
печень
.pdf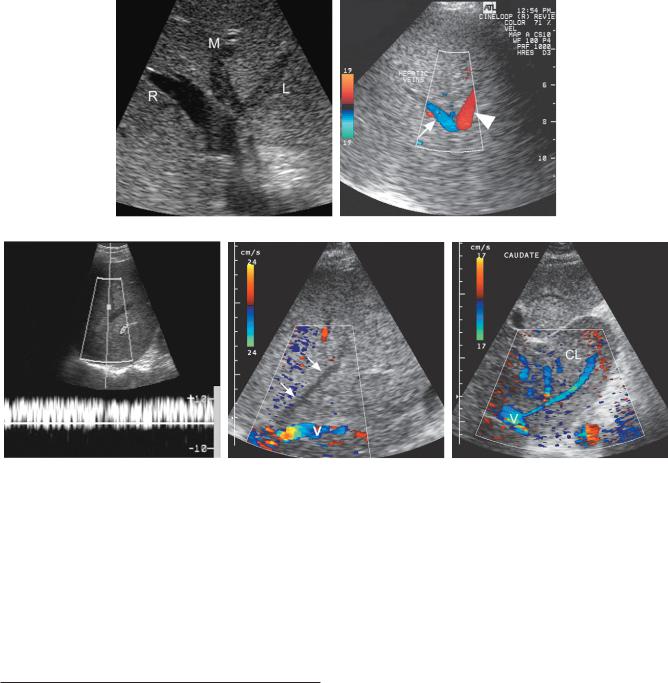
Liver 79
A B
C D E
Figure 3-42 Hepatic vein thrombosis in different patients. A, Transverse gray-scale view of the hepatic vein confluence demonstrates hypoechoic thrombus within the left (L) and middle (M) hepatic veins. The right hepatic vein (R) remains patent. B, Color Doppler view of a hepatic vein bifurcation demonstrates normal direction flow in one branch (arrow) and reverse flow in the other branch (arrowhead). The vessel with reversed flow must be supplying a collateral somewhere in the liver. C, Pulsed Doppler waveform from a hepatic vein shows reversed flow that has lost all of its normal pulsatility. D, Color Doppler view showing flow in the inferior vena cava (V) but no detectable flow within the middle hepatic vein (arrows). E, Transverse color Doppler view shows an enlarged caudate lobe (CL). Multiple venous collaterals are seen draining from the caudate lobe into the vena cava (V).
Hepatic Transplant Complications
Improvements in surgical techniques have decreased, but not eliminated, the incidence of complications after liver transplantation. The major complications that sonography is capable of detecting are vascular and biliary. Identification of biliary obstruction and bile leaks depends on the same principles that apply to the native bile ducts (see Chapter 4).
Vascular thrombosis and stenosis can affect the hepatic artery, portal vein, hepatic veins, and the IVC after transplantation. Arterial lesions are especially
critical because the bile ducts are supplied exclusively by the hepatic arteries, and significant interruption of arterial flow results in biliary necrosis. Hepatic artery thrombosis is suspected when no arterial signal is detected on either duplex or color Doppler imaging. Because thrombosis can affect the main hepatic artery or the right or left branch, all three vessels should be studied. Collateral arterial flow can develop and result in an arterial signal despite complete thrombosis of the main hepatic artery. Arterial stenosis can also be detected. Peak systolic velocities greater than 200 cm/sec or focal increases in velocity of greater than threefold suggest a
80 ULTRASOUND: THE REQUISITES
A B C
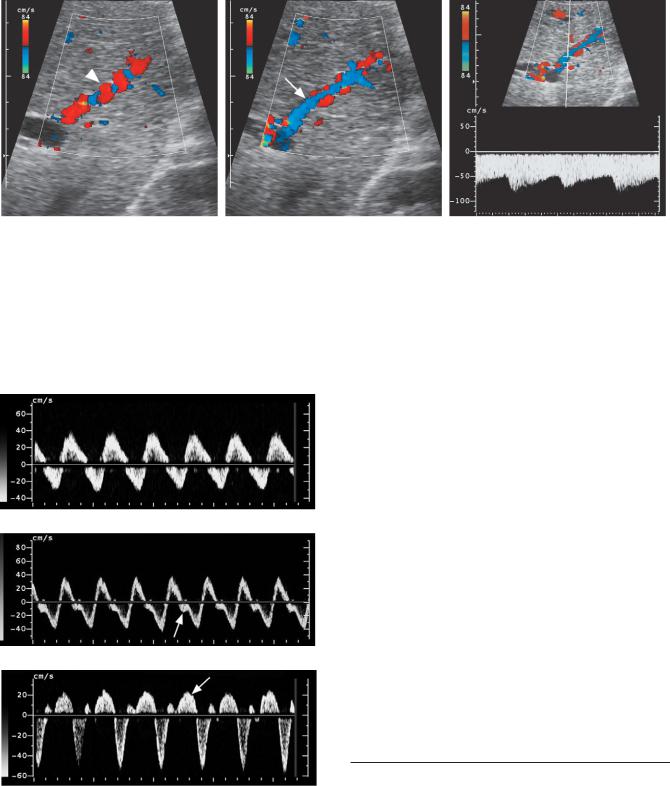
Figure 3-43 Arterial portal fistula secondary to Osler-Weber-Rendu syndrome. A, Magnified view of the peripheral aspect of the liver shows an enlarged tortuous intrahepatic artery (arrowhead). B, Similar view immediately adjacent to the hepatic artery shown in A shows an adjacent portal vein (arrow) with reversed flow, opposite in direction to the hepatic artery. C, Pulsed Doppler waveform from the portal vein shows arterialization of the portal vein flow as well as reversal of flow. This indicates a more distal arteriovenous fistula.
