
МОНОГРАФИИ ВОЗ Т 4
.pdf
Fructus Myrtilli
References
1.European Pharmacopoeia, 5th ed. Strasbourg, Directorate for the Quality of Medicines of the Council of Europe, 2005.
2.Upton R et al., eds. Bilberry fruit. Vaccinium myrtillus L. In: American herbal pharmacopeia. Santa Cruz, CA, American Herbal Pharmacopeia, 2001.
3.Farnsworth NR, ed. NAPRALERT database. Chicago, University of Illinois at Chicago, IL (an online database available directly through the University of Illinois at Chicago or through the Scientific and Technical Network [STN] of Chemical Abstracts Services), 30 June 2005.
4.Bedevian AK. Illustrated polyglottic dictionary of plant names. Cairo, Medbouly Library, 1994.
5.Bisset NR, Wichtl M, eds. Herbal drugs and phytopharmaceuticals, English ed. Boca Raton, FL, Medpharm, 1994.
6.Chandra A, Rana J, Li Y. Separation, identification, quantitation, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. Journal of Agriculture and Food Chemistry, 2001, 49:3515– 3521.
7.Ichiyanagi T et al. Structural dependence of HPLC separation pattern of anthocyanins from bilberry (Vaccinium myrtillus L.). Chemical and Pharmaceutical Bulletin, 2004, 52:628–630.
8.WHO guidelines on assessing quality of herbal medicines with reference to contaminants and residues. Geneva, World Health Organization, 2007.
9.Guidelines for predicting dietary intake of pesticide residues, 2nd rev. ed. Geneva, World Health Organization, 1997 (WHO/FSF/FOS/97.7).
10.Bruneton J. Pharmacognosy, phytochemistry, medicinal plants. Paris, Lavoisier, 1995.
11.Colombo D, Vescovini R. Studio clinico controllato sull’efficacia degli antocianosidi del mirtillo nel trattamento della dismenorrea essenziale [Controlled trial of anthocyanosides from Vaccinium myrtillus in primary dysmenorrhea]. Giornale Italiano di Ostetricia e Ginecologia, 1985, 7:1033–1038 [in Italian].
12.Coget J, Merlen JF. Etude clinique d’un nouvel agent de protection vasculaire le difrarel 20, compose d’anthocyanosides extraits du vaccinum myrtillus. Phlébologie, 1968, 21:221–228.
13.Bratman S, Kroll D. Bilberry (Vaccinium myrtillus). In: The natural pharmacist: clinical evaluation of medicinal herbs and other therapeutic natural products. Roseville, CA, Prima Publishing, 1999.
14.Ghiringhelli C, Gregoratti L, Marastoni F. Attivitá capillarotropa di antocianosidi ad alto dosaggio nella stasi da flebopatia [Capillarotropic activity of anthocyanosides in high doses in phlebopathic stasis]. Minerva Cardioangiologia, 1978, 26:255–276 [in Italian].
15.Jayle GE, Aubert L. Action des glucosides d’anthocyanes sur la vision scotopique et mésopique du sujet normal. Thérapie, 1964:19:171–185 [in French].
223

WHO monographs on selected medicinal plants
16.Mian E et al. Antocianosidi e parete dei microvasi nuovi aspetti sul modo d’azione dell’effetto protettivo nelle sindromi da absorme fragilitá capillare [Anthocyanosides and the walls of the microvessels: further aspects of the mechanism of action of their protective effect in syndromes due to abnormal capillary fragility]. Minerva Medica, 1997, 68:3565–3581 [in Italian].
17.Bravetti G. Preventive medical treatment of senile cataract with vitamin E and anthocyanosides: clinical evaluation. Annali di Ottalmologia e Clinica Oculistica, 1989, 115:109.
18.Head K. Natural therapies for ocular disorders part two: cataracts and glaucoma. Alternative Medicine Review, 2001, 6:141–166.
19.Perossini M et al. Diabetic and hypertensive retinopathy therapy with Vaccinium myrtillus anthocyanosides (Tegens): Double-blind placebo controlled clinical trial. Annali di Ottalmologia e Clinica Oculistica, 1987, 113:1173 [in Italian].
20.Blumenthal M et al., eds. The complete German Commission E monographs: therapeutic guide to herbal medicines. Austin, TX, American Botanical Council, 1998.
