
PAGE PROOF: 2ND PASS
C H A P T E R 4 Soils, Mineral Nutrition,
and Belowground Interactions
Most terrestrial plants are rooted in the soil and depend on it for support, water, and mineral nutrients. What is soil, and how does it affect plants? What are the characteristics and properties of soils? How is
soil created, and how long does it take for soil to be made? What are some of the differences among soils in different places?
In this chapter, we look down and examine some of the surprisingly complex things going on right under our feet. We look first at the characteristics and properties of soil, and will see how those properties affect the availability of water to plants. Since plants depend on soil for mineral nutrients, we examine what these minerals are and how the plant uses them. We also introduce the ecologically important underground interactions between plants and two other group of organisms: nitrogen-fixing bacteria and mycorrhizal fungi.
Soil Composition and Structure
You might think of soil as “dirt,” a sort of ground-up mixture of sand, dust, and crumbled bits of rock. If so, your image of soil would be very far from reality. Soil is a complex, often highly structured system that is a unique product of the interaction between living organisms and a physical matrix. Because soils are formed by the interaction among living things, rocks, air, water, and other materials, they occur only on Earth and nowhere else in our Solar System (at least as far as we know!).
What materials are found in soil, and what is it made of? To begin with, there are mineral particles, derived from rock. These particles can range from very large ones—stones and boulders—to, in progressively smaller sizes, pebbles, gravel, sand particles (>2.0–0.02 mm diameter), silt particles (0.02–0.002 mm), and clay particles (< 0.002 mm). (Conventionally, only sand, silt, and clay are considered to be part of the soil itself.) Besides these rock-derived particles, there is organic matter in varying states of decomposition. Air and water containing dissolved minerals are found in the pores between the mineral particles.
Finally, soils are, to one extent or another, the major environment of various kinds of living things: fungi, bacteria, photosynthetic prokaryotes (such as cyanobacteria), single-celled eukaryotes such as diatoms, protists, visible and microscopic insects and other arthropods (see Chapter 15), and many other kinds of small (slugs, earthworms, nematodes) and larger (groundhogs,

64 Chapter 4 |
PAGE PROOF: 2ND PASS |
gophers) animals. And, of course, soils are filled with the roots and rhizomes of plants. The living things in the soil continually alter its physical and chemical properties. Plants, for example, take up large volumes of water from the soil, changing the amount and distribution of water within the soil. The various soil organisms act and interact in a wide variety of ways. Some benefit one another; others consume one another or defend themselves to avoid being consumed. In the process of metabolism, soil organisms respire, altering the chemistry of their environment, and produce waste products and other substances, which become part of the soil. These activities affect plants in many ways, from making nutrients available to causing disease. We examine some of these effects in this chapter.
Soil Texture
The properties of soil, and its effects on plants, depend, first, on soil texture: the relative proportions of the different particles making up the soil. Soils are composed of sand, silt, and clay particles. Depending on which particle size dominates the character of a soil, the soil texture is categorized as sandy, silty, clayey, or loamy (Figure 4.1). The properties of loamy soils are a balance between sand, silt, and clay particles, and are generally considered the most desirable soils for agriculture. Sandy soils, with more than about 50% sand particles, have a coarse texture. These soils drain rapidly after a rain, and they hold water and minerals poorly. Water and air penetrate sandy soils easily. Because of these characteristics, they warm readily in spring and cool quickly in autumn.
Clay particles have very distinctive properties, and even a soil with as little as 35–40% clay will exhibit those properties (a little clay goes a long way). Clayey soils can hold a large volume of water, and they retain water and minerals exceptionally well. Similarly, clay-dominated soils retain pesticides, pollutants, and other substances. They are much less permeable to air and water than are sandy soils, which can result in puddling, greater runoff, poor drainage, and poor aeration (due to water filling up the pore spaces and excluding air). As a consequence, clayey soils are very slow to warm up in spring and cool more slowly in
(A) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
fpo |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Composition (%) |
|
|
|
Soil type |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sand |
|
|
|
Silt |
|
Clay |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92 |
|
|
|
|
|
|
|
|
|
|
|
|
4 |
4 |
|
Sandy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
85 |
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
5 |
|
Loamy sand |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
45 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40 |
|
|
|
15 |
|
|
Loam |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60 |
|
|
|
|
|
|
|
|
20 |
|
|
|
Silt loam |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
28 |
|
|
|
|
|
|
|
|
|
|
|
|
|
37 |
|
|
|
|
|
|
|
35 |
|
|
|
|
|
Clay loam |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25 |
|
|
|
|
|
|
|
|
|
30 |
|
|
|
|
|
|
|
|
|
|
45 |
|
|
|
|
|
|
Clay |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
10 |
|
|
20 |
|
|
30 |
|
|
40 |
50 |
|
60 |
70 |
80 |
90 |
|
100 |
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Percent composition by dry weight |
|
|
|
|
|
|
|
||||||||||||||||||||
(B) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Clay |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
fpo |
|
|
|
|
|
|
|
|
|
|
|
|
90 |
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
80 |
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
(%) |
60 |
|
70 |
|
|
|
|
|
|
|
Clay |
|
|
|
30 |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40 |
Silt |
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
content |
50 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
50 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
content |
|
|
||||
|
|
|
|
|
|
|
Clay |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silty |
|
|
|
(%) |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
40 |
|
Sandy |
|
|
|
|
|
|
|
|
|
|
clay |
60 |
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
clay |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silty clay |
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Clay loam |
|
|
|
|
|||||||||||
|
|
|
30 |
|
|
Sandy clay |
|
|
|
|
|
|
|
|
|
|
loam |
|
|
|
70 |
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
loam |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loam |
|
|
|
Silt loam |
80 |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
Loamy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
10 |
|
|
|
|
Sandy loam |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
90 |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
Sand |
|
sand |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Silt |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100% |
||||||||
100% |
90 |
|
|
80 |
70 |
|
|
|
60 |
|
|
50 |
|
40 |
30 |
20 |
|
10 |
0 |
Silt |
|||||||||||||||||||
Sand
Sand content (%)
Figure 4.1
(A) Examples of soil particle size of several different soil types. (B) The soil texture triangle, which shows how soil types are classified according to the percentage of sand, silt, and clay they contain (by dry weight). The values for clay are drawn parallel to the sand side of the triangle, those for silt are parallel to the clay side, and those for sand are parallel to the silt side. The points on the grid at which the three lines intersect defines the type of soil; for example, the grid lines for a soil with 20% clay, 40% sand, and 40% silt intersect in the region that shows a soil with this particular composition is loam.