A
B
Passive congestion in different patients. A, Portal vein waveform showing markedly abnormal venous pulsatility. B, Hepatic vein waveform demonstrating increased pulsatility and a diminished systolic pulse (arrow). C, Hepatic vein waveform showing inversion of the systolic pulse (arrow).
stenosis of greater than 50%. Waveforms obtained distal to the stenosis demonstrate blunting of the systolic peak that can be quantified with resistive index measurements. Values less than 0.4 are almost always due to arterial obstruction, and values between 0.4 and 0.5 are suspicious. Blunting of the intrahepatic arterial waveforms occurs with both a proximal stenosis and complete thrombosis with collateral flow (Fig. 3-45). Reversal of arterial flow is a sign of collateral flow and indicates thrombosis or severe stenosis. As with other stenotic arteries, turbulent flow can cause perivascular soft tissue vibration that may be visible both on color Doppler and on pulsed Doppler.
Portal vein, hepatic vein, and IVC thrombosis appear as they would in native livers. Portal vein stenosis can occur at the anastomosis and should be suspected when there is a threefold to fourfold focal increase in the flow velocity in the portal vein. Stenosis of the IVC at the superior anastomosis causes focal velocity elevation, loss of pulsations in the hepatic veins and in the proximal IVC, and hepatic vein flow reversal. Loss of hepatic vein pulsatility has also been reported in rejection.
Portosystemic Shunts
A variety of portosystemic shunts can be created surgically to decompress the portal system in patients with portal hypertension. These generally involve a shunt between the portal vein or superior mesenteric vein and the IVC or between the splenic vein and the left renal
Liver 81
A B
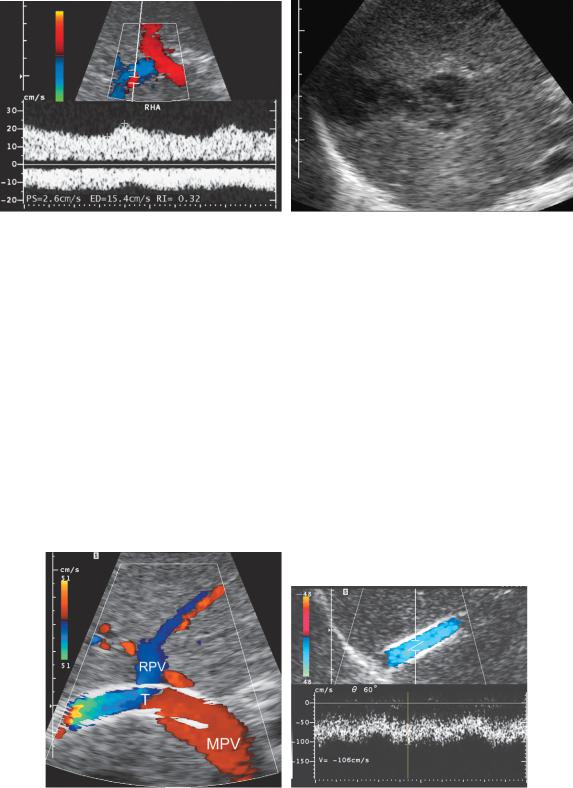
Figure 3-45 Post liver transplant hepatic artery thrombosis. A, Color Doppler image and pulsed Doppler waveform of the right posterior segment hepatic artery shows an extremely blunted hepatic arterial waveform with a resistive index value of 0.32. Also note that hepatic arterial flow is on the opposite side of the baseline from the portal venous flow. This implies that the right hepatic artery is functioning as a collateral vessel and has reversal of flow. B, Gray-scale view of the liver parenchyma demonstrates a complex fluid collection due to a biloma. This occurred because of bile duct necrosis related to the hepatic artery thrombosis.
