
PAGE PROOF: 2ND PASS
C H A P T E 13R Disturbance and Succession
The living world is in a constant state of flux. In previous chapters, we looked at processes of change at the level of the individual (Part I) and the population (Part II). Change also occurs at the level of the commu-
nity. Communities change as the species present change, as the populations change in number, age structure, or size structure, resulting in changes in physiognomy and ecosystem function.
In Chapter 12 we described a variety of techniques for quantifying community characteristics. In this chapter we explore patterns of community change and their causes. We also explore a variety of other aspects of communities: their continuity in time, their predictability, and whether they reach an equilibrium.
The process of succession has been central to plant ecologists’ study of plant communities. Succession is directional change in community composition and structure over time. The term denotes changes over periods longer than a single season, although very long-term trends, such as those due to climate change, are not regarded as part of succession. Succession begins when a disturbance—an event that removes part or all of a community—is followed by colonization or regrowth of the disturbed site by plants. Consider the change in the plant community following the abandonment of an agricultural field in North Carolina (Figure 13.1; Oosting 1942; Keever 1950). In the first year, a variety of species are likely to colonize the field. The dominant species are typically short-lived ones—annuals and biennials such as Erigeron canadense (daisy fleabane, Asteraceae) and Gnaphalium purpureum (purple cudweed, Asteraceae). Over the next few years these species tend to be replaced by herbaceous perennials such as Andropogon virginicus (broomsedge, Poaceae) and Aster ericoides (white aster, Asteraceae). By 10 years after abandonment, shrubs such as Rhus radicans (poison ivy, Anacardiaceae; this species also grows as a vine) and trees such as Pinus taeda (loblolly pine, Pinaceae) begin to dominate the field. Eventually, after 150 to 200 years, the pines are replaced by hardwood tree species— Quercus rubra (red oak, Fagaceae) and Carya spp. (hickory, Juglandaceae). If no subsequent large-scale disturbance occurs, these hardwood species will remain the dominant species.
Discussions about the causes and nature of the successional process are part of the debate about the nature of communities (see Chapter 12). One extreme view is that succession is an orderly and predictable process that is a result of emergent community properties. An alternative view is that succes-

252 Chapter 13
(A)
(B)
(C)
sion is a rather unpredictable series of events that result from interactions between individuals and the abiotic environment. As we will see, succession involves a number of different patterns, mechanisms, and causes. Ecologists have amassed a great deal of information about successional processes. Rather than a single, unified the-
PAGE PROOF: 2ND PASS
Figure 13.1
Old-field succession in the Piedmont region of North Carolina. (A) This field, three years after abandonment, is dominated by perennial species, Andropogon virginicus (broom-sedge, Poaceae) and Solidago sp. (goldenrod, Asteraceae). (Photograph courtesy of N. Christensen.) (B) This field, eight years after abandonment, has been colonized by Pinus taeda (loblolly pine, Pinaceae). (C) A mature oak-hickory forest with a canopy of Quercus alba (white oak, Fagaceae), Q. rubra (red oak), Carya glabra (pignut hickory, Juglandaceae), and C. tomentosa (mockernut hickory). Note the absence of a ground layer, a characteristic of the infertile soils of this region. (Photographs courtesy of R. Peet.)
ory, what we have is a complex set of interlocking models (Figure 13.2). These models can be applied to particular systems, providing descriptions of the patterns of succession and the processes responsible.
Theories of the Causes
of Succession
Several issues form the core of discussion about the processes responsible for succession. One group of questions flows directly from the sharply divergent views of communities advocated by Clements and Gleason (see Chapter 12). First, what processes are responsible for successional changes? Do emergent properties and interactions among species play a major role? Or is succession the mere unfolding of the life history of each species independent of the others? If species interactions are important, are they mainly in the form of mutualisms, or is competition among species more important?
Second, how predictable is succession? If two adjacent areas, or the same area at two different times, were to undergo succession, would the same changes occur, leading to the same end point? A climax is the hypothetical
deterministic end point of a successional sequence. Do plant communities undergoing succession usually reach a stable climax state? To the extent that they do not, is this mainly because extrinsic disturbances (such as storms) prevent it, or because of internal dynamics (such as population cycles) inherent in the communities?