21.Lietti A, Cristoni A, Picci M. Studies on Vaccinium myrtillus anthocyanosides. I. Vasoprotective and antiinflammatory activity. Arzneimittel-For- schung, 1976, 26:829–832.
22.Laplaud PM, Lelubre A, Chapman MJ. Antioxidant action of Vaccinium myrtillus extract on human low density lipoproteins in vitro: initial observations. Fundamental & Clinical Pharmacology, 1997, 11:35–40.
23.Martín-Aragón S et al. Antioxidant action of Vaccinium myrtillus L. Phytotherapy Research, 1998, 12:S104–S106.
24.Prior RL et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. Journal of Agricultural and Food Chemistry, 1998, 46:2686–2693.
25.Wasek M et al. Electron spin resonance assessment of the antioxidant potential of medicinal plants. Part I. Contribution of anthocyanosides and flavonoids to the radical scavenging ability of fruit and herbal teas. Acta Polaniae Pharmaceutica, 2001, 58:283–288.
26.Lietti A, Forni G. Studies on Vaccinium myrtillus anthocyanosides. II. Aspects of anthocyanins pharmacokinetics in the rat. Arzneimittel-Forschung, 1976, 26:832–835.
27.Eandi M. Post-marketing investigation on Tegens preparation with respect to side effects. 1987. Cited in Morazzoni P, Bombardelli E. Vaccinium myrtillus I. Fitoterapia, 1996, 67:3–29.
28.Detre Z et al. Studies on vascular permeability in hypertension: Action of anthocyanosides. Clinical Physiology and Biochemistry, 1986, 4:143–149.
29.Kadar A et al. Influence of anthocyanoside treatment on the cholesterolinduced atherosclerosis in the rabbit. Paroi Arterielle, 1979, 5:187–205.
30.Cohen-Boulakia F et al. In vivo sequential study of skeletal muscle capillary permeability in diabetic rats: effect of anthocyanosides. Metabolism, 2000, 49:880–885.
224

Fructus Myrtilli
31.Bertuglia S, Malandrino S, Colantuoni A. Effect of Vaccinium myrtillus anthocyanosides on ischaemia reperfusion injury in hamster cheek pouch microcirculation. Pharmacology Research, 1995, 31:183–187.
32.Colantuoni A et al. Effects of Vaccinium myrtillus anthocyanosides on arterial vasomotion. Arzneimittel-Forschung, 1991, 41:905–909.
33.Caselli L. Studio clinico ed elettroretinografico sull’attivitá degli antocianosidi [Clinical and electroretinographic study on activity of anthocyanosides]. Archivio di Medicina Interna, 1985, 37:29–35 [in Italian].
34.Scharrer A, Ober M. [Anthocyanosides in the treatment of retinopathies (author’s translation)]. Klinische Monatsblatter für Augenheilkunde, 1981, 178:386–389.
35.Politzer M. [Experiences in the medical treatment of progressive myopia (author’s translation)]. Klinische Monatsblatter für Augenheilkunde, 1977, 171:616–619.
36.Muth ER, Laurent JM, Jasper P. The effect of bilberry nutritional supplementation on night visual acuity and contrast sensitivity. Alternative Medicine Review, 2000, 5:164–173.
37.Jayle GE et al. Étude concernant l’action sur la vision nocturne [Study concerning the action of anthocyanoside extracts of Vaccinium myrtillus on night vision]. Annales d’oculistique, 1965, 198:556–562 [in French].
38.Terrasse J, Moinade S. Premiers résultats obtenus avec un nouveau facteur vitaminique P «les anthocyanosides» extraits du Vaccinium myrtillus. Presse Medicale, 1964, 72:397–400 [in French].
39.Gloria E, Peria A. Effetto degli antocianosidi sulla soglia visiva assoluta [Effect of anthocyanosides on the absolute visual threshold]. Annali di Ottalmologia e Clinica Oculistica, 1966, 92:595–607 [in Italian].
40.Sala D et al. Effect of anthocyanosides on visual performances at low illumination. Minerva Oftalmologia, 1979, 21:283–285.
41.Levy Y, Glovinsky Y. The effect of anthocyanosides on night vision. Eye, 1998, 12:967–969.
42.Zadok D, Levy Y, Glovinsky Y. The effect of anthocyanosides in a multiple oral dose on night vision. Eye, 1999, 13:734–736.