vein. Of the surgical shunts, the portocaval shunts are in general the easiest to evaluate sonographically for shunt patency. Splenorenal shunts are more difficult to visualize, owing to left upper quadrant bowel gas.
A transjugular intrahepatic portosystemic shunt (TIPS) is now used commonly in patients with complications of portal hypertension. These shunts are very easy to evaluate for patency because the liver provides an acoustic window and overlying bowel gas is rarely a
problem. The normal stent should have detectable flow throughout its lumen. Because the stent decompresses the portal system directly into the low pressure hepatic venous system, portal flow in the right and left portal vein usually reverses after stent placement and is directed into the stent instead of into the liver (Fig. 3-46A). Flow velocities in the stent are higher than typical for venous structures and range between 90 and 190 cm/sec (see Fig. 3-46B). Common problems with
A B
Figure 3-46 Normal TIPS stent. A, Longitudinal view of the porta hepatis shows the TIPS stent
(T) entering the portal vein near the junction of the main portal vein (MPV) and right portal vein (RPV). Note that flow is seen throughout the lumen of the stent and note the reversal of flow in the right portal vein. B, Pulsed Doppler waveform from the middle aspect of a TIPS stent shows mild pulsatility and normally high velocity of 106 cm/sec.
82 ULTRASOUND: THE REQUISITES
A B C
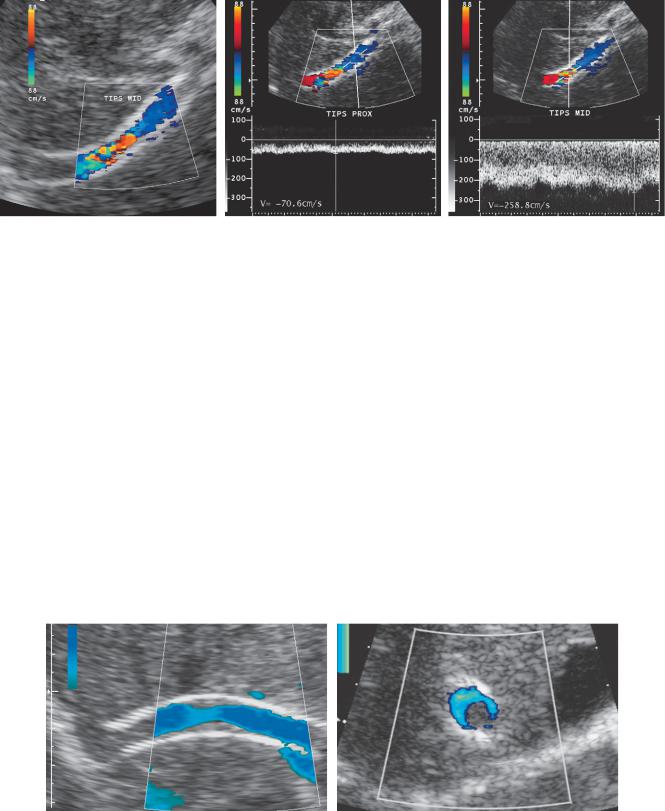
Figure 3-47 TIPS stenosis. A, Longitudinal color Doppler view of the stent shows a focal area of color aliasing manifest as a red color assignment in the middle aspect of the stent. This indicates elevated frequency shifts in this region and, because the Doppler angle is relatively constant, must be due to elevated velocities. B, Pulsed Doppler waveform from the proximal aspect of the stent shows an abnormally low velocity of 70.6 cm/sec. C, Pulsed Doppler waveform from the area of aliasing in the mid aspect of the stent shows abnormally elevated velocity of 258.8 cm/sec.
