
Кононов / 7
.pdf
transitions in organic compounds

Interpretation of Molecular UV-Visible Spectra
 UV-Visible spectra can be interpreted to help determine molecular structure, but this is presently confined to the analysis of electron behavior in known compounds.
UV-Visible spectra can be interpreted to help determine molecular structure, but this is presently confined to the analysis of electron behavior in known compounds.
 Information from other techniques (NMR, MS, IR) is usually far more useful for structural analysis
Information from other techniques (NMR, MS, IR) is usually far more useful for structural analysis
 However, UV-Vis evidence should not be ignored!
However, UV-Vis evidence should not be ignored!
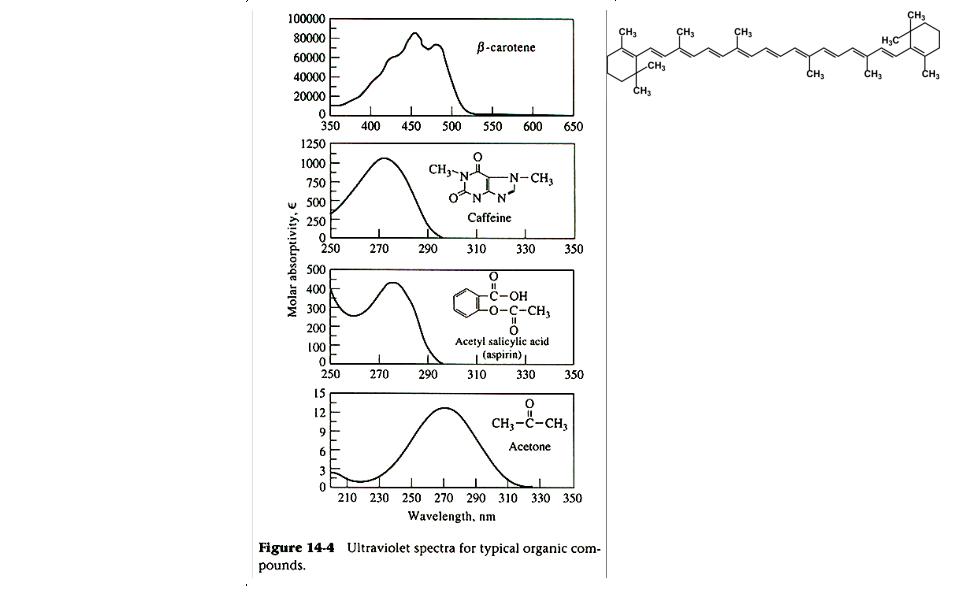
UV-vis spectra are useful but do not have the information content of other spectroscopic techniques. Typical UV-Visible spectra of small organic molecules are shown here, from Skoog et al. Figure 14-4. Note the lambda(max) and differences in molar absorptivity for each of the spectra.
Sometimes compounds in non-viscous solvents (and especially in the gas phase) will show fine structure in the UV-Visible spectrum caused by vibrational energy levels splitting the electronic transitions.

• * and
* and  * transitions: high-energy, accessible in vacuum UV ( max <150 nm). Not usually observed in molecular UV-Vis.
* transitions: high-energy, accessible in vacuum UV ( max <150 nm). Not usually observed in molecular UV-Vis.
n  * and
* and  * transitions: non-bonding electrons (lone pairs), wavelength ( max) in the 150-250 nm region.
* transitions: non-bonding electrons (lone pairs), wavelength ( max) in the 150-250 nm region.
n  * and
* and  * transitions: most common transitions observed in organic molecular UV-Vis, observed in compounds with lone pairs and multiple bonds with max = 200-600 nm.
* transitions: most common transitions observed in organic molecular UV-Vis, observed in compounds with lone pairs and multiple bonds with max = 200-600 nm.

 d/f orbitals – transition metal complexes
d/f orbitals – transition metal complexes
–UV-Vis spectra of lanthanides/actinides are particularly sharp, due to screening of the 4f and 5f orbitals by lower shells.
Charge transfer (CT) – occurs when electron-donor and electron-acceptor properties are in the same complex – electron transfer occurs as an “excitation step”
–MLCT (metal-to-ligand charge transfer)
–LMCT (ligand-to-metal charge transfer)
–Ex: tri(bipyridyl)Ru(II), which is red – an electron is exicted from the d-orbital of the metal into a * orbital on the ligand