43.Pennarola R et al. The therapeutic action of the anthocyanosides in microcirculatory changes due to adhesive-induced polyneuritis. Gazzette Medicina Italiana, 1980, 139:485–491 [in Italian].
44.Erlund I et al. Consumption of black currants, lingonberries and bilberries increases serum quercetin concentrations. European Journal of Clinical Nutrition, 2003, 57:37–42.
45.Puilliero G et al. Ex vivo study of the inhibitory effects of Vaccinium myrtillus anthocyanosides on human platelet aggregation. Fitoterapia, 1989, 60:69–75.
225

Radix Panacis Quinquefolii
Definition
Radix Panacis Quinquefolii consists of the dried roots of Panax quinquefolius L. (Araliaceae) (1–3).
Synonyms
Aralia quinquefolia Dec. & Planch., Ginseng quinquefolium Wool, Panax americanum Raf. (4).
Selected vernacular names
Ameerika zensen, American ginseng, American white ginseng, Amerika ninjin, Amerikanischer Ginseng, Amerikanne Ginseng, Canadian ginseng, Canadian white ginseng, Chinese seng, five fingers, garantogen, ginseng americano, ginseng d’Amerique, hsi-yang-shen, hua-ch’i-seng, hua- ch’i-senh, little Indian, man root, matc’etasa, ninsin, North American ginseng, seng, seiyou ninjin, shang, wenane, wild American ginseng, xi yang shen, xi-yang-shen (5, 6).
Geographical distribution
Native to Canada and the United States, and cultivated in France and northern China (7).
Description
Herb, up to more than 1 m high; rootstock fusiform, up to 2 cm in diameter; stem straight, slender, subterete, often striate; leaves 3 or 4, 5 (3–7)- foliolate, petiole slender, up to 10 (rarely to 15) cm long, leaflets thin, elliptic to obovate, up to 16 cm long and 8.5 cm broad (the basal ones smaller, often ovate), acute to rounded at the base, acuminate at the apex, conspicuously and often doubly serrate, the teeth deltoid, acute, the petiolules up to 4.5 cm long, the principal veins slightly raised on both surfaces, lateral veins 5–9, ascending; peduncle slender, straight, up to 10 (rarely to 30) cm long, the bractlets deltoid to lanceolate, acute, 2–5 mm long; pedicels 15–40 per umbel, up to 12 mm long, swollen distally; calyx
226

Radix Panacis Quinquefolii
carnose, cupuliform, at anthesis about 2 mm long and 2 mm in diameter, the lobes deltoid, acute, about 0.5 mm long; petals greenish-white, membranous, slightly granular-papillose distally, oblong, about 1.5 mm long and 1 mm broad, subacute and slightly incurved at the apex; filaments carnose, narrowed distally, 1–1.5 mm long, the anthers oblong, about 1 mm long, obtuse at both ends; summit of the ovary flattened or concave, styles 2, carnose, slightly curved, 1–1.5 mm long; locules 2; fruit laterally flattened, transversely oblong, up to 7 mm long and 10 mm broad, longitudinally sulcate, the wall at length dry, seeds 2, oblong, 4–5 mm long, 3–4 mm broad (4).
Plant material of interest: dried roots
General appearance
Fusiform, cylindrical or conical, 1–12 cm, sometimes up to 20 cm in length, and up to 2.5 cm in diameter at the crown, with one or more stem scars. Externally pale yellow to golden, exhibiting transverse striations and linear lenticels, and showing fine and dense longitudinal wrinkles, and rootlet scars. If stem base is present, then scales thin and perishing (differs from P. ginseng, in which scales at base of stem are fleshy and persistent). The middle and lower part of the main root with 1 or more lateral roots, mostly broken off. Sometimes, the upper part with rhizome showing distinct annulations, rounded or semi-rounded stem scars, and bearing adventitious roots, or broken off. Texture heavy and hard, not easily broken, fracture short, fractured surface is white to ivory, with distinct aromatic odour and rings of secretory canals present in secondary phloem, slightly starchy, bark exhibiting yellowish brown dotted resin canals, cambium ring brownish yellow, wood exhibiting less distinct radiate striations (1, 2).
Organoleptic properties
Odour: slight and characteristic; taste: slightly bitter and sweet (1, 2).
