
PAGE PROOF: 2ND PASS
C H A P T E 16R Communities in Landscapes
Communities exist as components of a larger landscape. The populations that make up a community are linked to populations of the same species in other communities by migration (see Chapter 5). Communities are
also linked by the movement of air, water, and nutrients (see Chapter 15). Landscape ecology is the study of these larger-scale relationships among communities. In this and the following chapter we will examine ecological patterns and processes that occur at the level of landscapes. In this chapter we explore methods for examining patterns of variation among communities within a landscape.
How can we quantify differences in community composition and structure? How are species distributed among communities? Can we relate these differences to variation in the environment? These types of questions and analyses are some of the oldest in plant ecology. Comparing communities across a landscape and looking for environmental causes of differences and similarities goes back to the origins of plant ecology at the end of the nineteenth century. While technological advances have enhanced ecologists’ ability to collect and analyze complex data to use in making these comparisons, many of the basic underlying questions and approaches have remained the same for the past 100 years.
Comparing Communities
The first question we might want to ask about the communities in a landscape is how different they are from one another. In Chapter 12 we described several ways of characterizing communities. Those characteristics can be compared among two or more communities. The techniques used for such comparisons depend on the nature of the variables being compared. If each community is described by a single characteristic (such as total biomass or mean canopy height), then univariate (single dependent variable) statistical procedures are used. In contrast, if each community is described by multiple parameters (such as a species list), then multivariate (multiple dependent variable) procedures are needed. The latter methods can be complex and have been the subject of much debate among ecologists.

318 Chapter 16 |
PAGE PROOF: 2ND PASS |
Table 16.1 Using the Jaccard index to measure the similarity of sites based on presence/absence data
(A) Typical presence/absence dataa |
|
|
(B) Reordered matrixb |
|
|
|
|
(C) Matrix of Jaccard similarity values |
||||||||||||
|
|
|
|
|
|
for the data in (A) or (B) |
|
|
||||||||||||
|
|
|
Sites |
|
|
|
|
|
|
|
Sites |
|
|
|
|
|
|
Sites |
|
|
Species |
A |
B |
C |
D |
E |
|
Species |
A |
B |
E |
D |
C |
|
Site |
A |
B |
C |
D |
E |
|
1 |
1 |
1 |
0 |
0 |
1 |
|
8 |
1 |
1 |
0 |
0 |
0 |
|
A |
1.00 |
0.57 |
0.33 |
0.13 |
0.13 |
|
2 |
1 |
1 |
1 |
0 |
0 |
|
1 |
1 |
1 |
1 |
0 |
0 |
|
B |
0.57 |
1.00 |
0.30 |
0.00 |
0.25 |
|
3 |
0 |
0 |
1 |
1 |
1 |
|
9 |
1 |
1 |
0 |
0 |
1 |
|
C |
0.33 |
0.30 |
1.00 |
0.57 |
0.22 |
|
4 |
0 |
0 |
1 |
1 |
0 |
|
2 |
1 |
1 |
0 |
0 |
1 |
|
D |
0.13 |
0.00 |
0.57 |
1.00 |
0.33 |
|
5 |
1 |
0 |
1 |
1 |
0 |
|
6 |
0 |
1 |
1 |
0 |
0 |
|
E |
0.13 |
0.25 |
0.22 |
0.33 |
1.00 |
|
6 |
0 |
1 |
0 |
0 |
1 |
|
10 |
0 |
1 |
0 |
0 |
1 |
|
|
|
|
|
|
|
|
7 |
0 |
0 |
1 |
1 |
1 |
|
5 |
1 |
0 |
0 |
1 |
1 |
|
|
|
|
|
|
|
|
8 |
1 |
1 |
0 |
0 |
0 |
|
3 |
0 |
0 |
1 |
1 |
1 |
|
|
|
|
|
|
|
|
9 |
1 |
1 |
1 |
0 |
0 |
|
7 |
0 |
0 |
1 |
1 |
1 |
|
|
|
|
|
|
|
|
10 |
0 |
1 |
1 |
0 |
0 |
|
4 |
0 |
0 |
0 |
1 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
aThe presence of a species in a site is indicated by a 1.
bA reordered matrix attempts to group sites that share species and species that share sites.
Non-numerical Techniques
While the methods used in plant community ecology have changed considerably in response to the availability of powerful computers, the questions asked have not. One such basic question is, which communities in a landscape are most similar to each other? Later in this chapter we will show how we can quantify the answer to this question. However, we can also address this question in a more qualitative way, using a method favored by European ecologists during the mid-twen- tieth century.
We might begin, for instance, with a survey of five sites that contain a total of ten species (Table 16.1A). The order of the sites and species in Table 16.1A is arbitrary. (Because this is a hypothetical example, we use numbers for the species, rather than names.) We can then try to change the order of the sites and species so that we group the sites that share the most species and group the species that are most often found together. This reordering is shown in Table 16.1B. The reordering has produced some patterns, with a group of species that are shared by sites A and B appearing in the upper left-hand corner and a group of species that are shared by C and D in the lower right-hand corner, while site E shares species with both groups. Species 9, 2, 10, and 5 are shared by both groups, while some of the species might be considered indicators of each group (8 and 4) since they each appear in just one group. With a much larger table showing many more sites and species, such patterns usually become even more apparent.