TIPS are stenoses of the stent or the hepatic vein. In most cases it is possible to detect these stenoses in asymptomatic patients by performing regular Doppler evaluations of the shunt and the portal veins. This allows for intervention before symptomatic decompensation. Signs of stenosis include elevated velocities across the narrowed segment, typically seen on color Doppler imaging as focal areas of color aliasing (Fig. 3-47A). When an abnormality is seen on color Doppler analysis, pulsed Doppler waveforms can be obtained through the stenotic and non-stenotic segments and velocities can be calculated. Elevated maximum and depressed minimum stent velocities are signs of stent stenosis (see Fig. 3-47B and C). One system uses 90 cm/sec and 190 cm/sec as the lower and upper limits, of normal stent velocities.
Additional signs of dysfunction are low portal vein velocity, a temporal increase or decrease in maximum and minimum stent velocities on sequential examinations, and reversal of flow in the draining hepatic vein. Conversion of left and/or right portal flow from the normal pattern of flow toward the stent to a pattern of flow away from the stent on follow-up scans indicates decreased flow going through the shunt. This type of flow conversion in the right and left portal veins is usually a late manifestation of shunt dysfunction. In some patients neointimal hyperplasia and hepatic vein stenosis can also be imaged directly. It is seen as a narrowing in the flow lumen on color or power Doppler imaging (Fig. 3-48). Minimal deviations from normal in single Doppler parameters usually do not indicate a
A B
Figure 3-48 TIPS stenosis with visible luminal narrowing in different patients. A, Longitudinal power Doppler view of the stent shows an area of narrowing in the middle aspect of the stent. B, Transverse power Doppler view shows partial occlusion and only eccentric blood flow in the lumen of the stent.

Figure 3-49 TIPS thrombosis. Longitudinal power Doppler view of the TIPS (T) shows no detectable flow within the stent. Readily detectable flow is seen in the right portal vein (PV) and in the hepatic vein (HV).
significant stenosis. However, when multiple parameters are abnormal, a stenosis is likely and intervention should be considered. When stent stenosis is not detected, it can progress to complete thrombosis. This is usually easy to diagnose with Doppler analysis because normal stent flow is relatively easy to detect (Fig. 3-49).
Liver 83
SUGGESTED READINGS
Abu-Judeh HH: The “starry sky” liver with right-sided heart failure. AJR Am J Roentgenol 178:78, 2002.
Abu-Yousef MM: Duplex Doppler sonography of the hepatic vein in tricuspid regurgitation. AJR Am J Roentgenol 156:79-83, 1991.
Abu-Yousef MM: Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. J Ultrasound Med 11:263-268, 1992.
Abu-Yousef MM, Milam SG, Farner RM: Pulsatile portal vein flow: A sign of tricuspid regurgitation on duplex Doppler sonography. AJR Am J Roentgenol 155:785-788, 1990.
Atri M, et al: Incidence of portal vein thrombosis complicating liver metastasis as detected by duplex ultrasound. J Ultrasound Med 9:285-289, 1990.
Avva R, Shah HR, Angtuaco TL: US case of the day: Giant hemangiomas of the liver. Radiographics 19:1689-1692, 1999.
Bennett GL, Krinsky GA, Abitbol RJ, et al: Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: Correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol 179:75-80, 2002.