Microscopic characteristics
In contrast to Panax ginseng, transverse sections of P. quinquefolius show abundant large, thin-walled cork parenchyma cells. Secondary phloem is characterized by conspicuous air lacunae; abundant starch-containing storage parenchyma; few sieve elements, found in small groupings; and rings of schizogenous secretory canal lined with 6–8 epithelial cells that lack starch. Xylem is characterized by abundant starch-containing storage parenchyma and a few tracheary elements, composed of non-lignified tracheids and slightly lignified spiral or reticulated vessels lacking secretory canals and found in isolation or in small groupings. Druse crystals
227

WHO monographs on selected medicinal plants
are sometimes present within vascular parenchyma cells. Diarch or triarch primary xylem is in the centre of root starch grains, simple or 2–5- compound, range from 5–6 μm in diameter (1, 8).
Powdered plant material
Pale yellowish brown powder with a slightly aromatic odour. Shows cork cells; yellowish secretory canals and yellowish brown secretory substances in and outside the canal; fragments of parenchyma cells contain starch grains, simple or 2–5-compound, range from 5–6 μm in diameter; tracheids with spiral or reticulate thickening rings; irregular shaped bast fibres, lumen smooth, 200–1200 μm in length; rosettes of calcium oxalate 30–33 μm in diameter (1, 8).
General identity tests
Macroscopic (1, 2) and microscopic examinations (1, 8); DNA analysis (9); and thin-layer chromatography (1, 2, 10) and high-performance liquid chromatography (1, 2, 11, 12) for the presence of 24(R)-pseudogin- senoside F11 and the absence of ginsenoside Rf.
Purity tests
Microbiological
Tests for specific microorganisms and microbial contamination limits are as described in the WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues (13).
Foreign organic matter
Not more than 2.0% (1).
Total ash
Not more than 8.0% (1).
Acid-insoluble ash
Not more than 1% (2).
Water-soluble extractive
To be established in accordance with national requirements.
Alcohol-soluble extractive
Not less than 30% (2).
Loss on drying
Not more than 10.0% (1).
228

Radix Panacis Quinquefolii
Pesticide residues
The recommended maximum limit of aldrin and dieldrin is not more than 0.05 mg/kg (14). For other pesticides, see the European pharmacopoeia (14) and the WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues (13) and pesticide residues (15).
Heavy metals
For maximum limits and analysis of heavy metals, consult the WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues (13).
Radioactive residues
Where applicable, consult the WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues (13).
Chemical assays
Not less than 2% total ginsenosides of Rg1, Re and Rb1 (2).
Major chemical constituents
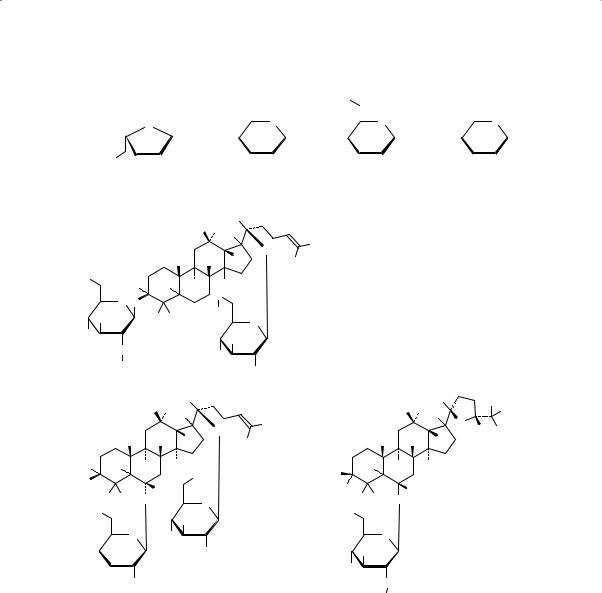
Major constituents of the root are the damamrane triterpene saponins collectively known as ginsenosides. As in the case of Panax ginseng, the ginsenosides of P. quinquefolius are derivatives of protopanaxadiol or protopanaxatriol, with the majority of these compounds (e.g. ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1 and Ro) being common to both species. However, there are quantitative and qualitative differences. The total ginsenoside content of Panax quinquefolius is higher than that of Panax ginseng whereas the ginsenosides Rf and Rg2 do not occur in P. quinquefolius. On the other hand, 24(R)-pseudoginsenoside F11 is found in P. quinquefolius, but not in P. ginseng. In cultivated P. quinquefolius, however, the dominant ginsenosides are malonyl (m)-Rbl, Rb1 and Re with the percentages of m-Rb1 and Rb1 being almost identical. (Rg1 levels and total ginsenosides are much higher in wild than in cultivated P. quinquefolius.) (6). Furthermore, the combined amount of Rbl and m-Rb1 often exceeds half of the total ginsenoside content with the total malonyl ginsenoside (m-Rb1, m-Rb2, m-Rc and m-Rd) content being approximately 40% (5, 6). In a study of wild American ginseng, total ginsenosides range from 1–16%, with the majority being in the range of 4–5% (16). Polysaccharides of biological significance include quinquefolans A, B and C (17). The structures of ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1 and pseudoginsenoside F11 are presented below.