Using this sort of reordering method, plant community ecologists tried to discern patterns of commu-
nities across landscapes. Dissatisfaction was expressed with this method, however, because it is partially subjective. For example, site C shares more species with site A than site E shares with site A. So one might rearrange the columns in the table to place sites A and C next to each other. Also, we decided that there were two groups of sites, (A, B) and (C, D), even though there is considerable overlap in the species shared by sites in different groups. To avoid this subjectivity, ecologists developed a variety of quantitative techniques.
Univariate Techniques
Univariate techniques are used whenever a single type of measurement is made, such as biomass per unit area. We might determine biomass in ten quadrats within each of six communities. A typical question would be, “Do these communities differ in their average biomass per square meter?” There are many standard statistical techniques for analyzing such data, such as analysis of variance (ANOVA). A number of statistics textbooks describe these methods in detail (e.g., Sokal and Rohlf 1995; Zar 1999; Scheiner and Gurevitch 2001). Most often, though, we compare communities by measuring more than one variable.
Multivariate Techniques
All multivariate techniques rely on the same basic principles and approach. Each is concerned with comparing how different the members of a group of objects (such as communities) are, based on the values obtained for a set of characteristics measured for all of the objects. The mathematical techniques then arrange those objects in

PAGE PROOF: 2ND PASS
Communities in Landscapes 319
one or more dimensions, based on their differences in the entire set of characteristics taken together.
Let’s begin with a simple univariate (one-dimen- sional) example. Imagine that we wish to compare three plant communities (A, B and C) with mean biomasses of 500 g/m2, 725 g/m2 and 625 g/m2. We can put them in order based on their mean biomass (A C B). Another way of accomplishing the same goal is to first determine the difference between each pair of means (dAB = 225, dAC = 125, dBC = 100). Using this information, we determine that A and B are most different, or that the order is
(A C B). Note that dAC + dBC = dAB because there is only one way to put these numbers in order from smallest
to largest—they fall on a line. While the second method, using differences, seems more complicated than the first, it is actually what you do when you use ANOVA to determine whether groups differ from one another.
With two descriptive variables, the process becomes a bit more complicated, but the basic principles are the same. Let’s assume that we also measured mean foliage height for the three communities and found that the values are A = 3.2 m, B = 3.5 m, and C = 5.6 m. Instead of a single number, or scalar, giving the difference between two communities, we now need an ordered list of numbers, or vector (see Chapter 7), to give the difference. In this case, we can use that old standby, the Pythagorean theorem (x2 + y2 = z2), where x and y are the lengths of the sides of a right triangle and z is the length of the hypotenuse. The measure of the difference between each pair of communities is referred to as the Euclidean distance. If we let x represent mean biomass and y represent mean foliage height, then for communities A and B, x = 725 – 500, y = 3.5 – 3.2, and z = 225.0002 = dAB (Figure 16.1). Similarly, dAC = 125.0230 and dBC = 100.0220. Now the distances do not sum because the points no longer lie on a straight line. Typically units of measure are not used because the distances are a complex combination of the units of measure for each descriptive variable.
With only two dimensions, it is easy to graph and visualize our data (Figure 16.1). But what if we have many descriptive variables? Information on species presences and abundances constitutes just such a large set of descriptive variables. Think of the two axes in Figure 16.1 as representing the abundances of species 1 and species 2. Now, imagine that we add a third axis projecting out of the page, on which the abundance of species 3 is graphed. We might not be so readily able to imagine a fourth axis for the abundance of species 4. Typical data include, however, not just three or four, but tens to hundreds of species. Clearly, we cannot graph such a multidimensional object. Instead, we resort to various techniques that reduce the problem from n dimensions (n = the number of species) to the two or three dimensions that we can visualize.
|
6.0 |
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
5.5 |
|
|
|
|
|
|
(m) |
5.0 |
|
|
|
|
|
|
height |
|
|
|
|
|
|
|
|
|
dAC |
|
|
dBC |
|
|
foliage |
4.5 |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
Mean |
4.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.5 |
|
|
dAB |
|
|
B |
|
|
|
|
|
|
|
|
|
3.0 |
A |
|
|
|
|
|
|
500 |
550 |
600 |
650 |
700 |
750 |
|
|
450 |
||||||
|
|
|
Mean biomass (g/m2) |
|
|||
Figure 16.1
Three plant communities (A, B, and C) measured for biomass and foliage height. Euclidean distances between the points are given by dAB, dAC, and dBC.