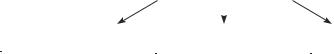
PAGE PROOF: 2ND PASS |
|
|
|
|
Disturbance and Succession 253 |
|||||
|
|
|
|
|
|
|
|
|||
Figure 13.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VEGETATION DYNAMICS |
|
|
|||
A hierarchical framework for a theo- |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
ry of plant community dynamics. |
|
|
|
|
|
|
|
|
|
|
Three main processes affect commu- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DIFFERENTIAL SPECIES |
|
|
DIFFERENTIAL SPECIES |
||
nity dynamics: site characteristics, |
|
SITE AVAILABILITY |
|
|
|
|
|
|||
availability of species, and species |
|
|
|
|
|
AVAILABILITY |
|
|
PERFORMANCE |
|
|
|
|
|
|
|
|
|
|
|
|
characteristics. Each of these process- |
|
Course-scale |
|
|
|
Dispersal |
|
|
Resource availability |
|
es is, in turn, driven by particular |
|
disturbance |
|
|
|
Agents |
|
|
Soil |
|
interactions and conditions. (After |
|
Size |
|
|
|
Landscape |
|
|
Microclimate |
|
Pickett et al. 1987.) |
|
Severity |
|
|
|
|
|
|
|
Ecophysiology |
|
Timing |
|
|
|
Propagule pool |
|
|
|||
|
|
|
|
|
|
|
||||
|
|
Dispersion |
|
|
|
Decay rate |
|
|
Germination |
|
|
|
|
|
|
|
Land use |
|
|
Assimilation |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Growth rate |
|
|
|
|
|
|
|
|
|
|
Life history |
Third, are most plant communities static and |
|
|
|
|
|
|
|
Allocation |
||
|
|
|
|
|
|
|
Reproductive time |
|||
unchanging, or are they in a state of dynamic equilibri- |
|
|
|
|
|
|
|
Reproductive mode |
||
um, or are they always in a fundamental state of flux? A |
|
|
|
|
|
|
|
Stress |
||
stable equilibrium implies that they would return to the |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Climate |
|||
same structure and composition after small to moderate |
|
|
|
|
|
|
|
Prior occupants |
||
perturbations, remaining in that |
state over time. |
|
|
|
|
|
|
|
Competitors |
|
Clements’s view was that communities are unchanging. |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Identity |
|||
Dynamic equilibrium implies very different processes, |
|
|
|
|
|
|
|
Consumers |
||
but in some ways is a modern substitute for Clements’s |
|
|
|
|
|
|
|
Disturbance |
||
|
|
|
|
|
|
|
Resource base |
|||
perspective. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Clements-Gleason dichotomy, however, does |
|
|
|
|
|
|
|
Allelopathy |
||
not account for many of the other questions we consid- |
|
|
|
|
|
|
|
Soil |
||
|
|
|
|
|
|
|
Microbes |
|||
er relevant to understanding succession today. For exam- |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Neighbors |
|||
ple, it seems obvious that one might ask about the role |
|
|
|
|
|
|
|
Consumers |
||
of competition (see Chapter 10) in successional process- |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Identity |
|||
es. This question, though, does not fit neatly into the |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Cycles |
|||
Clements-Gleason dichotomy, as neither of these scien- |
|
|
|
|
|
|
|
Plant defenses |
||
tists ascribed an important role to competition in suc- |
|
|
|
|
|
|
|
Patchiness |
||
cession. This is especially odd, given that Clements |
|
|
|
|
|
|
|
|
||
wrote extensively about competition, but (given his |
|
|
|
|
|
|
|
|
||
superorganism viewpoint) he thought of it in a rather |
the 1890s and early 1900s, largely through the work of |
|||||||||
different sense than modern ecologists do. Similarly, nei- |
Cowles (1899, 1911) and Clements (1916). These ideas |
|||||||||
ther Clements nor Gleason appears to have thought of |
were a major force in plant ecology in North America |
|||||||||
herbivory as an important factor affecting plant com- |
(and to some extent in the entire English-speaking |
|||||||||
munity structure and function, though they were cer- |
world) for the next half century or more. The move away |
|||||||||
tainly well aware of it. Thus, while it is useful to begin |
from these ideas took several directions. One influential |
|||||||||
with the historical differences between Clements’s and |
new perspective on community dynamics came from |
|||||||||
Gleason’s views on succession, it is a mistake to think |
Alex S. Watt (1947). In his view, a plant community is |
|||||||||
that those differences are all that one must consider in |
composed of a mosaic of patches, and the patches are |
|||||||||
trying to piece together a contemporary view of suc- |
dynamically related to one another (see Chapter 10). |
|||||||||
cessional processes. |
|
|
Community structure results from a balance between |
|||||||
As with much in ecology, the answer to all of the |
factors that are predictable and those that are unpre- |
|||||||||
questions posed above is that a variety of patterns and |
dictable (what we now might view as factors that drive |