229
WHO monographs on selected medicinal plants
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|||
|
|
|
O |
|
HO |
|
O |
|
|
|
O |
|
HO |
|
O |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Araf = |
OH |
Arap = |
|
OH |
Glc = |
OH |
Rha = |
|
CH3 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
OH |
|
|
|
OH |
|
|
|
OH OH |
|||||
-L-arabinofuranosyl -L-arabinopyranosyl -D-glucopyranosyl -L-rhamnopyranosyl
|
|
|
|
|
H3C |
|
|
|
|
|
HO |
H H |
|
|
|
|
|
|
CH3 H |
O |
|
|
|
|
CH3 |
H3C |
|
HO |
H |
|
H |
H |
CH3 |
|
|
|
|
||||
|
O O |
|
|
|
O |
|
|
|
|
|
R |
|
|
OH |
|
H3C |
CH3 |
|
||
|
|
O |
||||
|
|
|
|
|
|
|
HO |
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
O |
|
|
|
HO |
|
|
|
|
|
|
|
|
|
Glc |
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
H3C |
|
|
|
|
|
HO |
H H |
|
CH3 |
|
|
|
|
|
|
O |
|
|
|
|
|
H |
|
|
|
CH3 CH3 |
H3C |
|
|||
H |
H |
H |
CH3 |
|
|
|
HO |
|
|
|
OH |
|
|
|
H |
|
|
|
||
H3C |
CH3 |
|
|
|
||
O |
|
O |
|
|||
HO |
|
|
|
|
||
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
OHO
|
OH |
OH |
|
||||
|
|
||||||
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
O |
Ginsenoside Rg1 |
|
|||||
|
|
|
|||||
|
|
|
|
|
R = H |
||
|
|
R |
|||||
|
|
|
|
|
|
Ginsenoside Re |
R = Rha |
CH3 Ginsenoside Rb1 |
R = Glc |
|
|||||
Ginsenoside Rb2 |
R = Arap |
|
|||||
Ginsenoside Rc |
R = Araf |
|
|||||
Ginsenoside Rd |
R = H |
|
|||||
|
|
HO |
|
H |
H3C |
OH |
|
|
|
|
H |
|
CH3 |
||
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
H |
|
CH3 |
CH3 |
H |
|
||||
|
|
H |
|
|
CH3 |
|
|
H |
|
|
|
||||
HO |
|
|
|
|
|
|
|
H |
H |
|
|
|
|||
H3C CH3 |
|
|
|
||||
O |
|
|
|
|
|
||
HO
O
OH
HO
O
Rha
Pseudoginsenoside F11
Medicinal uses
Uses supported by clinical data
None.
Uses described in pharmacopoeias and well established documents
While some preliminary studies have indicated that the root may be useful as an adjunct for the management of postprandial hyperglycaemia in subjects with type 2 diabetes mellitus (18–22), the numbers of subjects participating in the studies was too small to enable any solid conclusions
230

Radix Panacis Quinquefolii
to be drawn. Further randomized controlled clinical trials with larger populations of patients with diabetes and better methodology are needed before therapeutic recommendations can be made.
Uses described in traditional medicine
Used orally as a diuretic, digestive, tonic and a stimulant (5, 6, 23). Used to enhance stress resistance, and to treat cough, loss of appetite, colic, vomiting, insomnia, neuralgia, rheumatism and headaches (5, 24).
Experimental pharmacology
Antihyperglycaemic activity
Intraperitoneal administration of an aqueous extract of the root exhibited significant hypoglycaemic activity in mice with alloxan-induced hyperglycaemia when administered at a dose of 10 g/kg body weight (bw) (p < 0.01). Activity-guided fractionation of the extract led to isolation of three glycans, the quinquefolans A, B and C, which displayed significant hypoglycaemic activity (p < 0.01, p < 0.05, p < 0.01, respectively) in normal mice and in mice with alloxan-induced hyperglycaemia at a dose of 10, 100 and 10 mg/kg bw, of quinquefolans A, B and C, respectively (17).