The first step is to devise a distance measure. If the descriptive variables are related monotonically (meaning that the abundances of species 1 and species 2 always increase together, for instance, and when species 1 increases, species 3 always decreases), and also increase or decrease together linearly (when graphed against one another, the points fall on a straight line), then we can use the multivariate equivalent of the Euclidean distance. This measure is the Pearson product-moment correlation, which is the familiar correlation coefficient (see Appendix A). However, species abundances are usually not related in such a simple fashion.
Consider two species with different soil moisture requirements: species 1 does best in wet soils, while species 2 does best in partly moist soils. Going from very dry soils to partly moist soils, both species would increase in abundance. But going from partly moist soils to wet soils, species 2 would continue to increase in abundance, while species 1 would decline in abundance (Figure 16.2). The descriptive variables would no longer be related in a monotonic fashion. In addition, many of the descriptive variables used are not continuous. Data on the presence and absence of species, for example— the information contained in a species list—are dichotomous, consisting of just 1’s and 0’s (see Table 16.1A).
For these types of data, ecologists have devised a number of distance metrics called similarity measures. Consider first those that are used for presence/absence data. For a pair of sites, we can define four types of species: those that are present in both sites (a), those that are present in the first site but absent in the second (b),
320 Chapter 16 |
PAGE PROOF: 2ND PASS |
those that are present in the second site and absent in the first (c), and those that are absent in both (d). This pattern can be represented as follows:
|
|
First site |
Second site |
Present |
Absent |
|
|
|
Present |
a |
b |
Absent |
c |
d |
For sites A and B in Table 16.1, a = 4, b = 1, c = 2, and d = 3. The simplest, and oldest, measure of similarity, invented by the French ecologist P. Jaccard, is the Jaccard index (Jaccard 1901). It is the percentage of species con-
tained in two sites that are shared by those sites:
Sj = + a + a b c
For sites A and B, SJ = 4/(4 + 1 + 2) = 0.57.
Over the years a number of similarity measures have been invented for both presence/absence and abundance data (Table 16.2). While various indices work best in different situations and with different types of data, for presence/absence data the Jaccard index consistently performs the best, or close to the best, in the widest number of situations. Using the Jaccard index, we can now proceed to the second step, measuring the distance between each of our sites (Table 16.1C).
|
50 |
Trientalis latifolia |
|
Xerophyllum tenax |
|
|
|
Galium ambiguum |
|
40 |
Iris bracteata |
|
Viola lobata |
|
(%) |
|
|
|
Eriophyllum lanatum |
|
30 |
|
|
Frequency |
|
|
20 |
|
|
|
|
|
|
10 |
|
|
0 |
Dry |
|
Moist |
Figure 16.2
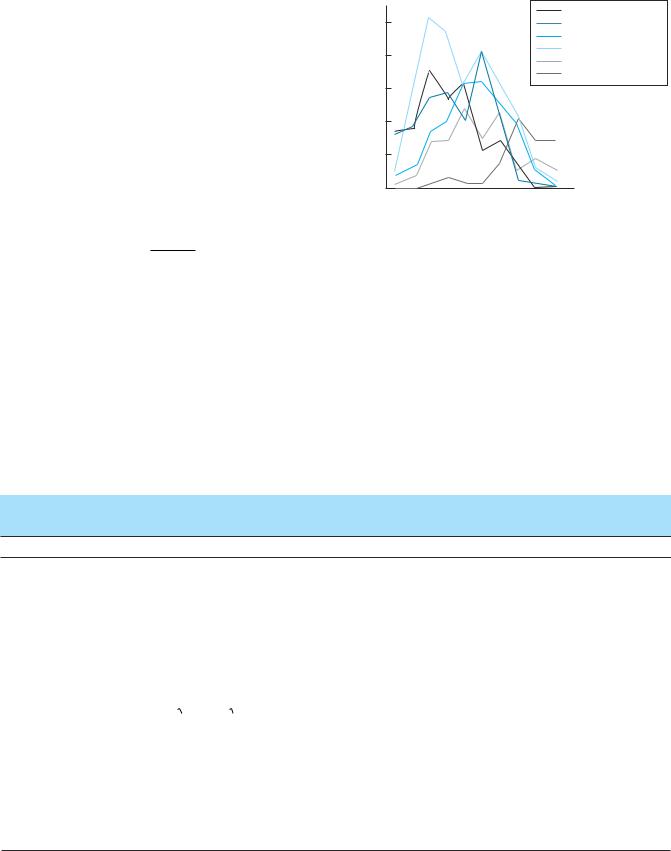
Patterns of species abundance along a moisture gradient in the Siskiyou Mountains, Oregon. As seen in this case, changes in the abundances of species along an environmental gradient are unlikely to be related monotonically. Species abundance was measured as the frequency of quadrats within a site in which a species appeared. (Data from Whittaker 1960; after Brown 1984.)