|||||||||
processes can be found in different communities and, to |
the community toward equilibrium and unpredictable |
|||||||||
some extent, at different times. We will return to these |
disturbances that disrupt that tendency). Watt was care- |
|||||||||
questions after we examine the factors that lead to suc- |
ful to distinguish this view—that the community is a |
|||||||||
cessional change. Then we will ask, is climax a fixed, |
dynamic mosaic of patches—from that of a succession- |
|||||||||
immutable state—an end point at which the communi- |
al sequence in which a homogeneous community tends |
|||||||||
ty remains for all time, barring a major catastrophic |
toward a final, determinate end point. He emphasized |
|||||||||
change? |
|
|
the ubiquitous patchiness of natural plant communities |
|||||||
The initial ideas about succession as a predictable |
and stated that the persistence of patches is a funda- |
|||||||||
process leading to a static climax were developed in |
mental characteristic of plant communities. |
|||||||||

254 Chapter 13
PAGE PROOF: 2ND PASS
Several other early ecologists shared this perspective. R. T. Fisher studied old-growth forests on Pisgah Mountain in New Hampshire, including both hardwood and coniferous forests, over several decades, beginning in 1910. The dynamic and patchlike character of these ancient forests was cogently summed up by Fisher in a preface to a study by his successors (Cline and Spurr 1942, as cited by Dunwiddie et al. 1996):
The primeval forests, then, did not consist of stagnant stands of immense trees stretching with little change in composition over vast areas. Large trees were common, it is true, and limited areas did support climax stands (or preclimax stands), but the majority of the stands were in a state of flux resulting from the dynamic action of wind, fire and other forces of nature. The various successional stages brought about, coupled with the effects of elevation, aspect, and other factors of site, made the virgin forest highly variable in composition, density and form.
Interestingly, a major hurricane on September 22, 1938, uprooted many of the trees in this forest, subsequently changing the vegetation, and ongoing studies suggest that such infrequent, large-scale disturbances are an important factor in forest community dynamics (Foster 1988).
By the 1980s, influenced in particular by work in tropical forests and by broader trends in ecological thinking, many ecologists had begun to view plant communities as mosaics of patches rather than as homogeneous entities. In other words, they ceased trying to average over the entire community and refocused on the smaller scale of gaps and patches (Forman and Godron 1986; Pickett and White 1985). At the same time, ecologists abandoned the search for idealized, static types—such as climax communities—to embrace concepts of variability and dynamic processes as essential to the true nature of communities. In the 1980s, this changing perspective intersected with a very different tradition from Europe. There, the floristic-sociological school was part of the development of landscape ecology. The European floristic-sociological approach focused on measuring static patterns. When wed to the thinking of North American ecologists interested in patch dynamics, the result was a dynamic outlook that helped put successional studies into a wider landscape framework. We discuss these issues in more depth in Chapter 17.
Disturbance
Let’s begin by considering the mechanisms that underlie successional change. Complex as the questions listed above may seem, a loose consensus has emerged among ecologists since the early 1980s on at least two crucial points. First, most ecologists now think that most communities do not tend to stable equilibria. Moreover,
different kinds of disturbances over a range of scales clearly play a crucial role in community ecology.
A disturbance can be defined as a relatively discrete event in time that causes abrupt change in ecosystem, community, or population structure, and changes resource availability, substrate availability, or the physical environment (White
and Pickett 1985, p. 7). Disturbances can result in the removal of a substantial portion of the existing vegetation in an area. They can range from damage to a single plant to the destruction of forests or prairies over thousands of hectares. Sources of disturbance include fire, storms (windstorms, ice storms, tornadoes, etc.), landslides, earthquakes, mudslides, volcanic eruptions, floods, the activities of animals, and disease. Because of the wide diversity of disturbances and their effects, it is almost always more useful to discuss specific kinds of disturbance than disturbance as a single phenomenon.