Antioxidant activity
The antioxidant activity of a root extract containing 8% ginsenosides was assessed in the metal chelation, affinity to scavenge 1,1-diphenyl-2- picrylhydrazyl stable free radical, and peroxyl and hydroxyl free radical assays. Dissociation constants (Kd) for the extract to bind transition metals were of the order of Fe2+ > Cu2+ > Fe3+ and corresponded to the affinity to inhibit metal-induced lipid peroxidation. In a metal-free linoleic acid emulsion, the extract exhibited a significant concentrationdependent (0.01–10 mg/ml) mitigation of lipid oxidation as assessed by the ammonium thiocyanate method (p α 0.05). The extract also showed strong 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity up to a concentration of 1.6 mg/ml (r2 = 0.996). The extract also inhibited the non-site-specific deoxynucleic acid (DNA) strand breakage caused by Fenton agents, and suppressed the Fenton-induced oxidation of a 66 Kd soluble protein obtained from mouse brain over a concentration range of 2.0–40.0 mg/ml (25). The free radical scavenging capacity and antioxidant effects of the crude drug appear to be related to the concentration of ginsenosides Rb1 and Rb2 in the plant (26).
The effect of saponins, isolated from the root, on the oxidation of lowdensity lipoprotein (LDL) was measured. The potential of the saponins for reducing LDL oxidation as well as limiting the ability of oxidized-LDL
231

WHO monographs on selected medicinal plants
(Ox-LDL) to impair endothelium-dependent relaxation in rat aortic rings was specifically assessed. Native LDL (0.2 or 0.3 mg/ml) was incubated with the saponins (0.25–1.0 mg/ml), with vitamin C (50 μM) as a control. Oxidation was initiated with copper sulfate at 37 ºC for 0–24 h. The results demonstrate that the saponins reduced lipid peroxide levels concentrationdependently and also reduced alterations in relative electrophoretic mobility of Ox-LDL in a similar manner. Furthermore, measurement of content of phospholipid fractions indicated that the saponins reduced the conversion of phosphatidylcholine to lysophosphatidylcholine in Ox-LDL (27). Further investigations indicated that lower concentrations of saponins (1.0–100.0 μg/ml) in combination with vitamin C (1.0–10.0 μM) significantly enhanced the protective effects of the saponins. Pretreatment with the saponins (1.0–100.0 μg/ml) resulted in concentration-dependent inhibition of LDL oxidation and prolongation of lag time (28).
Antithrombotic activity
The effects of a root extract on thrombin-induced endothelin release were investigated using cultured human umbilical vein endothelial cells. Endothelial cells were pretreated with 1, 10 and 100.0 μg/ml of the extract and then incubated for 4 and 24 h with thrombin. The results demonstrated that the concentration of endothelin was significantly decreased in a concentration-dependent, time-related manner (at 4 h, 50% inhibitory concentration (IC50) = 5.1 μg/ml; at 24 h, IC50 = 6.2 μg/ml). Furthermore, pretreatment of cultured endothelial cells with NG-nitro-L-arginine, a nitric oxide synthetase inhibitor, significantly (p < 0.05) inhibited throm- bin-induced endothelin release by the extract indicating that the effect was partly due to release of nitric oxide (29).
Hormonal activity
A methanol extract of the root (100 g root in methanol 600 ml) did not significantly bind to either estrogen receptor Α or Β at a concentration of 20.0 μg/ml, but did increase expression of pS2 (an estrogen-sensitive gene) in S-20 cells (30).
The expression of the estrogen-regulated gene pS2 in MCF-7 breast cancer cells was assessed after treatment of the cells with a decoction of the roots. The extract and estradiol were equally able to induce RNA expression of pS2, and the extract caused a dose-dependent decrease in cell proliferation (p < 0.005) (31). The estrogenic effects of a decoction of the roots on the expression of pS2, an estrogen-regulated gene, in breast cancer cell lines MCF-7, T-47D and BT-20 was evaluated by Northern and Western blot analyses. Competitive studies were also performed with ginseng in combination with tamoxifen. The ginseng extract (600.0 μg) and
232