 Although UV-Visible spectra are no longer frequently used for structural analysis, it is helpful to be aware of well-developed interpretive rules.
Although UV-Visible spectra are no longer frequently used for structural analysis, it is helpful to be aware of well-developed interpretive rules.
 Examples:
Examples:
– Woodward-Fieser rules for max dienes and polyenes
–Extended Woodward rules for a,b-unsaturated ketones
–Substituted benzenes ( max base value = 203.5 nm)
X
Substituent (X) |
Increment (nm) |
|
|
-CH3 |
3.0 |
|
|
-Cl |
6.0 |
|
|
-OH |
7.0 |
|
|
-NH2 |
26.5 |
|
|
-CHO |
46.0 |
|
|
-NO2 |
65.0 |
There are several well-developed (and famous) rules for the interpretation of UV-Visible spectra, which were derived by synthesizing a variety of related compounds, recording their spectra, and determining trends in the results, usually in conjunction with Huckel or Extended Huckel Theory. It is worth remembering these rules so that a given UV-Visible spectrum can be checked for consistency. It is especially useful to know these rules in HPLC analysis, to help select wavelengths for UV detection of chromatographic peaks.

There are numerous applications reliant upon the ultraviolet and visible (UV/Vis) wavelength range. For example, absorption spectroscopy is applied to analyze and identify polymers and copolymers containing chromophores that absorb in this wavelength range, such as aromatic or carbonyl groups. In this context, the investigation of photochemical reactions, for instance of reactions occurring in degradation processes, is noteworthy. Moreover, absorption measurements allow the monitoring of alterations in the tertiary structure of macromolecular systems, for instance, in the case of the denaturation of biomacromolecules, especially proteins and nucleic acids. Figure 1.14 demonstrates the increase in the optical absorption observed upon heating an aqueous solution of lysozyme, a globular protein that acts as an enzyme in the cleavage of certain polysaccharides The absorption change reflects the unfolding of the polypeptide chains due to the destruction of intramolecular interactions such as hydrogen bonds (see Scheme 1.3).

The thermal denaturation of other superstructures, such as those of collagen and deoxyribonucleic acid (DNA), may also be monitored by following the increase
in the optical absorption. Collagen is the most abundant protein in connective tissues and constitutes a major part of the matrix of bones. In its native state, it adopts a three-stranded helical structure. Dissociation of the three
chains at temperatures above 40 C is accompanied by an increase in optical absorption. DNA, the carrier of genetic information and an essential constituent
of the nuclei of biological cells, contains the bases adenine, guanine, cytosine, and thymine, and hence absorbs UV light. The intensity of its absorption spectrum (max=260 nm) is reduced by about 30% when single strands combine to
form the double-stranded helix. Conversely, the optical absorption increases upon denaturation [41]. This is illustrated in Fig. 1.15.
As regards nucleic acids in solution, hypochromicity applies to a decrease in optical absorbance when single-stranded nucleic acids combine to form double-stranded helices. The hypochromic effect is not restricted to nucleic acids, proteins, and other polymers, but has also been observed with aggregates of dyes and clusters of aromatic compounds. In interpreting this effect, it has been assumed that the electron clouds of chromophores brought into close proximity are strongly interacting. The resulting alteration in the electron density causes changes in the absorption spectrum.

Electronic spectroscopy
II. Luminescence spectroscopy
Principle of Fluorescence and Phosphorescence:
10-8 – 10-9s
M* M + heat
10-14 to 10-15 s
-Excitation of e- by absorbance of h .
-Re-emission of hv as e- goes to ground state.
-Use h 2 for qualitative and quantitative analysis
Method |
Mass detection |
Concentration |
Advantages |
For UV/Vis |
|||
|
limit (moles) |
detection limit |
|
need to |
|||
|
|
|
(molar) |
|
observe Io and |
||
UV-Vis |
10-13 |
to 10-16 |
10-5 |
to 10-8 |
Universal |
I difference, |
|
which limits |
|||||||
|
|
|
|
|
|
||
|
|
|
|
|
|
detection |
|
fluorescence |
10-15 |
to 10-18 |
10-7 |
to 10-10 |
Sensitive |
||
|
|||||||
|
|
|
|
|
|
|
|

a) Excitation:
•At room temperature, everything starts out at
the lowest vibrational energy level of the ground state
•Suppose a molecule is illuminated with light at a resonance frequency
•Light is absorbed; for dilute sample, Beer-Lambert law applies
Energy
A 
 c
c  l
l
nuclear configuration
Excitation - following light absorption, a chromophore is excited to some higher vibrational energy level of S1 or S2
•The absorption process takes place on a time scale (10-15 s) much faster than that of molecular vibration → “vertical” transition (Franck-Condon principle).