One way of classifying disturbances is by whether or not they completely remove a community, including any organic soil. These two categories of disturbance lead to two categories of succession. Primary succession occurs when plants colonize ground that was not previously vegetated. Examples include the establishment of plant communities on lava fields, earth laid bare by the retreat of a glacier, rock outcrops, sand dunes, and newly formed beaches and sandbars in rivers, or, at a much larger scale, islands newly emerged from the sea. Primary succession also occurs where human activity results in massive soil disturbance, as on mine wastes, roads blasted through rock outcrops, and other sites that are left with undifferentiated soil parent material (Walker 1999). A key aspect of primary succession is that the process begins with the development of soil, which forms as the plant community develops (see Chapter 4).We will take a closer look at primary succession later in this chapter.
Secondary succession occurs when plants colonize ground previously occupied by a living community. In this case, soil already exists, and plant propagules, such as seeds and ramets, are readily available. Forest regrowth following a catastrophic fire is an example of secondary succession, as is the colonization of an abandoned agricultural field, called old-field succession. Once soils are well established, there is no fundamental difference between primary and secondary succession, as long as sources of colonizing plants are readily available.
Since disturbances are normal characteristics of all ecosystems, ecologists often discuss the disturbance regime of an ecosystem—the characteristics of the dis-

PAGE PROOF: 2ND PASS
(A)
(B) |
(C) |
Disturbance and Succession 255
Figure 13.3
(A) A surface fire in a forest in northern lower Michigan, in which only groundlevel vegetation is burnt. (B) A crown fire in central New Jersey in 1986 in which entire trees were killed. (C) A crown fire occurred three years earlier, killing most of the mature trees in this spruce-fir forest in the Rocky Mountains. At this time only low-growing herbs and shrubs are found. (Photographs A and C by S. Scheiner; B by D. Burgess.)
turbances occurring in that ecosystem. We can describe disturbance regimes using three characteristics: intensity, size, and frequency. The intensity of a disturbance is the amount of change that it causes. A forest fire (Figure 13.3), for example, can be a light surface fire, which runs along the soil surface or ground vegetation and destroys only herbaceous plants or low-growing shrubs, or it can be a severe crown fire that spreads from the canopy of one tree to another, killing most of the canopy trees as well as most of the shrubs and other vegetation. The size (spatial extent) of a disturbance is the amount of area affected. A windstorm, for example, might blow down a single tree, while a hurricane might blow down hundreds or even thousands of trees over an entire section of a forest. The frequency of a disturbance—also called the return interval—quantifies how often, on average, it occurs in a particular place. These three characteristics are often correlated: small disturbances of low
intensity are generally much more frequent than large, intense disturbances. These factors vary, however, depending on the type of community and disturbance. The timing of the disturbance can also be important. For example, a fire that occurs in early spring before plants are actively growing can have very different effects from one in the late summer when they are just beginning to set seed (Biondini et al. 1989; Howe 1995).
Gaps
The size of a disturbance is a major factor in the types of species that can colonize a disturbed site. Disturbances create gaps in communities that can be filled by colonizing species. In any given community, gaps of various sizes can exist at any given time, so that succession may be occurring on a variety of scales. For example, mounds made by badgers (Taxidea taxus) in North American prairies are 0.2–0.3 square meters in size. Each year about

256 Chapter 13
0.01% of the prairie is turned over by the creation of new mounds (Platt and Weis 1977). The mounds are colonized by nearby plants whose seeds have limited dispersal abilities. In contrast, a massive forest fire that burns thousands of hectares may leave no nearby seed sources. In this case, the initial colonists will be seeds that have traveled long distances by wind or animal or have remained dormant in the soil.