PAGE PROOF: 2ND PASS |
Soils, Mineral Nutrition, and Belowground Interactions 65 |
|
(A) |
Figure 4.2 |
|
|
(A) Sand grains are irregular in size and shape, and are composed largely of quartz |
|
|
with some other secondary minerals. Silt particles are similar to sand grains in min- |
|
|
eral composition and shape, but are smaller in size. (After Buckman and Brady 1969.) |
|
|
(B) Clay particles have a distinctive crystalline structure. Three different kinds of clay |
|
|
particles are shown here. Of the three, montmorillonite has the greatest cation |
|
|
exchange capacity (ability to hold cations) because in addition to binding cations on |
|
|
its surface, it has a large exchange surface available between its plates. Contrast the |
|
|
highly structured form of these clay particles with the rather formless sand and silt |
|
|
particles in part A. The clay particles are also far smaller. (After Etherington 1982.) |
|
(B)Kaolinite
Si tetrahedra
No water between lattice layers
7.2Å |
Al octahedra |
Si tetrahedra
Montmorillonite
Si tetrahedra
Water and exchangeable cations between lattice layers
Si tetrahedra
14Å
Al octahedra (Isomorphous Mg)
Si tetrahedra
Illite
Si tetrahedra
Potassium ions between lattice layers
Si tetrahedra
10Å
Al octahedra (Isomorphous Fe, Mg)
Si tetrahedra
autumn; plant growth in these soils may be slow to get started early in the growing season. Silty soils tend to be intermediate in their characteristics and properties between sandy and clayey soils. Because soils often possess characteristics of more than one of these texture classes, they may be described by a combination of these terms, such as sandy loam, silty clay loam, or sandy clay.
To understand why different soil textures have the properties they do, we need to look more closely at the soil particles themselves. Sand and silt particles are generally irregular in shape, ranging from somewhat rectangular or blocky to chunky, spherical shapes (Figure 4.2A). In contrast, clay particles (Figure 4.2B) have a much more specific structure. These particles are made up of plates, whose size, shape, and arrangement depends on the minerals they contain and the conditions under which they were formed. A clay particle is made up of two or three flat crystalline plates, layered or laminated together. These particles can be hexagonal in shape with distinct edges, or they may form irregularly shaped flakes or even rods. Soils may also contain particles similar in size to clays, but without the distinctive crystalline structure of clay particles. For example, an amorphous material called allophane, with particles about the same tiny size as clay particles, is prevalent in soils developed from volcanic ash.
Most of the clay particles in soil exist in a colloidal state (sand and silt particles are much too large to form colloids). In a colloid, one or more materials in a finely divided state are suspended or dispersed throughout a second material. (Other examples of colloids are gelatin, fog, cytoplasm, blood, milk, and rubber.) Clay particles have an enormous amount of external surface area because they have a large surface-to-volume ratio and because there are so many of them in a given volume of soil. In some kinds of clays, there is additional internal surface area between the plates (see Figure 4.2B). Thus, the tremendous surface area that characterizes clays is due to the fineness of the particles and to their plate-like structure. The external surface area of a gram of fine clay is at least 1000 times that of a gram of coarse sand; the total surface area in the clay present in the
66 Chapter 4
top 10 cm of soil in less than a half hectare of clayey soil, if spread out, would cover the continental United States.
Because sand particles have a low surface-to-volume ratio, sandy soils have large, open pores between the mineral particles. Water drains easily from these pores because there is nothing to hold the water against the pull of gravity, and air penetrates them readily. Clay-domi- nated soils have a much larger number of pores than do sand-dominated soils. The total amount of pore space— the total proportion of the soil occupied by air and water—is greater in clayey soils (50–60%) than in sandy soils (35–50%), not only because of the smaller size of clay particles, but because of their arrangement. Clay particles (as well as particles of organic matter) tend to cluster together, forming porous aggregates, resulting in much more pore space than in sands, in which the particles lie close together. (Soils with native vegetation often have greater pore space than those that have been cultivated for crops because tilling damages some of the soil structure.)
The greater pore space is one factor contributing to the greater amount of water that can be held in claydominated soils, but there is another factor that is also very important. Unlike sand and silt particles, clay particles typically bear a strong negative electrochemical charge. Thus, they act as anions in the soil, attracting cations (positively charged ions, including the major plant nutrients), as well as water molecules, to their surfaces. The role of clay particles in adsorbing nutrient cations—attracting and holding them to their surface— is one of their most important effects for plants. Although there are many kinds of cations that are attracted to clay particles, certain ones are most prominent and most important for plant growth. In humid regions, hydrogen and calcium ions (H+ and Ca2+) are usually most abundant, followed by magnesium (Mg2+), potassium (K+), and sodium (Na+) ions. In soils of arid regions, hydrogen ions move to last place in this list, and sodium ions become more important. These positively charged ions attract numerous molecules of water to their surfaces, adding to the overall capacity of the clay particles to retain moisture in the soil.
The adsorbed cations on clay particles are partially available to be taken up by plants. There is a continuous, dynamic interaction between the ions adsorbed on clay and other colloidal particles and those in the soil solution— the water in the soil and its associated dissolved minerals. Ions that are displaced from their positions on the surface of a particle enter the soil solution, from which they can be taken up by plants, leached (lost from the surface soil as water drains), or adsorbed on another particle. These reactions differ greatly among soils of different origins, textures, and chemical compositions, and among regions differing in temperature and especially in rainfall.
PAGE PROOF: 2ND PASS
Soil pH
The pH of soil—the negative logarithm of the concentration of H+ ions in the soil solution—varies widely among different soils. The pH scale ranges from 1 to 14, where 7.0 is neutral (the pH of pure water); acid soils have lower pH numbers and higher H+ ion concentrations. Soils in the United States can range from less than 3.5 (for example, in the pine barrens of New Jersey) to as high as 10 (for example, in arid grasslands of the southwestern United States). For a soil to have a pH above 7 (neutral soil), it must be calcareous (containing CaCO3), sodic (containing Na2CO3), or dolomitic (containing CaCO3 • MgCO3). Most crops grow best in slightly acidic soils, but native vegetation can be adapted to anything from very acid to neutral to alkaline soils.
Soil pH has enormous effects on plant growth, and indeed, in determining what species can survive and grow in the soil. It acts on plants indirectly, however, through its strong effects on the availability of mineral nutrients and on the activity of some soil organisms (such as bacteria and fungi), changing the conditions for plant growth in a complex manner. Forest trees can grow over a range of soil pH, but are especially tolerant of acid soils. Conifers and some other tree species tend to increase the acidity of the soil in which they are growing, primarily through the properties of the litter they produce (shed needles, etc.). Grasslands tend to be found on relatively alkaline (high pH) soils, but this may be an indirect effect because low rainfall results in soils with high pH (as we will see below) and also favors grassdominated vegetation. There are other characteristic associations between soil pH and plants. Species in the Ericaceae, for example, such as heaths and blueberries, tend to grow only in very acid soils, while other taxa, such as Larrea tridentata (creosote bush, Zygophyllaceae), are typically found in alkaline soils.
Soil pH can strongly affect the availability of cations to plants. Cations bind loosely to clay particles, which are generally negatively charged. Under acid conditions, the excess H+ ions tend to bind more strongly to the clay particles than do the nutrient ions, displacing these nutrients into the soil solution. Thus mild acidity, which is characteristic of many soils, promotes nutrient availability. Under extreme acidity (whether natural or due to acid precipitation), however, the nutrient cations are so mobile that they are easily leached, and are carried off in groundwater—water found underground in aquifers, rock crevices, and so on.
What determines the pH of a soil? Two cations, hydrogen and aluminum, tend to increase soil acidity, while the other cations have the opposite effect, increasing soil alkalinity. Hydrogen ions are continually added to the soil by decaying organic matter, roots, and various soil organisms. They are actively exchanged between the surfaces of colloidal particles in the soil

