
Кононов / 6
.pdf
Intensity & Spape of Spectral Lines
What is the strength of an absorption line?
Absorption: A = - lg T , T=I/I0
A
Amax
( ) |
( ) |
The Bouguer-Lambert-Beer Law:
A( ) = ε( )∙C∙L

What determines the Shape of the line?
The Natural Lifetime and Homogeneous broadening
A=1/
h
the frequency dependence of the emission (absorption) intensity for a spectroscopic transition is given by:
I
a rigid rotor or a harmonic oscillator:
Let's suppose E decay exponentially in time:
The functional form is called a Lorentzian line shape, and holds for any system whose correlation function decays exponentially.

The uncertainty principle:
∙t |
ħ/2 |
|
∙t |
1/2 |
1/2t |
·(1/t)
Single atom: |
1/ |
0 |
|
|
|
|
|
|
|
Life-time reduced by collision: |
t = 1/ 0 |
collision |
||
Line width includes natural radiative decay via spontaneous emission, collisional broadening, which are all refereed to as homogeneous forms of line broadening.
Inhomogeneous broadening
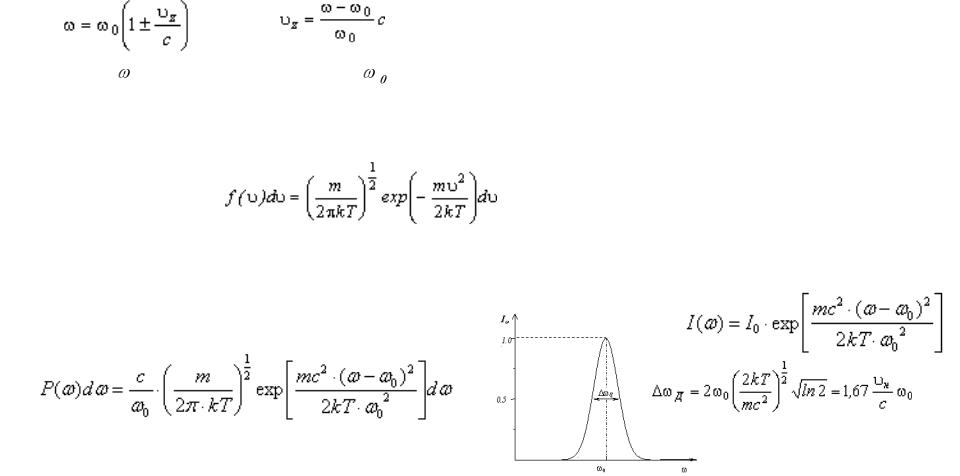
Doppler effect
When thermal motion causes a particle to move towards the observer, the emitted radiation will be shifted to a higher frequency. Likewise, when the emitter moves away, the frequency will be lowered. For non-relativistic thermal velocities, the Doppler shift in frequency will be:
and |
|
Where is the observed frequency, |
is the rest frequency, v is the velocity of the emitter towards |
the observer, and c is the speed of light.
In the case of the thermal Doppler broadening, the velocity distribution is given by the Maxwell distribution:
Since there is a distribution of speeds both toward and away from the observer in any volume element of the radiating body, the net effect will be to broaden the observed line.
Then the corresponding distribution of the frequencies is