Key Features
Echogenicity of the liver should be equal or slightly |
Acute hepatitis may cause a “starry sky” appearance. |
greater than that of the right kidney, equal or less than |
This is often a subtle finding and is not specific for |
that of the pancreas, and less than that of the spleen. |
hepatitis. |
The segments of the liver are divided by the hepatic |
Fatty infiltration is usually diffuse and causes |
veins, the gallbladder, the interlobar fissure, the |
increased hepatic echogenicity. When more |
fissure for the ligamentum venosum, and the |
severe, it attenuates the sound pulse and makes it |
ligamentum teres. |
difficult to see the diaphragm and the hepatic |
Hepatic cysts are easily seen and characterized with |
vessels. Fatty infiltration often localizes adjacent to |
sonography. They frequently have partial septations |
the ligamentum teres or portal bifurcation. Focal |
and peripheral wall puckering. |
sparing frequently localizes around the gallbladder |
Hemangiomas are typically homogeneous and |
and portal bifurcation. |
hyperechoic. In a patient at low risk for malignancy, |
Cirrhosis causes the hepatic parenchyma to be |
this type of lesion requires no further evaluation. |
coarsened and inhomogeneous and the liver |
Focal nodular hyperplasia is usually nearly isoechoic to |
surface to be nodular. High-resolution views often |
the liver. The classic vascularity seen on color and |
show distinct small nodules. |
power Doppler analysis is a spoke-wheel pattern. |
The sonographic signs of portal hypertension are |
Hepatic adenomas are rare and exhibit a variety of |
splenomegaly, ascites, portosystemic collaterals, |
sonographic appearances. |
and reversal of portal venous flow. |
Target lesions are masses with a hypoechoic peripheral |
The umbilical vein and the coronary vein are the easiest |
halo. These are very likely to be malignant. |
portosystemic collaterals to visualize. |
Most liver metastases are target lesions, but there is a |
The diagnosis of portal vein thrombosis requires the |
wide range of appearances. |
combined use of gray-scale analysis and color- |
Hepatocellular cancer should be suspected whenever a |
Doppler imaging and relies on the absence of |
solid mass is seen in a patient with chronic liver |
detectable blood flow or the visualization of intralu- |
disease (especially cirrhosis and chronic hepatitis). |
minal filling defects. |
There is a propensity to invade the portal vein and, |
Hepatic vein thrombosis may appear as an intraluminal |
to a lesser extent, the hepatic veins. |
hepatic vein thrombus, reversal of hepatic vein flow, |
Lymphoma typically appears as a hypoechoic mass or |
no detectable hepatic vein flow, and hepatic vein |
masses. Rarely it can appear anechoic and simulate a cyst. |
collaterals. |
84 ULTRASOUND: THE REQUISITES
Brancatelli G, Federle MP, Grazioli L, et al: Benign regenerative nodules in Budd-Chiari syndrome and other vascular disorders of the liver: Radiologic-pathologic and clinical correlation. Radiographics 22:847-862, 2002.
Buetow PC, Pantograg-Brown L, Buck JL, et al: From the Archives of the AFIP: Focal nodular hyperplasia of the liver: Radiologic-pathologic correlation. Radiographics 16:369-388, 1996.
Buetow PC, Buck JL, Ros PR, Goodman ZD: From the Archives of the AFIP: Malignant vascular tumors of the liver: Radiologic-pathologic correlation. Radiographics 14:153-166, 1994.
Birnbaum BA, et al: Definitive diagnosis of hepatic hemangiomas: MR imaging versus Tc-99m-labeled red blood cell SPECT. Radiology 176:95-101, 1990.
Bolondi L, et al: Liver cirrhosis: Changes of Doppler waveform of hepatic veins. Radiology 178:513-516, 1991.
Bree RL, et al: The varied appearances of hepatic cavernous hemangiomas with sonography, computed tomography, magnetic resonance imaging, and scintigraphy. Radiographics 7:1153-1175, 1987.
Casillas VJ, Amendola MA, Gascue A, et al: Imaging of nontraumatic hemorrhagic hepatic lesions. Radiographics 20:363-378, 2000.
Caturelli E, Pompili M, Bartolucci F, et al: Hemangioma-like lesions in chronic liver disease: Diagnostic evaluation in patients. Radiology 220:337-342, 2001.
Cerri GG, de Oliveira IRS, Machado MM: Hepatosplenic schistosomiasis: Ultrasound evaluation update. Ultrasound Q 15(4):210-215, 1999.
Chehida FB, Gharbi HA, Hammou A, et al: Ultrasound findings in hydatid cyst. Ultrasound Q 15(4):216-222, 1999.
Cho KJ, Lunderquist A: The peribiliary vascular plexus: The microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology 147:357-364, 1983.
Cronan JJ, et al: Cavernous hemangioma of the liver: Role of percutaneous biopsy. Radiology 166:135-138, 1988.
Dodd GD III, et al: Detection of malignant tumors in end-stage cirrhotic livers: Efficacy of sonography as a screening technique. AJR Am J Roentgenol 159:727-733, 1992.
Dodd GD III, et al: Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 192:657-661, 1994.
Dodd GD III, Baron RL, Oliver JH III, Federle MP: Pictorial review: Spectrum of imaging findings of the liver in end-stage cirrhosis: I. Gross morphology and diffuse abnormalities. AJR Am J Roentgenol 173:1031-1036, 1999.
Dodd GD III, Baron RL, Oliver JH III, Federle MP: Pictorial review: Spectrum of imaging findings of the liver in end-stage cirrhosis: II. Focal abnormalities. AJR Am J Roentgenol 173:1185-1192, 1999.