PAGE PROOF: 2ND PASS Soils, Mineral Nutrition, and Belowground Interactions 67
(such as clay and organic matter) and the soil solution, contributing directly to the acidity of the soil. Aluminum ions (Al3+) indirectly cause hydrogen ions to be released from colloidal particles by reacting with water to form Al(OH)2+ and Al(OH)2+ plus H+. These hydrogen ions are then added to the H+ ions in the soil solution. High rainfall levels favor the predominance of aluminum and hydrogen ions because these ions are held very strongly by colloidal particles, while the other cations are more readily leached and thus lost from the soil.
Most of the other cations, called exchangeable bases, contribute to making the soil more alkaline. The cation bold exchange capacity (CEC) of a soil is a measure of the total ability of the soil colloids to adsorb cations (in units of centimoles of positive charge per kilogram of dry soil [cmolc/kg]), and the percentage base saturation is the proportion of the CEC that is occupied by exchangeable bases (Figure 4.3). In arid regions, the bases are not leached out of the soil by rainfall, so the percentage base saturation is very high (90–100%), H+ concentration is low, and the soils tend to be alkaline. In areas with higher rainfall, the bases are leached more easily while H+ and Al3+ ions are retained, so the percentage base saturation
is much lower (50–70%), and the soils tend to be acid. Plants and soil organisms are an important source
of soil acidity. When roots or soil organisms respire, they generate CO2. In wet soils, this CO2 goes into solution immediately, creating a weak acid, carbonic acid. This is also the reason why rainwater is naturally a bit acid: CO2 from the atmosphere dissolves into the raindrops. In tropical rainforests, where conditions favor massive amounts of respiration and there are often few clay particles to bind cations, this added CO2 can help make the soils quite acid and promotes the mobility of cations. The consequence is rapid uptake of nutrients by plants in undisturbed forests, and rapid loss of those nutrients when forests are cleared.
Horizons and Profiles
Soils are not homogenous; they contain characteristic layers, or horizons, that differ from one soil type to another. If you look at roadcuts or excavations dug for construction, you can often see the horizons quite clearly. The sequence of horizons that characterizes a soil is called the soil profile (Figure 4.4). The horizons are grouped under four categories: O, A, B, and C. Subcategories of these four categories are numbered according to their particular characteristics (Figure 4.5).
The O horizons consist of organic material formed above the mineral soil, derived from decaying plant materials, microbial matter, and the remains and waste products of animals. The A horizons—the surface layer of mineral soil— represent the region of maximum leaching, or eluviation. The uppermost A horizon, the A1, is often darker than the rest of the soil and may contain
|
|
Clay loam |
|
|
|
Clay loam |
|||
|
|
|
|
|
|
|
+ lime |
||
CEC = 20 cmolc/kg |
|
CEC = 20 cmolc/kg |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
H+ |
|
||
|
|
|
|
|
|
|
|||
10 |
H+and |
|
|
|
|
|
|
||
|
|
|
|
|
|
||||
|
|
Al3+ |
|
|
|
|
|
|
|
|
|
|
Sandy loam |
|
|
CEC = 10 cmolc/kg |
|
|
16 |
2 |
H+ |
|
Bases |
|
|
10 |
Bases |
8 |
Bases |
|
|||
|
|
||
|
|
|
|
Base saturation = 50% |
80% |
80% |
|
pH = 5.5 |
6.5 |
6.5 |
|
Figure 4.3
Percentage base saturation in three soils: a clay loam (left), the same clay loam with agricultural lime (calcium carbonate) added to raise the pH (center), and a sandy loam with low cation exchange capacity (right). The CEC for each soil is indicated. CEC is measured in the SI units, centimoles of positive charge per kilogram of dry soil (cmolc/kg), indicating the numer of centimoles (1/100th of a mole) of positive charge adsorbed per unit mass of the soil; 1 mole of negative charge attracts 1 mole of positive charges whether the charges come from H+, K+, Ca2+ or Al3+. See a current soils text for further explanation. (After Buckman and Brady 1969.)
highly decayed organic matter. The B horizons, deeper in the soil, represent the region of maximum illuviation, or deposition of minerals and colloidal particles leached from elsewhere. Clays, iron, and aluminum oxides often accumulate in the B2 horizon. The C horizon is the undeveloped mineral material deep in the soil; it may or may not be the same as the material from which the soil develops. Below that may be bedrock, or just deep accumulations of mineral material deposited by wind, water, or glaciers.
No one soil has all of the horizons pictured in Figure 4.5. Some horizons may be much more distinct and better developed than others. Plowing and the activity of earthworms may obscure the distinction between the upper horizons. The soil profile may also not be fully developed, and some horizons may be absent or indistinct, if the soil is relatively young. Upper horizons may have been lost through erosion, leaving the deeper horizons exposed at the surface.
Erosion is a particular problem where forests have been clear-cut, on soils that have been long cultivated

