
Кононов / 8
.pdf
Схема Яблонского

Quantum yield and life-time of fluorescence
Quantum yield of fluorescence, Ff, is defined as: |
|
number of photons emitted |
|
f |
|
||
number of photons absorbed |
|||
|
|
Another definition for f is |
|
kr |
f |
k |
|
|
|
where kr is the radiative rate constant and k is the sum of the rate constants for all processes that depopulate the S1 state.
In the absence of competing pathways f=1
Radiative lifetime, |
r, is related to kr |
|
1 |
|
r |
|
|||
kr |
||||
|
|
|||
|
|
|
The observed fluorescence lifetime, is the average time the molecule spends in the excited state, and it is
1
f
k

Laws of Luminescence:
Stokes’ Law of Luminescence: a law, set forth by G. G. Stokes in 1852, stating that the wavelength of photoluminescence is greater than the wavelength of the exciting light. Stokes’ law is not always obeyed. In many cases anti-Stokes lines, whose wavelengths are shorter than the wavelength of the exciting light, are observed in photoluminescence spectra.
(Закон Стокса: люминесценция смещена в длинноволновую сторону относительно длины волны возбуждающего света)
Kasha’s rule: Fluorescence comes mostly from the lowest singlet state
(Правило Коши: интенсивность флуоресценции из высших синглетных состояний мала по сравнению с флуоресценцией из состояния S1)
Vavilov’s law: Quantum yield is independent on excitation wavelength
(закон Вавилова: квантовый выход люминесценции постоянен при изменении в широких пределах длины волны возбуждающего света в стоксовой области)
Mirror image rule: Fluorescence emission spectrum is mirror image of absorption spectrum (Закон зеркального отражения: спектр люминесценции зеркально симметричен спектру поглощения)
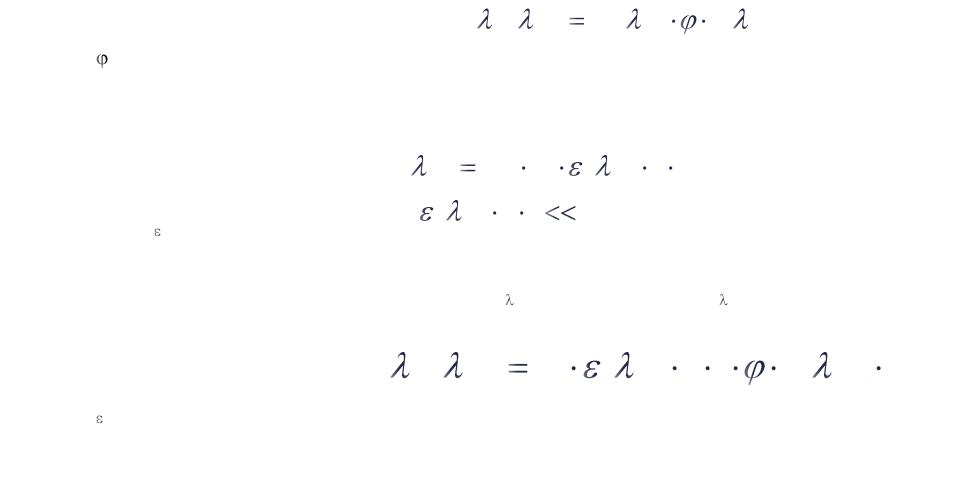
Fluorescence intensity
The fluorescence intensity (F) at a particular excitation (lx) and emission wavelength (lm) will depend on the absorption and the quantum yield:
where, |
F( ex , em ) I A ( ex ) |
I ( em ) |
IA – light absorbed |
|
|
– quantum yield |
|
|
I – light emitted |
|
|
From the Beer-Lambert law, the absorbed intensity for a dilute solution (very small absorbance)
|
I A ( |
ex ) |
|
2.3 |
I0 |
( ex ) c l |
where, |
for |
( |
|
) c |
l |
1 |
Io – Initial intensity |
ex |
|||||
– molar extinction coefficient |
|
|
|
|
|
|
|
|
|
|
|
|
|
c – concentration |
|
|
|
|
|
|
l– path length |
|
|
|
|
|
|
The fluorescence intensity (F) at a particular excitation ( x) and emission wavelength ( m) for a dilute solution containing a fluorophore is:
where, |
F( ex , em ) Io |
( ex ) c l |
I ( em ) Z |
Z – instrumental factor |
|||
Io – incident light intensity |
|
|
|
– molar extinction coefficient |
|
|
|
c = concentration |
|
|
|
l – path length |
|
|
|

Fluorescence spectra
F( ex , em ) Io ( ex )  c
c  l I ( em )
l I ( em )  Z
Z
•Emission spectrum
•Hold excitation wavelength fixed, scan emission
•Reports on the fluorescence spectral profile
•Excitation spectrum
•Hold emission wavelength fixed, scan excitation
•Reports on absorption structure
Fluorescence Intensity Fluorescence Intensity

Fixed Excitation Wavelength
Emission Wavelength (nm)
Fixed Emission Wavelength
Excitation Wavelength (nm)

Emission spectrum |
Excitation-emission matrix |
Fluorescence Intensity |
Fixed Excitation Wavelength |
|
Emission Wavelength (nm)
Excitation Wavelength (nm)
Emission Wavelength (nm)

550 |
|
|
|
|
|
|
530 |
|
|
|
|
|
2.216e+007 |
|
|
|
|
|
|
|
510 |
|
|
|
|
|
1.575e+007 |
490 |
|
|
|
|
FAD |
9.335e+006 |
470 |
|
|
|
|
|
|
|
|
|
|
|
|
|
450 |
|
|
|
|
|
2.923e+006 |
Excitation(nm)370 |
|
|
|
|
|
|
430 |
|
|
|
|
|
1.761e+006 |
410 |
NADH |
|
|
|
|
|
|
|
|
|
|
|
|
390 |
|
|
|
|
|
1.120e+006 |
350 |
|
|
|
|
|
4.785e+005 |
|
|
|
|
|
|
|
330 |
Tryp. |
|
|
|
|
1.947e+005 |
310 |
|
|
|
|
|
1.306e+005 |
|
|
|
|
|
|
|
290 |
|
|
|
|
|
|
270 |
|
|
|
|
|
6.646e+004 |
250 |
300 |
350 |
400 |
450 500 550 600 650 700 9.767e-001 |
||
|
||||||
|
|
|
|
Emission(nm) |
|
TCL-1 |
Courtesy of N. Ramanujam |
|
|
|
|||
|
|
|
|
|||

FAD |
FADH2 |

–Endogenous Fluorophores
amino acids structural proteins
enzymes and co-enzymes vitamins
lipids porphyrins
–Exogenous Fluorophores
Cyanine dyes Photosensitizers
Molecular markers – GFP, etc.

GFP and gene expression