Dodd GD III, Baron RL, Oliver JH, Federle MP: End-stage primary sclerosing cholangitis: CT findings of hepatic morphology in 36 patients. Radiology 211:357-362, 1999.
Dodd GD III, Memel DS, Baron RL, et al: Portal vein thrombosis in patients with cirrhosis: Does sonographic detection of intrathrombus flow allow differentiation of benign and malignant thrombus? AJR Am J Roentgenol 165:573, 1995.
Duerinckx AJ, et al: The pulsatile portal vein in cases of congestive heart failure: Correlation of duplex Doppler findings with right atrial pressures. Radiology 176:655-658, 1990.
Feldstein VA, Patel MD, LaBerge JM: TIPS shunts: Accuracy of Doppler US in determination of patency and detection of stenoses. Radiology 201:141-147, 1996.
Freedman AM, Sanyal AJ, Tisnado J, et al: Complications of transjugular intrahepatic portosystemic shunt: A comprehensive review. Radiographics 13:1185-1210, 1993.
Filly RA, Reddy SG, Nalbandian AB, et al: Sonographic evaluation of liver nodularity: Inspection of deep versus superficial surfaces of the liver. J Clin Ultrasound 30: 399-407, 2002.
Gallix BP, Taourel P, Dauzat M, et al: Flow pulsatility in the portal venous system: A study of Doppler sonography in healthy adults. AJR Am J Roentgenol 169:141-144, 1997.
Gazelle GS, Lee MJ, Mueller PR: Cholangiographic segmental anatomy of the liver. Radiographics 14:1005-1013, 1994.
Gibney RG, Hendin AP, Cooperberg PL: Sonographically detected hepatic hemangiomas: Absence of change over time. AJR Am J Roentgenol 149:953-957, 1987.
Gibson PR, et al: A comparison of duplex Doppler sonography of the ligamentum teres and portal vein with endoscopic
demonstration of gastroesophageal varices in |
patients |
with chronic liver disease or portal hypertension, |
or both. |
J Ultrasound Med 11:327-331, 1992. |
|
Gibson RN, et al: Identification of a patent paraumbilical vein by using Doppler sonography: Importance in the diagnosis of portal hypertension. AJR Am J Roentgenol 153:513-516, 1989.
Glockner JF, Forauer AR: Pictorial essay: Vascular or ischemic complications after liver transplantation. AJR Am J Roentgenol 173:1055-1059, 1999.
Goyal AK, Pokharna DS, Sharma SK: Ultrasonic measurements of portal vasculature in diagnosis of portal hypertension: A controversial subject reviewed. J Ultrasound Med 9:45-48, 1990.
Grazioli L, Federle MP, Brancatelli G, et al: Hepatic adenomas: Imaging and pathologic findings. Radiographics 21:877-894, 2001.
Herold C. Reck T, Ott R, et al: Changes in hepatic hemodynamics after orthotopic liver transplantation: Color Doppler sonography. Abdom Imaging 26:32-35, 2001.
Hung C-H, Changchien C-S, Lu S-N, et al: Sonographic features of hepatic adenomas with pathologic correlation. Abdom Imaging 26:500-506, 2001.
Ichikawa T, Federle MP, Grazioli L, et al: Fibrolamellar hepatocellular carcinoma: Imaging and pathologic findings in 31 recent cases. Radiology 21:352-361, 1999.
Ito K, Honjo K, Fujita T, et al: Liver neoplasms: Diagnostic pitfalls in cross-sectional imaging. Radiographics 16:273-293, 1996.
Kane R, Eustace S: Diagnosis of Budd-Chiari syndrome: Comparison between sonography and MR angiography. Radiology 195:117-121, 1995.
Kanterman RY, Darcy MD, Middleton WD, et al: Doppler sonographic findings associated with transjugular intrahepatic portosystemic shunt (TIPS) malfunction. AJR Am J Roentgenol 168:467-472, 1997.
Keogan MT, McDermott VG, Price SK, et al: Pictorial essay: The role of imaging in the diagnosis and management of biliary complications after liver transplantation. AJR Am J Roentgenol 173:215-219, 1999.
Kliewer MA, Sheafor DH, Paulson EK, et al: Percutaneous liver biopsy: A cost-benefit analysis comparing sonographic and CT guidance. AJR Am J Roentgenol 173:1199-1202, 1999.
Konno K, Ishida H, Sato M, et al: Liver tumors in fatty liver: Difficulty in ultrasonographic interpretation. Abdom Imaging 26:487-491, 2001.