A forest can be thought of as a set of patches of widely varying sizes that have experienced disturbances of different types and intensities. The size of a patch has major effects on species composition, the successional trajectory within that patch, and ecosystem processes. In an experimental study on gaps of different sizes in the southern Appalachian Mountains in North Carolina, solar irradiance was two to four times higher in large than in small gaps, and soil and air temperatures were much higher (Phillips and Shure 1990). Standing crop biomass and aboveground net primary productivity (see Chapter 15) were three to four times greater in the largest than in the smallest gaps. Species richness was also greater in large gaps, and species composition differed among gaps of different sizes. We will return to the question of how gap formation and gap size influence overall community species richness in Chapter 14.
Ecologists’ thinking about disturbance has changed substantially over the past few decades. Early ecologists, for example, thought of disturbances as being unusu- al—or unnatural—occurrences that disrupted the ordinary and orderly processes of a community. In the past quarter century, however, disturbance has been recognized as a natural part of many communities. This change in viewpoint has altered management practices, as we will see in the next section.
PAGE PROOF: 2ND PASS
Fire
Fire is a major source of disturbance in many communities. Fires can vary tremendously in intensity, size, and frequency. One measurement of fire intensity is the amount of heat transferred per unit area per unit time. The most intense forest fires can release as much as 500,000 kJ/m2 in a few minutes, which is enough heat to melt an aluminum-block engine. The Mack Lake fire (discussed below) released approximately 3 × 1012 kJ of energy, as much as 90 thunderstorms, or 9 times the energy of the atomic bomb dropped on Hiroshima (Pyne et al. 1996). The speed with which a fire spreads is another aspect of its intensity. Surface fires can move quickly through an area. In a North American short-grass prairie on a very windy day, a fire can move as fast as 22 km/hr—as fast as an Olympic sprinter—for at least short periods. Crown fires have been clocked at 12 km/hr.
Fire frequency is a key determinant of community structure and composition. In mesic prairies, fires can recur as often as every 2 to 3 years. As we will see in Chapter 18, these frequent fires destroy colonizing trees and so maintain the prairie as grassland. Chaparral communities in southern California burn approximately once every 25 years. Average fire return intervals in different community types vary greatly—from every few years to once a century to once every millennium (Table 13.1).
Many plant species have adaptations to fire. One such adaptation is the location of the meristems (see Chapter 8) in a place protected from fire. Prairies, where fires recur frequently, are dominated by grasses and forbs, which have meristems at or below the ground surface. A light, quickly moving surface fire is unlikely to
Table 13.1 Examples of fire regimes in various community types in North America
Fire regime |
Communities |
|
|
Natural fires rare or absent |
Pacific Northwest coastal forests; wetter regions of eastern deciduous |
|
forests; southwestern deserts |
Infrequent, low-intensity surface fires with a ±25-year return interval
Frequent, low-intensity surface fires with a 1- to 25-year return interval combined with high-intensity surface fires with a 200to 1000-year interval
Infrequent, intense surface fires with a +25-year return interval combined with crown fires with a 100to 300-year interval
Most eastern deciduous forests; pinyon-juniper woodlands of the Southwest; some montane meadows in the Rockies and Sierras
Sierra mixed coniferous forests; western montane-zone pine forests; southeastern pine forests; prairies of Nebraska and Oklahoma; sawgrass prairie in the Florida Everglades
Pine forests and boreal forests in the Great Lakes region; lower-elevation forests of the Rockies; California redwood forests
Frequent, intense surface and/or crown fires with a |
Most boreal forests; higher-elevation western forests; chaparral from |
25to 100-year return interval |
California to Texas |
Infrequent crown fires with a return interval >100 years |
Northwestern wet coastal montane forests; subalpine forests of the western |
|
mountains; rainforests of Hawaii |
Source: Davis and Mutch 1994.

PAGE PROOF: 2ND PASS
harm these meristems, although the tops of the plants may be destroyed. (Having meristems at ground level also protects these plants from damage by grazers, which may have been the primary selective force acting on this adaptation; see Chapter 11.) Several tree and shrub species have the ability to resprout from roots, rhizomes, or buds underneath the bark if the aboveground portions of the plant are killed or severely damaged by fire. Examples from the frequently burned pine barrens of the eastern United States are Pinus rigida (pitch pine, Pinaceae) and Quercus ilicifolia (scrub oak, Fagaceae). Other species with such adaptations include Eucalyptus spp. (Myrtaceae) in Australia, Populus tremuloides (quaking aspen, Salicaceae) at high latitudes or high altitudes in North America, and Adenostoma fasciculatum (chamise, Rosaceae) in southern California. Other species, such as Quercus velutina (black oak) in the eastern United States and Pinus ponderosa (ponderosa pine) in the western United States, have very thick bark that protects the tree’s cambium (the living, growing part of the trunk just under the bark) during a surface fire. As they grow, these species also tend to shed their lower branches, which
(A)
(B)
Disturbance and Succession 257
could serve as a “fire ladder,” with the result that fire cannot as easily spread to the canopy.