Landscape Patterns
Ordination: Describing Patterns
Ordination is the process of taking information such as that contained in Table 16.1C—points arrayed in an n-dimensional space—and reducing it to fewer dimen-
Table 16.2 Some similarity measures used by plant ecologists
Index |
Formula |
Index |
Formula |
Presence/absence indices
Jaccard index |
S J = |
|
|
|
a |
|
|
|
|
|
||
|
|
|
|
a + b + c |
|
|
|
|||||
Sørensen-Dice index |
SSD |
= |
|
2a |
|
|
|
|
|
|||
|
2a + b |
+ c |
||||||||||
|
|
|
|
|
|
|||||||
Simple matching |
SSM |
= |
|
|
a + d |
|
|
|
||||
|
a + b + c |
+ d |
||||||||||
|
|
|
|
|
|
|||||||
Ochioi index |
SO = |
|
|
|
|
|
a |
|||||
|
|
(a |
+ b) + (a + c) |
|||||||||
|
|
|
|
|
|
|||||||
Asymmetrical similarity |
SAS |
= |
|
b |
|
|
|
|
|
|
||
|
2a + b |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||
Definitions of symbols:
a= number of species in both sites
b= number of species in second site only
c= number of species in first site only
d= number of species in neither site
Abundance indices
Percentage of similarity
Asymmetrical percentage of similarity
Minimum similarity
Bray-Curtis index
Morisita’s index
SPS = ∑ | p1i − p2i | |
|
|
|
|||||||||
|
|
|
|
|
∑ |
(n1i − n2i) |
|
|
|
|||
SAPS |
= |
|
|
|
|
|
, for n1i ≠ 0 |
|||||
|
∑ |
n |
|
|
||||||||
|
|
|
|
|
1i + ∑ n2i |
|
|
|
||||
SMS = ∑ |
|
min(p1i, p2i) |
|
|
|
|||||||
SBC |
= |
∑ |
|
min(n1i, n2i) |
|
|
||||||
|
∑ |
n1i + ∑ n2i |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|||||
SM |
= |
|
|
|
2∑ |
(n1in2i ) |
λ |
j = |
∑ n ji (n ji −1) |
|||
|
|
|
|
|
|
, |
|
|||||
|
(λ 1 |
+λ |
|
N j (N j −1) |
||||||||
|
|
|
2 )N1N2 |
|
|
|||||||
p1i = the proportion of individuals in the ith species in sample 1 (p1i = n1i/N) n1i = the number of individuals of species i in sample 1
N = the total number of individuals sampled min(x,y) = the smaller of the two values
Note: These indices differ in the factors they emphasize (e.g., common versus rare species) and their robustness to deviations in assumptions (Whittaker 1972; Janson and Vegelius 1981; Wolda 1981; Austin and Belbin 1982; McCulloch 1985).

PAGE PROOF: 2ND PASS |
Communities in Landscapes 321 |
Figure 16.3
The principle of dimension reduction. The flashlight causes the balls, arrayed in three-dimensional space, to cast a two-dimensional shadow on the back wall.
Axis 3
sions; in other words, ordination is just a quantitative redescription of the data. Often the results are shown as a twoor three-dimensional graph. There are many ordination techniques, each of which has had its advocates. They are usually known by acronyms: PO (polar ordination), PCA (principal components analysis), PCoA (principal coordinates analysis), RA (reciprocal averaging), DCA (detrended correspondence analysis), NMDS (nonmetric multidimensional scaling). Each technique uses somewhat different mathematical approaches and has different assumptions, limitations, and advantages. While the techniques differ in their mathematical details, they all rely on the same basic principles, and they all provide broadly similar information. The distinctions between and details of these methods are beyond the scope of this book, but those who wish to explore them further will find the topic covered in depth by Legendre and Legendre (1998).
The process of dimension reduction—taking highly multivariate data and collapsing them into a small number of dimensions—can be visualized as follows. Imagine a physical representation of a three-dimensional data set like that shown in Figure 16.3, in which each axis shows the abundance of a species and each ball represents a different site. Now, we shine a flashlight through the model so that a shadow falls on one wall. Each ball (site) is now represented by a point on that wall. We have reduced the data from three dimensions to two. In the process we have lost some information. Two points that were actually far apart in the three-dimensional model may now appear to be close to each other because of the way their shadows fall. We can minimize this problem, though, by carefully choosing the angle at which we shine the flashlight. Most likely the balls are not arrayed
2 Axis
Axis 1
randomly throughout the model. Rather, they are probably arrayed in some more compact shape, such as an elongated ellipse. If we shine the flashlight so that it is at right angles to the long axis of the ellipse, then points that were the farthest apart in the original model are still far apart in the shadow.
Of course, with actual data, the points are arrayed in a much more complicated n-dimensional space, and the “flashlight” is a set of mathematical steps that determine the coordinates of those points in the reduced twoor three-dimensional space. Figure 16.4 shows two ordinations of the data from Table 16.1C using two different techniques; notice how both techniques produce the same overall picture of the relationship between the sites. Since ecologists are usually interested in broad patterns, rather than the exact numerical relationships between sites, the choice of distance metric and ordination technique is often a matter of convenience.