68 Chapter 4 |
PAGE PROOF: 2ND PASS |
Figure 4.4
Profile of a forest soil at the edge of the Adirondack Mountains in northern New York State (in the Spodosol soil order). This soil is a stony loam, forested with birch, hemlock, and spruce, and is quite acidic throughout the profile. There is a thick layer of organic material at the top of the profile, and a number of distinct horizons, each with particular properties and a characteristic appearance. Roots are shown reaching down to the top of the B3 horizon at about 45 cm deep, although the deeper roots of large trees would certainly penetrate more deeply into this soil. (After Buckman and Brady 1969.)
Depth |
Horizon |
Extent |
Composition |
pH |
|
|
3 cm |
Litter |
4.78 |
|
O |
12 cm |
Fermentation zone of |
3.48 |
|
|
partially decayed organic |
|
|
|
|
|
|
|
|
|
|
matter; reddish-brown |
|
15 cm |
|
3 cm |
Raw humus; black |
3.45 |
|
A2 |
12 cm |
Silicious gray layer |
4.20 |
30 cm |
B21 |
3 cm |
Precipitated humus; |
3.93 |
|
|
|
dark brown to black |
|
|
|
12 cm |
Compact sesquioxide |
4.50 |
|
B22 |
|
accumulation; yellowish |
|
|
|
to reddish-brown sandy |
|
|
45 cm |
|
|
loam |
|
|
|
|
|
using poor farming practices, on slopes, |
|
|
and on lands that have been severly |
|
|
overgrazed. Soil erosion has been enor- |
B3 |
|
mously important in human history |
|
|
with both economic and social conse- |
60 cm |
|
quences, and even precipitated the col- |
C |
|
|
|
|
lapse of several Old and New World civ- |
|
|
ilizations (Figure 4.6). It is also one of the |
|
|
most critical (and most widely ignored) environmental |
|
|
problems today. It occurs in many different parts of the |
|
|
world and affects many natural and semi-natural ecosys- |
fpo |
|
tems. Rebuilding soils to replace losses to erosion can |
||
take thousands of years. The consequences of soil erosion for our ability to raise food crops and for the sustainability of natural ecosystems can be devastating.
Soils vary in depth, from thin layers of soil barely covering a rock substrate (for example, in many alpine areas) to very deep soils of close to 2 meters (for example, in some well-developed prairie soils). Soil depth has a great deal of influence on vegetation and plant growth: The greater the soil depth, the more favorable the soil for plant growth, all other things being equal. Deeper soils can hold more water and nutrients and can retain water for a longer period of time without rainfall, and they allow greater development of plant root systems.
Figure 4.5
An abstract, general soil profile showing the major horizons that might be present in particular soils. No one soil is likely to have all of the horizons shown, and particular soils may have greater development of subhorizons than what is shown. Only the upper part of the C horizon is considered to be part of the soil proper. The depth of soils varies tremendously with the location and nature of the soil, but to gain some perspective, readers might picture this illustrated profile as being about a meter deep to the top of the bedrock. (After Buckman and Brady 1969.)
Horizon
O1
O2
A1
A2
A3
B1
B2
B3
C
R
18 cm Friable yellow sandy |
4.55 |
|
|
loam |
|
|
|
|
|
Friable yellow sand |
4.80 |
|
|
|
Properties
Organic; original forms recognized
Organic; original forms not recognized
Mineral mixed with humus; dark colored
Horizon of maximum eluviation of silicate clays; Fe, Al oxides, etc.
Transition to B; more like A than B Transition to A; more like B than A
Maximum illuviation of silicate clays; Fe, Al oxides, some organic matter
Transition to C; more like B than C
Zone of least weathering; accumulation of Ca, Mg carbonates; cementation
Bedrock