Some species release their seeds from fruits or cones following a fire. Some pines have a trait called serotiny, in which they retain their seeds in tightly sealed cones for many years, releasing them only after exposure to fire. The cones are sealed by resin, which melts during a fire, releasing the seeds. Following a fire, these trees can release many years of accumulated seed production. The sealed cones, therefore, serve as an aboveground seed bank. Cleared mineral soil may be required for germination in many serotinous species. Among the pine species with serotiny are Pinus contorta (lodgepole pine) in western North America, P. banksiana (jack pine) in north-central North America, and some populations of P. rigida in the eastern United States (Figure 13.4). Serotiny also occurs in other fire-adapted taxa unrelated to pines, such as many Australian species in the Proteaceae. A related adaptation occurs in a number of annual and short-lived perennial herbs that appear after fires. Some of these plants have a soil seed bank (see Chapter 8) in which seed dormancy is broken by chemical compounds found in ash or smoke.
Pinus palustris (longleaf pine) is a dominant tree in forests of the southeastern United States, where surface fires recur approximately every 3 to 5 years. Young seedlings are especially vulnerable to these fires. P. palustris has an unusual life history that appears to be an adaptation to these frequent fires: after the first year or so, the young plant exists as a small tuft of needles grow-
(C)
Figure 13.4
Serotiny occurs in some Pinus rigida (pitch pine, Pinaceae) populations in the pine barrens of Long Island, NY. (A) Ordinary open cones on a typical, non-serotinous tree. (B) Closed, serotinous cones on a tree belonging to a frequently burned population with a high frequency of serotiny, which is a genetically determined trait. (C) A serotinous cone that has opened as a result of a forest fire, releasing its seeds. (Photographs by J. Gurevitch.)

258 Chapter 13
Figure 13.5
A seedling of Pinus palustris (longleaf pine, Pinaceae) looks very much like a clump of grass—hence the term “grass stage.” The apical meristem is located out of sight, just below the ground, where it is protected from frequent fires. (Photograph © G. Grant/Photo Researchers, Inc.)
ing at ground level, looking very much like a clump of grass (Figure 13.5). The apical meristem sits just below the ground, where it is undamaged if a low-intensity fire occurs. During this time, the plant grows a large root system and creates a large nutrient store. Finally, when its nutrient store is large enough, it grows very quickly. Within a few years, the tree is big enough, and has bark thick enough, to be quite fire-resistant. Thus, its growth pattern minimizes the number of years that it is vulnerable to fire.
If weather conditions are favorable to fire, the probability of a fire occurring increases with the fuel load, the amount of combustible plant material in the community. In prairies, the grasses die back each winter, and highly combustible dead grass builds up. This buildup of material increases the probability of fire with time, as well as increasing the intensity of fire when it finally occurs. Likewise, when exotic grasses invade nongrassdominated communities, they can contribute to increased fire frequency by creating fuel where it did not previously exist, which in turn appears to facilitate further grass invasion (D’Antonio and Vitousek 1992).
In many Eucalyptus forests in Australia, the pine barrens of the eastern United States, and chaparral in California and other parts of the world, similar processes of fuel buildup occur. Here the buildup is of living twigs,
PAGE PROOF: 2ND PASS
stems, and leaves of the dominant species. In some other community types, the probability of fire does not increase with time. In these communities, weather conditions, rather than fuel availability, limit fire frequency and intensity. In the boreal forests of North America, for example, the chance of a fire occurring in a given stand is roughly independent of the time since the last fire in that stand. There is usually adequate fuel for a fire, but weather is only sometimes conducive to forest fires (Johnson 1992). Interestingly, a roughly constant chance of fire also occurs in stands of California chaparral that have long been unburned: apparently, once the fuel load reaches a critical level, fuel availability no longer strongly affects the chance of fire (Johnson 1992; Keely et al. 1999).