Multivariate statistical methods started to permeate plant community ecology in the mid-1950s, sometimes by importation of techniques being used in other fields and sometimes by independent invention. Perhaps the most influential work was the development of polar ordination by J. Roger Bray in the mid-1950s while he was a student of John Curtis (Bray 1955; Bray and Curtis 1957). Independently, David Goodall (1954) showed how factor analysis (now referred to as principal components analysis) could be applied to community surveys.
Robert Whittaker developed a general approach called direct gradient analysis, in which the ecologist chooses environmental axes and orders vegetation samples (stands) along those axes, examining the resulting patterns. Bray and Curtis adopted a different approach,
322 Chapter 16 |
PAGE PROOF: 2ND PASS |
(A) |
|
|
|
|
|
|
|
|
|
||||
component |
0.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
0.4 |
|
|
|
|
A |
|
|
C |
D |
||||
|
|
|
|
|
|
||||||||
principal |
|
|
|
|
|
|
|
|
|
|
|||
0.0 |
|
|
|
B |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|||
Second |
–0.4 |
|
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
–0.8 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
–0.8 |
|
–0.4 |
0.0 |
0.4 |
0.8 |
||||||
|
|
|
|
First principal component |
|||||||||
(B) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 16.4 |
|
0.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ordinations of the data in Table |
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
16.1C using two different ordination |
|
|
0.4 |
|
|
A |
|
|
|
|
|
|
|
|
|
|
techniques: (A) principal coordinates |
|
|
|
|
|
|
|
|
|
|
|
|
D |
analysis (PCoA), and (B) nonmetric |
||||
|
|
|
|
|
|
|
|
|
|
|
||||||
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
multidimensional scaling (NMDS). |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Dimension |
0.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notice that the two techniques pro- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
duce the same broad pattern of rela- |
||
|
–0.4 |
|
|
|
|
|
|
|
|
|
|
|
|
tionship between the sites. |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
–0.8 |
|
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
–1.2 |
–0.8 |
–0.4 |
0.0 |
0.4 |
0.8 |
1.2 |
|
||||||||
|
|
|
|
|
|
Dimension 1 |
|
|
|
|
|
|||||
called indirect gradient analysis, in which the stands are ordered by their similarity in species composition and the environmental factors responsible for the resulting patterns are inferred. The latter approach (a form of ordination) has the advantage of being more objective, but it cannot be used to examine patterns of vegetation along particular environmental axes that are known in advance to be of interest.
Ordination and related methods were further refined in the 1960s, 1970s, and 1980s by a number of ecologists, most notably Whittaker’s students and collaborators in the United States (e.g., Gauch and Whittaker 1972), Michael Austin in Australia (e.g., Austin 1977), and Cajo ter Braak in the Netherlands (e.g., ter Braak 1986). Over the same period, the development of multivariate techniques in systematics (under the heading of numerical taxonomy; Sneath and Sokal 1973) further influenced their use in ecology. Advances in computer hardware and software at this same time were particularly important in the spread of these techniques. During the 1960s and 1970s, using these methods required writing complex programs, making stacks of punch cards, and gaining access to a mainframe computer.
Today, these techniques are still commonly used, especially for pattern detection and hypothesis generation. A series of communities might be sampled to document relationships involving a particular ecological phenomenon—for example, nitrogen deposition, invasion by exotic species, or damage by a pathogen—with the object of relating the phenomenon to species compositions and environmental variables across communities. The patterns that are revealed can form the basis of experiments to test hypotheses about the mechanisms causing the patterns.
Determining Causes of Patterns
Ordination can describe patterns of species distribution among communities in a landscape. But what are the causes of those patterns? For plant communities, the
primary causes of differences in species composition are climatic, topographic, and edaphic factors—the physical factors that determine plant growing conditions (see Part I). A number of techniques exist for determining which of these factors are most important in a given set of communities. All of these techniques use basically the same strategy, looking for correlations between species distributions and environmental variables.
Consider a simple problem, that of determining the causes of variation in the abundance of a particular species across a landscape. One strategy for attacking this problem is to sample a number of sites across the landscape. At each site we measure the abundance of the species as well as a number of environmental variables, such as temperature, precipitation, elevation, slope, aspect, soil texture, and various soil nutrients. (Ideally, the climatic data would be based on long-term averages.) Then we calculate the correlation of each environmental variable with species abundance. This procedure contains two unstated assumptions: (1) that the pattern of species distribution is based on a long-term equilibrium with the environment, and that the current pattern is close to that equilibrium, and (2) that we have measured the correct environmental variables.
Of course, correlation does not demonstrate causation, but a strong correlation provides one piece of supporting evidence for a possible cause of the observed pattern. The method just described has a problem, however: the environmental variables are themselves correlated with one another. Sites at higher elevations, for example, are also likely to have lower temperatures. To account for these correlations, we use a technique called multiple regression. This technique determines the regression (Appendix A) of the abundance of our species with each environmental variable while correcting for correlations among the environmental variables themselves. The limitation of this technique is that it cannot correct for very large correlations. For example, if the abundance of our species decreased with increasing ele-

PAGE PROOF: 2ND PASS
(A)
FPO
vation and temperature also decreased with increasing elevation, then we could not determine whether the cause of decreasing abundance was elevation or temperature. However, our analysis would have narrowed the possible factors responsible for decreasing abundance, although the possibility would remain that the actual cause was some unmeasured third variable that is also correlated with elevation and temperature.