PAGE PROOF: 2ND PASS
DM looking for more up-to-date photo to replace this one.
Origins and Classification
Soils are formed by the action of weathering on parent material, the upper layers of the heterogeneous mass that is left over after the action of weathering and other forces on rocks. The physical and chemical actions of temperature, rainfall, and other climatic factors further grind up, move, and leach the parent material until it begins to develop into true soil. Parent material is originally derived from rocks, which are classified as igneous (of volcanic origin), sedimentary (from the deposition and recementation of material derived from other rocks), or metamorphic (changed by the action of great pressures and temperatures on igneous or sedimentary rocks deep underground).
The parent material can be broken up by the mechanical action of temperature changes, including repeated freezing and thawing, by the direct action of water, wind, and ice in abrading and eroding rock fragments, and by the actions of plants, fungi, and animals. Chemical reactions occurring in the disintegrating rock material accelerate the physical breakdown of the fragments and begin the process of decomposition and chemical alteration (Figure 4.7). Large and small fragments of rock and rock-derived debris are moved by ice (particularly by glaciers), wind, and water. They are then redeposited in the form of glacial till and outwash, loess (pronounced “luss”) and other aeolian (wind) deposits, alluvial (river and stream) deposits, and lake and marine sediments. These deposits may eventually form soil parent material.
The process of soil formation from parent material takes thousands of years. A young soil may be 10,000
Soils, Mineral Nutrition, and Belowground Interactions 69
Figure 4.6
During the 1930s, soil erosion in the midwestern United States led to massive dust storms that destroyed farms and even entire towns. What came to be known as the “Dust Bowl” displaced thousands of families, who left the region in search of a new livelihood. This shows the remains of a farm lot in South Dakota in 1936. (Photograph courtesy of the U.S. Department of Agriculture.)
years old; an old soil may be 100,000 years old or more. As a soil ages, the primary minerals that came from the parent material undergo chemical changes, and secondary minerals are formed. The structure of the soil develops, and then changes, as materials are leached, redeposited, and lost from the soil, as well as being physically and chemically altered. The mineral constituents of the soil shift, as minerals that are most stable at Earth’s surface gradually come to dominate. Young soils are found where the parent material is still present, as in areas that were glaciated relatively recently (such as much of the northern part of North America) or in areas of recent alluvial deposits (such as the bottomlands of the world’s great river systems). Old soils are most commonly found in the Tropics and Subtropics. Some of the world’s oldest soils are found, for instance, in parts of Africa.
Five major factors are responsible for determining the kinds of soils that develop in an area: climate, the nature of the parent material, the age of the soil, topography (which acts to alter the effects of climate),and living organisms. These factors do not operate in isolation, but rather interact in complex ways with one another. Vegetation, with its associated soil organisms, has an especially strong influence on soil development, but the nature of the vegetation that is present is also dependent on both soil and climate.
Soils are classified according to a comprehensive taxonomic system developed in the United States, although some soil scientists and ecologists still rely on earlier classification systems. The broadest category in the modern system is the soil order. There are ten soil orders worldwide (Table 4.1).