Some plant species are pyrogenic—that is, their accumulated leaf or twig litter tends to promote fire more than one would expect based on the mass of the litter alone. Good examples include many Eucalyptus, some chaparral shrubs, and possibly some pines. Oils and other flammable chemicals produced by these plants are often of central importance in pyrogenicity. Mutch (1970) proposed that this property might be an evolved adaptation. This proposal remains quite controversial, however, as the conditions required for such a trait to evolve as a direct adaptation to fire may be quite restrictive (see Chapter 6; Kerr et al. 1999). Regardless of whether pyrogenicity is an evolutionary adaptation in itself or a byproduct of selection on other traits, pyrogenic species reestablish themselves following a fire either because adults resprout or because the population has a seed bank (which may be located in the soil or in serotinous cones or fruits). Meanwhile, competing species are often killed by the fire. Thus, these pyrogenic plants create an environment that enhances their own persistence (or facilitates their invasion into a new community).
The problem of increasing fuel load with time led to a strenuous debate about how best to manage forests in the United States. For most of the twentieth century, the policy of the U.S. government was to suppress fire as much as possible. As a result, fuel loads and the density of young trees increased. Therefore, when fires did occur, rather than light surface fires, they often became raging crown fires, often killing mature trees and threatening human lives and property. In the 1970s, this management practice began to change for two reasons. First, the problems and dangers associated with accumulating fuel loads were recognized. Second, because of the increased understanding gained by ecologists, U.S. government agencies started to recognize that disturbance and fire are natural parts of ecosystems. In the absence of the natural disturbance regime, many properties of a community can change, including species composition. In the southeastern United States, for example, in the absence of fire, hardwood-dominated forests eventually replace longleaf pine forests.

PAGE PROOF: 2ND PASS
The new U.S. government management practices include letting naturally occurring forest fires burn themselves out (if they are not threatening human lives or property) and setting prescribed or controlled burns to reduce accumulated fuel loads (to avert a much bigger, uncontrolled fire). Both of these practices are controversial, however. The extensive wildfires in Yellowstone National Park in the summer of 1988, which began naturally and were allowed to burn, were critical in turning public opinion against a policy of allowing fires to burn. Prescribed burns, too, have become a subject of controversy.
Prescribed burns are planned by foresters and set only after careful evaluation of weather and other conditions. Most have been safe and effective in reducing fuel load and in facilitating regeneration of fire-adapted species such as longleaf pine. They are used routinely to manage forests in many regions of the United States and elsewhere. There have been occasional but spectacular mistakes, however, in which controlled burns were set without the proper precautions and became uncontrolled wildfires. The Mack Lake fire on May 5, 1980, in the Huron National Forest of Michigan began as a prescribed burn, set to help manage the jack pine community. Unfortunately, the fire got out of control. In 12 hours, the fire had burned 10,000 hectares; tragically, it also killed a firefighter and destroyed 44 homes (Pyne et al. 1996). More recently, a prescribed burn in the spring of 2000 near Los Alamos, New Mexico, escaped control, burning thousands of hectares and destroying several dozen homes; fortunately, no lives were lost.
Managing forests and forest fires has become more complex and difficult as suburbanization and the rise in ownership of vacation and retirement homes in scenic areas has led to increasing numbers of homes encroaching into large, previously almost uninhabited forests. This was the case in Los Alamos, as well as in some of the areas devastated by the wildfires (started by lightning) that burned more than 2.5 million hectares in the western United States during the very dry La Niña summer of 2000 (see Chapter 18). Furthermore, incidents such as the Mack Lake and Los Alamos fires have made decisions regarding fire management political and contentious. Many of these discussions are underlain by profound disagreements between environmentalists, logging corporations, and loggers over the extent to which the U.S. government should facilitate the logging of public forests. The logging industry proposes that they be permitted to thin forests to prevent severe fires from occurring. Critics of this proposal say that this is just an excuse to continue extensive logging of public lands, as the type of logging that is actually done removes most of the larger, commercially (and ecologically) valuable trees, leaving the smaller trees that are most vulnerable to fire. An alternative that has been sug-
Disturbance and Succession 259
gested by some environmentalists and foresters is to thin commercially valueless small trees and saplings, which are the most likely to contribute to catastrophic fires, but this proposal has not been enthusiastically embraced by the logging industry because it is not profitable. The results of these controversies have been protracted court battles without any clear resolution at present. Even without a political, economic, or environmental agenda to consider, there is also the problem of determining what a “natural” fire regime for a forest community might be and attempting to get to that state without having a catastrophic wildfire.