We could also perform manipulative experiments (e.g., growing the plant in growth chambers at different temperatures) to further narrow the possible causes and to test specific hypotheses. While such experiments are an important source of information, they also have limitations. Because they are often done in highly artificial environments such as a growth chamber or greenhouse, they cannot account for the myriad of abiotic and biotic factors and their interactions in nature. Competitive interactions, for example, might differ at high and low temperatures. Manipulative experiments done in a field setting are technically more challenging, but can provide a more realistic test of the importance of an environmental factor.
We can do the same sort of analysis for whole communities as was just described for a single species. Now, instead of using species abundance, we use position along an ordination axis as our dependent variable. We can see how this works with data from a study of Pinus contorta (lodgepole pine, Pinaceae) forests in the Canadian Rockies. Lodgepole pine is the most widely distributed tree in western North America (Figure 16.5A). In Alberta, Canada, where this study took place, it is the dominant species following fire in mid-elevation forests (1000–2000 m). For this study, 63 stands were sampled in Banff and Jasper National Parks; measurements included the number and sizes of all trees, cover of all
Communities in Landscapes 323
Figure 16.5
(A) Stands of Pinus contorta (lodgepole pine, Pinaceae) dominate the landscape in the middle elevations of the Canadian Rockies. The cones open in response to fire, and the result is stands like this one near Allison pass. (Photograph © John Worrall.) (B) Direct gradient analysis of P. contorta stands in the Canadian Rockies. The stands are arrayed along two gradients: moisture and elevation. Green numbers indicate stands in Banff National Park. Black numbers indicate stands in Jasper National Park. (After La Roi and Hnatiuk 1980.)
(B)
|
1800 |
|
|
|
|
|
|
|
|
|
|
|
|
|
61 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
1600 |
|
|
|
|
|
|
|
|
|
|
|
|
|
62 |
63 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(m) |
|
|
|
|
|
|
|
|
|
|
|
|
|
52 54 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Elevation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
1400 |
|
|
|
|
|
|
|
|
|
|
51 |
36 |
|
|
|
|||
|
|
|
|
|
|
|
|
|
57 |
|
59 |
50 |
47 |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
46 |
|
|
|
|
49 |
|
|
|
|
|
|
|
|
|
|
|
|
40 |
29 |
|
48 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
45 |
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
23 |
58 |
|
|
|
||||
|
|
|
|
|
|
|
27 |
|
|
35 |
|
|
|
|||||
|
1200 |
|
|
|
|
|
|
|
42 38 |
2841 |
|
|
|
|||||
|
|
|
18 |
|
1653 |
|
|
31 |
2244 |
|
|
|
||||||
|
|
|
|
|
|
|
33 |
|
34 |
21 |
39 |
37 |
|
|
|
|||
|
|
|
|
|
|
|
1930 |
|
56 20 |
|
|
|
||||||
|
|
4 |
24 |
257 |
8 |
9 10 |
17 |
|
|
|
43 |
|
|
|
|
|||
|
|
|
|
5 |
|
6 |
26 |
|
|
|
|
32 |
|
|
|
|||
|
|
3 |
1314 |
|
11 55 |
|
|
|
|
|
|
|
||||||
|
|
1 |
12 |
|
15 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
1000 |
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.0 |
|
|
|
2.5 |
|
|
|
|
|
|
3.0 |
3.5 |
|||||
|
Drier |
|
|
|
|
Moisture index |
Wetter |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||
understory species, soil chemistry, soil temperature, soil moisture, elevation, slope, and aspect.
Figure 16.5B shows data from these stands arrayed along two environmental axes, elevation and moisture. Because each axis consists of a single environmental variable, this graph is a form of direct gradient analysis. If each axis consisted of combinations of multiple environmental variables, we would refer to this method as canonical correspondence analysis (CCA; ter Braak 1986). In effect, canonical analysis is a hybrid between

324 Chapter 16 |
PAGE PROOF: 2ND PASS |
direct and indirect gradient analysis. Two ordinations are carried out, one on the species data and one on the environmental data, and then the correlations between the axes of the two ordination sets are determined. If we are lucky, the first one or two axes of each set will be highly correlated with each other. An analysis of macrophytes growing on dikes in the Netherlands provides an example of this type of analysis (Figure 16.6). As with ordination, various distance metrics, dimension reduction techniques, and correlation procedures can be used (Ludwig and Reynolds 1985).