70 Chapter 4 |
PAGE PROOF: 2ND PASS |
(A)
Forests – Natural vegetation
Organic  matter
matter accumulation
accumulation


























 Zone of
Zone of maximum leaching
maximum leaching










 Disintegrated
Disintegrated
Relatively unweathered bedrock











 Hydrous clay, oxide
Hydrous clay, oxide 














 accumulation
accumulation
weathered |
soil |
material |
|
||
|
|
Fresh |
|
unweathered |
|
rock |
Time |
|
(B)






 Relatively unweathered loess
Relatively unweathered loess
Well developed ultisol (red-yellow podzolic)
Grass – Natural vegetation
















 Organic
Organic matter
matter
 accumulation
accumulation

































 Blocky structure
Blocky structure 

































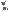 development
development
CaCO |
|
|
CaSO |
3 |
accumulation |
4 |
accumulation |
|
|
|
|
Fresh loess |
|
Well developed |
|
deposit |
|
mollisol |
|
|
Time |
(chernozem) |
|
|
|
|
|
Figure 4.7
Development of soil structure from two different parent materials: (A) bedrock and (B) loess deposited by wind. These soils are also developing under two different climatic and vegetation types. Over time, organic matter accumulates in the upper horizon, depending on the type of vegetation present. In the lower horizons, clay, iron oxides, or CaCO3 are deposited and accumulate, and characteristic structures develop. The soil orders (ultisol and mollisol) are described in Table 4.1. (After Buckman and Brady 1969.)
The soil classification categories most commonly used by plant ecologists are the soil series and soil type. These are used to classify local soils. A soil series is usually named after some local geographic area or feature, and generally consists of a dominant type with several associated other types. Soil types are based upon topography, parent material, and the vegetation under which the soils were formed. Soil series occur at regional scales, and soil types characterize landscape-scale features. In the United States, soil series are mapped for almost every county, and detailed maps are available for most locations showing the soil series and type, and often providing extensive information on the characteristics of
local soils. Such data can be highly valuable for many kinds of ecological studies, particularly at the community and ecosystem levels. The availability of information on local soils in other countries varies widely.
Organic Matter and the Role of Organisms
So far we have emphasized the role of physical processes in soil development and character. However, we began this chapter by emphasizing that soils are the unique product of living things acting on the physical environment (and in turn being affected by that environment). What are some of the ways in which organisms affect soils?