Kruskal JB, Thomas P, Nasser I, et al: Hepatic colon cancer metastases in mice: Dynamic in vivo correlation with hypoechoic rims visible at US. Radiology 215:852-857, 2000.
Kudo M, Tomita S, Tochio H, et al: Hepatic focal nodular hyperplasia: Specific findings at dynamic contrast-enhanced US with carbon dioxide microbubbles. Radiology 179:377-382, 1991.
Kurtz AB, Rubin CS, Cooper HS, et al: Ultrasound findings in hepatitis. Radiology 136:717-723, 1980.
Lafortune M, Patriquin HB: The hepatic artery: Studies using Doppler sonography. Ultrasound Q 15(1):9-26, 1999.
Liefer DM, Middleton WD, Teefey SA, et al: Follow-up of patients at low risk for hepatic malignancy with a characteristic hemangioma at US. Radiology 214:167-172, 2000.
Marn CS, Bree RL, Silver TM: Ultrasonography of the liver: Technique and focal and diffuse disease. Radiol Clin North Am 29:1151-1170, 1991.
Marsh JI, Gibney RG, Li DK: Hepatic hemangioma in the presence of fatty infiltration: An atypical sonographic appearance. Gastrointest Radiol 14:262-264, 1989.
McKenney KL: Role of US in the diagnosis of intraabdominal catastrophes. Radiographics 19:1332-1339, 1999.
McLarney JK, Rucker PT, Bender GN, et al: From the archives of the AFIP: Fibrolamellar carcinoma of the liver: Radiologicpathologic correlation. Radiographics 19:453-471, 1999.
Mergo PJ, Ros PR, Buetow PC, Buck JL: Diffuse disease of the liver: Radiologic-pathologic correlation. Radiographics 14:1291-1307, 1994.
Middleton WD, Hiskes H, Teefey SA, Boucher LD: Small (1.5 cm or less) liver metastases: US-guided biopsy. Radiology 205:729-732, 1997.
Millener P, et al: Color Doppler imaging findings in patients with Budd-Chiari syndromes: Correlation with venographic findings. AJR Am J Roentgenol 161:307-312, 1993.
Moody AR, Wilson SR: Atypical hepatic hemangiomas: A suggestive sonographic morphology. Radiology 188:413-417, 1993.
Liver 85
Mostbeck GH, et al: Hemodynamic significance of the paraumbilical vein in portal hypertension: Assessment with duplex US. Radiology 170:339-342, 1989.
Nascimento AB, Mitchell DG, Rubin R, Weaver E: Diffuse desmoplastic breast carcinoma metastases to the liver simulating cirrhosis at MR imaging: Report of two cases. Radiology 221:117-121, 2001.
Nelson RC, Chezmar JL: Diagnostic approach to hepatic hemangiomas. Radiology 176:11-13, 1990.
Nghiem HV, Bogost GA, Ryan JA, et al: Cavernous hemangiomas of the liver: Enlargement over time. AJR Am J Roentgenol 169:137-140, 1997.
Nghiem HV, Tran Khai, Winter TC III, et al: Imaging of complications in liver transplantation. Radiographics 16:825-840, 1996.
Pedrosa I, Saiz A, Arrazola J, et al: Hydatid disease; radiologic and pathologic features and complications. Radiographics 20:795-817, 2000.
Pena CS, Chew FS, Keel SB: Posttransplantation lymphoproliferative disorder of the liver. AJR Am J Roentgenol 171:192, 2000.
Platt JF, Yutzy GG, Bude RO, et al: Use of Doppler sonography for revealing hepatic artery stenosis in liver transplant recipients. AJR Am J Roentgenol 168:473-476, 1997.
Quinn SF, Gosink BB: Characteristic sonographic signs of hepatic fatty infiltration. AJR Am J Roentgenol 145:753-755, 1985.
Ralls PW: Color Doppler sonography of the hepatic artery and portal venous system. AJR Am J Roentgenol 155:517-525, 1990.
Richards JR, McGahan JP: Ultrasound for blunt abdominal trauma in the emergency department. Ultrasound Q 15(2): 60-72, 1999.
Rosenthal SJ, Harrison LA, Baxter KG, et al: Doppler US of helical flow in the portal vein. Radiographics 15:1103-1111, 1995.
Schneck CD: Embryology, histology, gross anatomy and normal imaging anatomy of the liver. In Friedman AC, Dachman AH (eds): Radiology of the Liver, Biliary Tract, and Pancreas. St. Louis, Mosby, 1994, pp 1-25.