Types of Data
We have described analyses that can use either presence/absence data or abundance data. Which type of data is most appropriate? The answer depends on the questions being addressed relative to the scale of variation. Different kinds of variation are expected to occur at different scales. By “scale” we do not mean geographic extent; rather, we mean ecological scale—how different the communities are ecologically. A set of neighboring old fields all abandoned within the past five years would represent small-scale ecological differences. All of the fields would have similar soil conditions and species composition. Differences among the fields would most likely show up as differences in species abundances rather than differences in species presences. In contrast, if those fields were included in an analysis with surrounding forest patches, or with fields abandoned decades ago, then we would have a much larger ecological scale. Now species presences might capture the differences among communities. Geographic extent is not synonymous with ecological scale, but the larger the geographic extent, the more likely that a wider range of ecological conditions will be captured in the analysis.
At larger scales, presence/absence data may be more informative than abundance data because of the sig- nal-to-noise problem. At large scales, we are usually trying to find the one or few causes of differences among very different types of communities. But abundances can vary for a number of reasons having to do with random demographic factors (see Chapters 7 and 14). While this variation may be of interest in relation to other questions, in this instance it acts as “noise” (random variation) in the analysis, obscuring the main pattern. By using presences and absences instead of abundances, we smooth out this noise. However, if we use presence/absence data, it is important that we include as many species as possible, especially rare species. The distribution of each species contains information about the environment and its effects on the plant community. The more species we include in the analysis, the more information we have. There is a redundancy to this information, so that the more species we use, the more confident we can be about our conclusions. A good rule of thumb is that in
Chloride |
|
ions |
|
Peat |
|
|
Electrical |
|
conductivity |
|
Phosphate |
Clay |
concentration |
Sand |
|
Figure 16.6
Canonical correspondence analysis of macrophytes growing on dikes in the Netherlands. The points show the average position of each species along the vegetation axes. The first vegetation axis is highly correlated with electrical conductivity (r = 0.83) and phosphate concentration (r = 0.86), while the second vegetation axis is correlated with chloride concentration (r = 0.86) and to a lesser extent with the amount of peat (r = 0.49) and sand (r = –0.40) in the soil. These correlations are indicated by how closely the environmental variables (arrows) line up with the vegetation axes. (After ter Braak 1986.)
any ordination, the number of species used should be at least three or four times the number of sites.
Classification
One use of landscape analysis is the classification of communities into larger groupings (Table 16.3). This classification can then be used for landscape management purposes. For example, if we wished to preserve particular types of communities, our classification scheme could help us choose which places to preserve to meet this goal. Classification techniques are complementary to those of ordination. In effect, what we are doing is taking the pattern revealed by the ordination and drawing circles around subsets of the points. For example, in Figure 16.4, we might put sites A and B into one group, sites C and D into another group, and site E into a third group. We could even construct larger groups from these smaller ones. Just because we have created groupings does not mean that the communities form discrete types; our different groups might gradually blend into one another. Yet, for planning purposes, defining discrete community types would still be useful.

PAGE PROOF: 2ND PASS
Communities in Landscapes 325
Table 16.3 An example of the classification of a North American plant community
Physiognomic categories
Class . . . . . . . . . . Woodlands
Subclass . . . . . . . Mainly evergreen woodlands
Group . . . . . . . . . Evergreen needle-leaved woodlands Subgroup . . . . . . Natural/seminatural
Formation . . . . . Evergreen coniferous woodlands with rounded crowns
Floristic categories
Alliance . . . . . . . . . . .Juniperus occidentalis
Association . . . . . . . .Juniperus occidentalis/Artemisia tridentata
Note: This classification follows the National Vegetation Classification system proposed by the Ecological Society of America. The classification uses a dual system in which higher categories are based on physiognomic criteria and finer-level categories are based on floristic criteria.
A variety of methods can be used to create groups. The two major types of classification techniques are mono- thetic-divisive and polythetic-agglomerative. A mono- thetic-divisive analysis begins by considering all of the sites and dividing them into two groups depending on the presence or absence of a single key species. Each of the new groups is further divided based on new key species contained within each. The process is continued until we have reached the desired number of groups, a set of groups that seems natural and useful, or until division is no longer possible. Thus, “monothetic” refers to the use of a single species as the criterion at each step, and “divisive” refers to the dividing process.
A polythetic-agglomerative analysis works in the opposite direction. Using some distance metric (such as the Jaccard index), the two most similar sites are grouped togther. Then the next two most similar sites are grouped together, and so on. The grouping may involve two individual sites, a single site and a previously created group, or two groups. The process stops when all sites have been grouped together. Thus, “polythetic” refers to the use of multiple species in the distance metric, and “agglomerative” refers to the joining process. Various distance metrics and joining algorithms can be used. The result is a tree diagram (Figure 16.7). As with the monothetic-divisive approach, this diagram
Distance between cluster centroids
11
6
5
4 |
CLUSTER |
1 |
|
|
|
2 |
3 |
4 |
|||
3 |
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
0 |
1 6 |
26 |
2 3 4 9 |
53 60 24 25 10 13 15 12 14 |
5 8 |
11 30 |
7 |
29 18 27 22 23 17 41 43 55 38 39 42 16 34 19 21 31 20 40 56 28 44 36 59 58 47 51 61 62 49 52 50 33 45 46 35 37 54 57 32 63 48 |
|||
|
|||||||||||
Pinus contorta forests of Banff and Jasper
Polythetic-agglomerative cluster analysis
Figure 16.7
A tree diagram developed by a cluster analysis using a polythetic-agglomerative method for the Pinus contorta stands in Figure 16.5. (After La Roi and Hnatiuk 1980.)