PAGE PROOF: 2ND PASS Soils, Mineral Nutrition, and Belowground Interactions 71
Table 4.1 Soil orders and some of their characteristics
Soil order |
Development and characteristics |
Found in |
|
|
|
Mollisols |
Develop under prairie vegetation; many of these soils |
Great Plains of the United States and Canada; |
|
are under cultivation, and they constitute some |
large areas in the heartland of Russia, Mongolia, |
|
of the most productive agricultural soils |
and northern China; northern Argentina, |
|
|
Paraguay, and Uruguay |
Spodosols |
Generally develop in cold-temperate humid regions |
|
under northern forests; usually strongly acid and |
|
of low fertility |
Northeastern and northern midwestern United States and adjacent areas in Canada; northern Europe, Siberia; also some soils in southern South America
Alfisols |
Develop in humid regions under deciduous forests; |
|
weathered somewhat less than spodosols but more |
|
than inceptisols; contain silicate clay-dominated |
|
horizon; often cultivated and highly productive |
|
agriculturally |
Oxisols |
Usually tropical soils, often supporting tropical rainforest |
|
vegetation. The oldest and most highly weathered soil |
|
order, with a deep subsurface horizon high in clays and |
|
other colloidal particles; intense leaching has removed |
|
most of the silica, leaving a high proportion of oxides |
|
of iron and aluminum, often with a red color |
Entisols |
Recent soils with lack of significant profile development; |
|
highly variable in fertility |
Inceptisols |
Young soils exhibiting limited weathering, but with |
|
greater profile development than entisols; many |
|
of these soils are under agricultural production |
Aridisols |
Develop in arid regions where soils are dry through |
|
most of the year and there is little leaching |
Vertisols |
High content of certain clays; sticky and plastic when |
|
wet and hard when dry, with extensive cracking; |
|
unstable for building and difficult to farm |
Ultisols |
Develop under warm to tropical climates under forest or |
|
savanna vegetation; generally highly weathered but still |
|
retaining some minerals; somewhat acid, with clay |
|
horizons; these are fairly old soils |
Histisols |
Wetland and bog soils; develop in a water-saturated |
|
environment; very high in organic matter content; |
|
sometimes also high in clay. Important soils not only in |
|
present-day wetlands, but in areas of ancient swamps |
|
and bogs that are currently forested, cultivated, or used |
|
for mining peat |
Large area from Baltic states to western Russia; southern half of Africa; eastern Brazil; England, France, central and parts of southern Europe; Michigan and Wisconsin to Pennsylvania and New York in the United States
One of the most widespread soil orders globally, and among the most important in terms of the size of the human population supported; also supports the greatest biodiversity of many groups of plants and animals. Covers much of Africa and South America
Sand hills of Nebraska; areas of northern and southern Africa; northern Quebec; parts of Siberia and Tibet; many areas that were covered by the most recent glaciation
All continents—north Africa, eastern China, western Siberia, Spain, central France, central Germany, northern South America, northwestern United States
Southwestern United States and northern Mexico; southern and central Australia; Bobi and Takla makan Deserts in China; Sahara Desert and also southwestern Africa; Pakistan; other arid regions
Large regions of India, Sudan, and eastern Australia; small areas in southeastern Texas and eastern Mississippi in the United States
Southeastern United States; northeastern Australia; Hawaii; southeastern Asia; southern Brazil
Not widespread, but may be locally important, as in the Everglades in southern Florida and in
many areas of northern Europe. The largest areas are in Canada southwest of Hudson and James Bay and in northwestern Canada into eastern Alaska
Note: These soil orders reflect the modern classification system currently used in the United States. Some of the major Great Soil Groups of the older classification system, with the soil orders with which they roughly correspond, are: podzols (spodosols and alfisols), chernozems and
brunizems (mollisols), latosols and lateritic soils (oxisols), lithosols, solonchak soils, and desert soils (aridisols), azonal soils (entisols), and wetland/bog soils (histisols).
Organic matter is the decaying and decomposed material in soil that comes from living things. It includes substances secreted by plants, microorganisms, and animals, products of excretion by animals, parts shed by animals and plants, and dead organisms and parts of organisms. Microscopic organisms, while tiny individually, collectively contribute a great deal of organic mat-
ter to the soil. The activities of various different kinds of microorganisms affect soil properties in many additional ways, including the recycling of mineral nutrients used by plants (see Chapter 15).
A property of soils that is of vital importance to plant life (and to animals, fungi, and the other organisms living in soils) is soil structure. Soil structure describes the