Singh, Y, Winic AB, Tabbara SO: Residents’ teaching files: Multiloculated cystic liver lesions: Radiologic-pathologic differential diagnosis. Radiographics 17:219-224, 1997.
Smith D, Downey D, Spouge A, Soney S: Sonographic demonstration of Couinaud’s liver segments. J Ultrasound Med 17:375-381, 1998.
Stoopen ME, Kimura-Fujikami K, Quiroz y Ferrari FA, BaroisBoullard V: Ultrasound imaging of hepatic amebic abscess. Ultrasound Q 15(4):189-200, 1999.
Stoupis C, Taylor HM, Paley MR, et al: The rocky liver: Radiologic-pathologic correlation of calcified hepatic masses. Radiographics 18:675-685, 1998.
Tchelepi H, Ralls PW, Radin R, Grant E: Sonography of diffuse liver disease. J Ultrasound Med 21:1023-1032, 2002.
Teefey SA, Hildebolt CC, Dehdashti F, et al: Detection of primary hepatic malignancy in liver transplant candidates: Prospective comparison of CT, MR imaging, US and PET. Radiology 226:533-542, 2003.
86 ULTRASOUND: THE REQUISITES
Teefey SA, Middleton WD, Crowe TM, Peters MG: Doppler evaluation of the portal vein: Effects of intravenous DDFP. J Ultrasound Med 16:641-645, 1997.
Tessler FN, et al: Diagnosis of portal vein thrombosis: Value of color Doppler imaging. AJR Am J Roentgenol 157:293-296, 1991.
Tessler FN, Tublin ME, Purdy S, Rifkin MD: US case of the day: Diffuse large B-cell lymphoma (posttransplant lymphoproliferative disorder [PTLD]). Radiographics 18:1307-1309, 1998.
Uggowitzer MM, Kugler C, Mischinger HJ, et al: Echo-enhanced Doppler sonography of focal nodular hyperplasia of the liver. J Ultrasound Med 18:445-451, 1999.
Vilgrain V, Boulos L, Vullierme MP, et al: Imaging of atypical hemangiomas of the liver and pathologic correlation. Radiographics 20:379-397, 2000.
VanSonnenberg E. Simeone JF, Mueller PR, et al: Sonographic appearance of hematoma in liver, spleen and kidney: A clinical, pathologic and animal study. Radiology 147:507-510, 1983.
Wachsberg RH: Sonography of liver transplants. Ultrasound Q 14(2):76-94, 1998.
Wachsberg RH, Simmons MZ: Coronary vein diameter and flow direction in patients with portal hypertension: Evaluation with duplex sonography and correlation with variceal bleeding. AJR Am J Roentgenol 162:637-641, 1994.
Wagner RC, Koenigsberg M, Wexler JP: US case of the day: Focal nodular hyperplasia (FNH). Radiographics 16:974-978, 1996.
Want SS, et al: Focal hepatic fatty infiltration as a cause of pseudotumors: Ultrasonographic patterns and clinical differentiation. J Clin Ultrasound 18:401-409, 1990.
Warshauer DM, Molina PL, Hamman SM, et al: Nodular sarcoidosis of the liver and spleen: Analysis of 32 cases. Radiology 195:757-762, 1995.
Weltin G, et al: Duplex Doppler: Identification of cavernous transformation of the portal vein, AJR Am J Roentgenol 144:999-1001, 1985.
Wernecke K, et al: Pathologic explanation for hypoechoic halo seen on sonograms of malignant liver tumors: An in vitro correlative study. AJR Am J Roentgenol 159:1011-1016, 1992.
Wernecke K, et al: The distinction between benign and malignant liver tumors on sonography: Value of a hypoechoic halo. AJR Am J Roentgenol 159:1005-1009, 1992.
White EM, et al: Focal periportal sparing in hepatic fatty infiltration: A cause of hepatic pseudomass on US. Radiology 162:57-59, 1987.
Yates CK, Streight RA: Focal fatty infiltration of the liver simulating metastatic disease. Radiology 159:83-84, 1986.
Yoshikawa J, et al: Focal fatty change of the liver adjacent to the falciform ligament: CT and sonographic findings in five surgically confirmed cases. AJR Am J Roentgenol 149:491-494, 1987.
Zweibel WJ: Sonographic diagnosis of diffuse liver disease. Semin US CT MRI 16:8-16, 1995.
