very little about how long the seeds of many species can remain dormant because the time scales necessary for such experiments are so long. In 1879, Edward Beal began a famous experiment at the University of Wisconsin. He took a set of 20 glass jars and in each put a mixture of soil and 50 seeds of each of 19 common weed species and one crop species. He then buried the jars uncapped, but upside down. The idea was to allow moisture, small arthropods, microbes, and so forth access to the seeds, while not allowing new seeds to get in. Beal and his successors dug up one jar every 5 years for the first 40 years, and at 10-year intervals after that time, and tested the seeds to see whether they were still viable (Priestley 1986). For 16 species, all seeds that germinated did so within 50 years, while the seeds of 4 species continued to germinate after that time. For two of these species—Malva pusilla (round-leaved mallow, Malvaceae) and Verbascum spp. (Scrophulariaceae; common mullein, V. thapsus, and moth mullein, V. blattaria, were not distinguished)—some germination occurred among 100-year-old seeds. There were also interesting patterns of germination and dormancy. For example, seeds of M. pusilla germinated after 5, 20, and 100 years of burial, but not at other time intervals. As the experiment was designed to look for maximum longevity rather than variation in germinability, we cannot estimate the latter. However, half the species showed gaps in germination like that seen in M. pusilla, albeit not so dramatic, suggesting that complex patterns of dormancy may exist in many species.
The existence of a seed bank can have important demographic consequences for a population. It can also help to buffer a population against year-to-year variation in demographic rates (bet hedging; see Chapter 9). Seed banks can also affect evolution in two ways. First, they slow the response to selection by maintaining genotypes produced in previous years. Second, mutation

PAGE PROOF: 2ND PASS
rates in seeds can actually be substantial, and seed banks can thus act as a source of genetic novelty (Levin 1990).
Summary
Growth and reproduction are key ways in which plants interact with the animals and the physical environment around them through their uptake of nutrients, use of space, pollination relationships, and dispersal of fruits and seeds. Plants grow by adding repeated modules such as leaves, flowers, and branches. This mode of growth leads to plasticity, allowing plants to vary their growth pattern in response to light availability or other environmental factors. There are a variety of ways of using this capacity, and clonal plants use it to explore their environments in varied ways—sometimes by following concentrations of resources, and sometimes by more active “foraging.”
Both sexual and asexual reproduction are common in plants. Some asexual reproduction is vegetative, but some is from seeds. Most seeds are produced sexually, however, and the evolution of pollen and seeds laid the basis for much of the diversification of plants. Many
Growth and Reproduction of Individuals 165
adaptations affect pollen transfer, including changes in the sizes, shapes, colors, and odors of flowers as well as in many pollen characteristics. These changes have led to some remarkable floral adaptations and cases of coevolution with specific pollinators, but most pollination in most species is probably by generalized animal taxa.
Plants have complex mating systems. The main causes of this complexity appear to be that inbreeding sometimes has important fitness consequences, that male and female floral functions may have different relative success rates, and that it is often beneficial to be able to adjust the relative allocation of resources to male and female functions.
The dispersal of fruits and seeds is affected by a large number of traits. Again, interactions with animals can be an important mode of dispersal, although plants must accomplish seed dispersal while avoiding seed consumption. Most seed dispersal is local, but rare long-dis- tance dispersal events can be demographically important. The seeds of some species remain dormant in the soil for many years. The movements of pollen, seeds, and fruits are key causes of spatial patterns in plant populations.
Additional Readings
Classic References
Darwin, C. 1877. The Various Contrivances by Which Orchids are Fertilized. John Murray, London.
Horn, H. S. 1971. The Adaptive Geometry of Trees. Princeton University Press, Princeton, NJ.
Contemporary Research
Barrett, S. C. H. and L. D. Harder. 1996. Ecology and evolution of plant mating. Trends Ecol. Evol. 11:73–79.
Schemske, D. W. and C. Horvitz. 1988. Plant-animal interactions and fruit production in a Neotropical herb: A path analysis. Ecology 69:1128–1137.
Waser, N. M., L. Chittka, M. V. Price, N. M. Williams and J. Ollerton. 1996. Generalization in pollination systems, and why it matters. Ecology 77:1043–1060.
Additional Resources
Charnov, E. 1982. The Theory of Sex Allocation. Princeton University Press,
Princeton, NJ.
Lanner, R. M. 1996. Made for Each Other: A Symbiosis of Birds and Pines. Oxford University Press, Oxford and New York.
Willson, M. F. 1983. Plant Reproductive Ecology. Wiley, Chichester, UK.
Willson, M. F. and N. Burley. 1983. Mate Choice in Plants: Tactics, Mechanisms, and Consequences. Princeton University Press, Princeton, NJ.