Wind
Wind can be another significant source of disturbance. At one end of the scale, wind can blow down a single branch or tree. Such windthrows range from blowdowns of branches or large parts of trees to losses of single trees and neighboring groups of trees. Windthrows are important in many tropical forests (Figure 13.6; Brokaw 1985), where trees can be very tall and are often
Figure 13.6
A large almendro tree (Dipteryx panamensis, Arecaceae) has created a gap in the tropical forest of La Selva Biological Station in Costa Rica. This photograph was taken approximately one year after the tree fell. (Photograph © G. Dimijian/Photo Researchers Inc.)

260 Chapter 13
PAGE PROOF: 2ND PASS
connected by vines. As a result, when one tree falls, it often brings down others (Putz 1983). In the MPassa forest of Gabon, Africa, for example, 51% of gaps were caused by single falling trees and were responsible for 38% of the total gap area (Florence 1981). Trees that fell in a domino-like fashion accounted for 14% of gaps and 16% of the total gap area. Once a gap forms, neighboring trees become more susceptible to being blown down. In MPassa, such adjacent treefalls made up 13% of the gaps and 36% of the total gap area. In that forest, the average time between gap formation at any one spot is about 60 years, and the average size of a gap is 3 ha. Treefall in such forests is clearly a very important source of disturbance.
Extremely powerful storms such as hurricanes and tornadoes, while rare, are an important source of wind damage. Hurricanes, typhoons, and cyclones are important in coastal regions. Caribbean hurricanes, for example, pass over any given patch of forest, on average, every 15–20 years. These storms can also be important in temperate regions. In the northeastern United States, hurricanes severe enough to blow down large tracts of forest recur once or twice a century. In 1938 a hurricane blew down 253,000 ha of forest in central New England (the same hurricane that destroyed most of the Pisgah forest mentioned above). Tornadoes are another important source of wind damage, especially in the midwestern U.S. “Tornado Alley” (Figure 13.7). In the forests of northern Wisconsin, catastrophic windthrows create, on average, 52 patches each year, ranging in size from 1 to 3785 ha, with a return interval of 1210 years (Canham and Loucks 1984).
The gaps and patches created by all of these kinds of wind damage are important factors in forest commu-
Figure 13.7
Locations of all tornadoes recorded the United States from 1981 to 1990. Tornadoes are most frequent in a region stretching from eastern Texas and Louisiana northward to Minnesota and Michigan, an area sometimes called “Tornado Alley.”
nity dynamics. Soil erosion by wind can also be a major disturbance factor in more open communities, from arid lands to plowed former grasslands (see Chapter 4).
Water
Water can be an important source of disturbance in both its liquid state, as floods and nonflood erosion (including soil erosion), and in its solid state, as snow and ice. Floods are most important in riparian habitats (areas adjacent to streams and rivers) and areas near swamps and bogs. In many such systems, annual floods are continually creating and destroying habitat. In Alaska, for example, the sandbar willow (Salix exigua, Salicaceae) grows on sandbars along rivers that are continually being destroyed and re-formed by snowmelt-caused floods. Heavy rainstorms can also lead to landslides. These types of disturbances can initiate primary succession because they tend to create new, previously unvegetated ground. Similarly, snow avalanches are responsible for disturbances in temperate mountain regions. In the Canadian Rockies, avalanches remove 1% of the forests each winter.
Ice storms are important sources of disturbance in many temperate regions. In temperate deciduous forests, such as those of the southeastern United States, they are often responsible for many small-scale disturbances, such as the removal of single branches from trees. Such disturbances create new patches in the forest. Ice storms can also cause large-scale disturbances. In January 1998, a massive ice storm covered large areas of New York and New England in the United States and Ontario, Quebec, and New Brunswick in Canada. Approximately 25% of the trees in the region were damaged moderately to severely in that single storm.