Inhomogeneous broadening Solvent effects
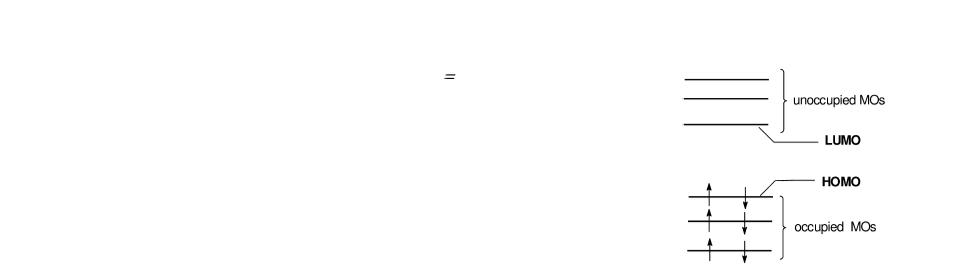
Electronic spectroscopy Molecular UV-Visible Spectroscopy
Molecular UV-Visible spectroscopy is driven by electronic absorption of UV-Vis radiation.  Molecular UV-Visible spectroscopy can:
Molecular UV-Visible spectroscopy can:
–Enable structural analysis
–Detect molecular chromophore
–Analyze light-absorbing properties (e.g. for photochemistry)
Photons are absorbed by matter on a time scale of about 10–15 s. During this very short time, the electronic structure of the absorbing molecule is altered, whereas the positions of the atomic nuclei in the molecule, vibrating on a time scale of 10–12 s, are not changed (Franck-Condon principle).
The molecule must contain a chromophoric group with excitable energy states corresponding to the photon energy according to:
h En - E0
En - E0
En and E0 denote the energies of the excited and the ground state, respectively.
Commonly, molecular orbitals are classified as occupied (doubly), singly occupied, and unoccupied. The acronyms HOMO and LUMO denote the frontier orbitals, i.e. the Highest Occupied and the
Lowest Unoccupied Molecular Orbital, respectively.
The molecular orbital model
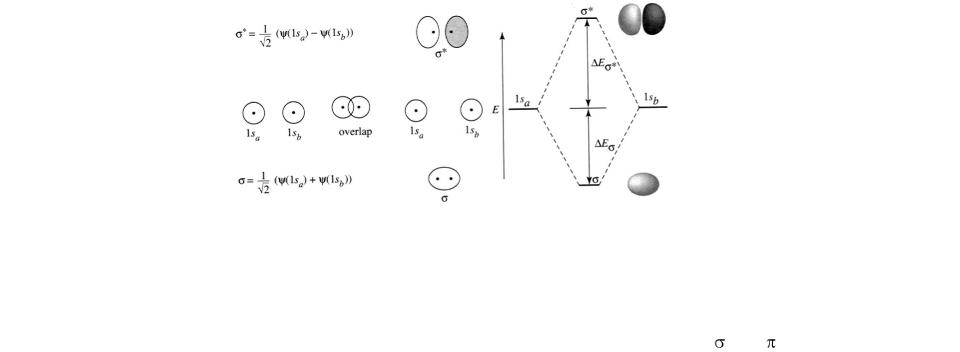
Changes in the electronic structure of a molecule can be visualized with the aid of the molecular orbital (MO) model. Molecular orbitals are thought to be formed by the linear combination of the valence shell orbitals of the atoms linked together in the molecule. The combination of two single orbitals of two
adjacent atoms results in two molecular orbitals, one of lower and the other of higher energy than before combination. The low-energy orbital, denoted as the bonding orbital, is occupied by a pair of electrons of antiparallel spin. The highenergy molecular orbital is called an antibonding orbital. It is unoccupied in the ground state, but may be occupied by an electron upon electronic excitation of the molecule.
There are different 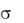
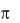 , nonbonding n orbitals, and antibonding
, nonbonding n orbitals, and antibonding 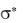 and
and 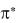 orbitals.
orbitals.
n orbitals, which are located on heteroatoms such as oxygen, nitrogen, or phosphorus, are nonbonding and are of almost the same energy as in the case of the isolated atom. A pair of electrons occupying an n orbital is regarded as a lone pair on the atom in question.
When a molecule in its ground state absorbs a photon, an electron occupying and , or n orbital is promoted to a higher-energy  or
or 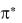 orbital. In principle,
orbital. In principle,
the following transitions are possible:

transitions in organic compounds

• * and
* and  * transitions: high-energy, accessible in vacuum UV ( max <150 nm). Not usually observed in molecular UV-Vis.
* transitions: high-energy, accessible in vacuum UV ( max <150 nm). Not usually observed in molecular UV-Vis.
n  * and
* and  * transitions: non-bonding electrons (lone pairs), wavelength ( max) in the 150-250 nm region.
* transitions: non-bonding electrons (lone pairs), wavelength ( max) in the 150-250 nm region.
n  * and
* and  * transitions: most common transitions observed in organic molecular UV-Vis, observed in compounds with lone pairs and multiple bonds with max = 200-600 nm.
* transitions: most common transitions observed in organic molecular UV-Vis, observed in compounds with lone pairs and multiple bonds with max = 200-600 nm.