326 Chapter 16
|
1800 |
|
|
|
|
|
|
|
|
|
|
61 |
|
|
1600 |
Ledum/Vaccinium |
|
|
||
|
|
|
|
63 |
|
|
|
|
|
|
|
|
|
(m) |
|
|
|
52 54 |
|
|
Elevation |
|
|
|
|
||
1400 |
Menziesia/Vaccinium |
47 |
|
|||
|
|
|||||
|
|
|
|
|
49 |
|
|
|
|
|
48 |
|
|
|
|
|
|
40 |
|
|
|
Shepherdia/Elymus |
42 |
58 |
|
||
|
1200 |
|
16 |
41 |
|
|
|
18 |
21 39 |
|
|||
|
|
|
Alnus/Linnaea |
|
||
|
|
8 |
9 |
17 |
|
|
|
|
5 |
|
|
|
|
|
2 |
Shepherdia/Arctostaphylos |
|
|||
|
1000 |
|
|
|
|
|
|
2.0 |
|
2.5 |
|
3.0 |
3.5 |
|
Drier |
|
|
Moisture index |
Wetter |
|
|
|
|
|
|
||
Figure 16.8
Classification of the Pinus contorta stands in Jasper National Park graphed in Figure 16.5, using a combination of techniques, including the cluster analysis shown in Figure 16.7 and indicator species analysis. The clusters are named by the dominate species. The number of clusters differs from that in Figure 16.7 based on the additional analyses. (After La Roi and Hnatiuk 1980.)
is used to devise some natural and useful classification scheme (Figure 16.8).
A useful adjunct to classification is the designation of indicator species. An ideal indicator species is found in all communities of a given type and not in any other community type. As with other procedures, there are various methods for picking indicator species (Dufrêne and Legendre 1997). The use of indicator species makes classification procedures much easier. An analysis of a landscape might proceed along the following lines. First, one would pick a number of representative sites in the landscape and do a complete species survey of each. Second, one would classify the sites and identify indicator species. Third, one would survey the rest of the landscape, looking just for the indicator species. By just looking for a much smaller number of species, many more sites could be surveyed in the same amount of time and effort. This variety of methods leads to three different approaches to classifying landscapes. The first approach relies on using all species. Polythetic agglomerative methods are typical of this approach and are a hallmark of the floristic-sociological school of central Europe. The second approach relies on using dominant or indicator species. Monothetic-divisive methods are typical of this
PAGE PROOF: 2ND PASS
approach and have been used primarily by Russian plant ecologists. The third approach relies on physiognomy rather than species identity. In this approach, all broad-leaved deciduous forests would be classified together, even if they shared no species in common. It is also possible to combine these approaches. The current National Vegetation Classification scheme for classification of North American vegetation (Table 16.3) combines aspects of the second and third approaches.
Which approach is most appropriate depends on the scale of classification and its purpose. At very broad continental or global scales, physiognomy-based classifications are the most useful because we are often looking for commonalities among communities that transcend species identities. Our discussion of global biomes in Chapter 19 uses this type of system. In contrast, at local scales, we often are interested in how closely-related species sort themselves into communities, so either of the other approaches would be useful. We may, on the other hand, wish to emphasize continuity among samples. If that is the case, ordination is most appropriate.
Remote sensing is the process of collecting data about an entity of interest without being in direct contact with that entity. It is especially useful for gathering and analyzing ecological information on a large scale, as in classifying vegetation over large areas. Data collection is done using airplanes or satellites, as in the case of the popular Landsat Thematic Mapper. A landscape is captured in an image that is divided into a grid. The most common grid size is 30 × 30 m2, although with newer technology 1 × 1 m2 grids are now available. Each grid square is called a pixel, like the pixels of a computer image. The data consist of the spectral (light wave) properties of each pixel (Box 16A). Different satelliteor air- plane-borne cameras record different numbers of wavelengths, from three or four to hundreds.
Classification using remote sensing proceeds by grouping pixels with similar spectral properties into a single category. There are two basic approaches. Unsupervised classification separates pixels into a userdefined number of classes based on statistical similarities between the pixels. This method is similar to the classification methods described previously. In this case, however, instead of each pixel (site) being characterized by the presence or abundance of a set of species, each pixel is characterized by the presence or intensity of a set of light waves. In contrast, supervised classification requires the groups to be defined explicitly by the user. Specific locations in the image (training regions) are characterized using ground-based knowledge. For instance, one begins by surveying a series of sites, which are then classified into groups using the techniques described above. Those sites are also mapped using a Global Positioning System (GPS) and located on the remotely sensed image. These sites then serve as the