72 Chapter 4 |
|
PAGE PROOF: 2ND PASS |
|
physical arrangement of soil particles into |
|
|
|
larger clusters, called aggregates (or peds). |
|
Transpiration |
|
One of the most important aspects of soil |
|
|
|
structure is the porosity of the soil, which |
Precipitation |
||
measures the total volume of pores and their |
|||
|
|
||
size, shape, and arrangement between and |
|
fpo |
|
within the aggregates (the aggregates are not |
Evaporation |
||
solid, and may be “fluffy” or packed to vary- |
|
|
|
ing degrees). Soil porosity determines how |
|
Evaporation |
|
much, and how easily, water and air can be |
|
|
|
held by and move around in the soil, and |
Infiltration |
Surface runoff |
|
thus is an important factor in the water-hold- |
|
||
Deep drainage |
|||
ing capacity of the soil. |
|||
Porosity and organic matter content also |
|
Evaporation |
|
|
Uptake by plants |
||
determine how easily roots or fungal hyphae |
|
||
Impermeable horizon |
|||
can penetrate soil, and how easy it is for ani- |
|||
mals to burrow and tunnel through it. The |
|
River |
|
actions of organisms (from animals to roots |
|
Groundwater |
|
|
|
||
and fungal hyphae) in moving through the |
|
|
|
soil create pores and soil structure. Organic |
|
Figure 4.8 |
|
matter is amorphous (it does not have a well-defined |
The fate of water falling on the ground from a rainstorm. |
||
structure, unlike clay particles, for example), and parti- |
(After Kramer 1983.) |
||
|
|||
cles of organic matter typically have very large surface |
|
||
areas. Organic materials bind mineral particles together |
|
||
to stabilize the aggregates so that the soil porosity is |
Rainwater immediately begins to drain downward |
||
maintained throughout the physical action of wetting, |
due to the pull of gravity, entering the groundwater and |
||
drying, freezing, thawing, and other processes. |
draining into the streams, rivers, and lakes of the water- |
||
Organic matter also supplies H+ ions, determining |
shed. After about a day, this rapid downward movement |
||
soil pH. Furthermore, soil organic matter is critically |
of water slows, and many of the large macropores in the |
||
important in plant nutrition, both by supplying essential |
soil refill with air (Figure 4.9A) The tiny micropores, how- |
||
nutrients and by providing physical particles that act, like |
ever, remain filled with water at this point, and the soil |
||
clay particles, to attract and retain ions because they are |
is at field capacity. The water potential (see Chapter 3) |
||
negatively charged and have very large surface areas. |
of the soil is now at –0.01 to –0.05 MPa (Figure 4.9B). |
||
Fresh and decayed organic matter in the soil also contains |
Water is held in the soil largely by attraction to the |
||
compounds, including various organic acids, that chem- |
surfaces of soil particles, particularly clays and organic |
||
ically alter essential plant mineral nutrients such as calci- |
matter. It moves through small pores by capillary action, |
||
um (Ca), iron (Fe), manganese (Mn), copper (Cu), and zinc |
a process that occurs within narrow tubes or in a surface |
||
(Zn), making them more readily available to plants. |
film of water (such as within soil pores, the xylem, and |
||
|
|
the substomatal chambers of leaves). Water is pulled |
|
Water Movement within Soils |
|
upward (or horizontally) by the attraction of the water |
|
|
|
molecules to the charged particle surfaces and to one |
|
Imagine a meadow in the summertime that has been |
another. As the plants in the hypothetical meadow of our |
||
without rain for some time. A thunderstorm sails in, |
example transpire, a water potential gradient (see Fig- |
||
offering a solid drenching. (A similar set of events would |
ures 3.2 and 3.3) is established, and soil water moves |
||
take place in a suburban lawn, a forest, an agricultural |
toward the roots and is absorbed by them. |
||
field, and even, to some extent, an urban vacant lot.) |
As the soil continues to become drier, the small |
||
What happens within the soil? Pores that had been filled |
pores begin to empty of water, filling with air. As the |
||
with air fill with water, first in the upper layers of the |
small pores empty, the once continuous film of water is |
||
soil profile, then deeper as more rain falls. Eventually |
broken in many places, and water movement is greatly |
||
almost all of the pores are filled with water. Some water |
slowed. Water can continue to move as water vapor |
||
runs off the soil surface, the amount depending on the |
through these emptied pores, but only small amounts of |
||
vegetation, slope, and other factors (Figure 4.8). The soil |
water can be transported in this way. Each day, as the |
||
is now saturated. Depending on the soil texture, soil |
plants transpire and the soil progressively dries, the |
||
structure, and soil depth, soils may hold very different |
water potential of the plants declines (becomes more |
||
amounts of water when they are saturated. |
|
negative), and each night, as stomata close, the water |
|
