
МОНОГРАФИИ ВОЗ Т 4
.pdf
Fructus Macrocarponii
rin was reported in a 69-year-old male patient with a mitral valve replacement (49). The patient was ingesting 2 l of cranberry juice per day in addition to warfarin and his INR ranged between 8 and 11. The patient was advised to stop drinking cranberry juice and his INR returned to 3 (normal) after 3 days.
The Medicines and Healthcare Products Regulatory Agency (MHPRA) in the United Kingdom has reported numerous cases of a possible interaction between cranberry juice and warfarin, leading to changes in the INR or to bleeding. In four cases, the increase in the INR or bleeding was not significant. Two cases of an unstable INR and one case of a decreased INR were reported (48).
Carcinogenesis, mutagenesis, impairment of fertility
No information was found.
Other precautions
No information was found.
Dosage forms
Crude drug, extracts, juice, tablets, capsules. Store in a well-closed container, in a refrigerator (2).
Posology
(Unless otherwise indicated)
For the prevention of UTIs in adults the recommended daily dose of cranberry juice is 30–300 ml of a 30% pure juice product; for the treatment of UTIs in adults the daily dosage range is 360–960 ml or equivalent (1).
Capsules containing a concentrated cranberry extract: 1–6 capsules daily, equivalent to 3 fluid ounces (90 ml) cranberry juice or 400–450 mg cranberry solids (1).
References
1.Upton R, Graff A, Swisher D, eds. Cranberry fruit. Vaccinium macrocarpon Aiton. In: American herbal pharmacopeia. Santa Cruz, CA, American Herbal Pharmacopeia, 2002.
2.The United States Pharmacopeia. 29. Rockville, MD, United States Pharmacopeia Convention, 2005.
3.Farnsworth NR, ed. NAPRALERT database. Chicago, University of Illinois at Chicago, IL (an online database available directly through the University of Illinois at Chicago or through the Scientific and Technical Network [STN] of Chemical Abstracts Services), 30 June 2005.
163

WHO monographs on selected medicinal plants
4.Ernst E et al., eds. The desktop guide to complementary and alternative medicine. Edinburgh, Mosby, 2001.
5.WHO guidelines on assessing quality of herbal medicines with reference to contaminants and residues. Geneva, World Health Organization, 2007.
6.European Pharmacopoeia, 5th ed. Strasbourg, Directorate for the Quality of Medicines of the Council of Europe, 2005.
7.Guidelines for predicting dietary intake of pesticide residues, 2nd rev. ed. Geneva, World Health Organization, 1997 (WHO/FSF/FOS/97.7).
8.Foda M et al. Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Canadian Journal of Urology, 1995, 2:98– 102.
9.Gibson L et al. Effectiveness of cranberry juice in preventing urinary tract infections in long term care facility patients. Journal of Naturopathic Medicine, 1991, 2:45–47.
10.Haverkorn MJ, Mandigers J. Reduction of bacteriuria and pyuria using cranberry juice [letters]. Journal of the American Medical Association, 1994, 272:590.
11.Jackson B, Hicks LE. Effect of cranberry juice on urinary pH in older adults.
Home Healthcare Nurse, 1997, 15:199–202.
12.Kahn HD et al. Effect of cranberry juice on urine. Journal of the American Dietetic Association, 1967, 51:251.
13.Kinney AB, Blount M. Effect of cranberry juice on urinary pH. Nursing Research, 1979, 28:287–290.
14.Kontiokari T et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women.
British Medical Journal, 2001, 322:1–5.
15.Papas PN, Brusch CA, Ceresia GC. Cranberry juice in the treatment of urinary tract infections. Southwestern Medicine, 1966, 47:17–20.
16.Reid G et al. Cranberry juice consumption may reduce biofilms on uroepithelial cells: pilot study in spinal cord injured patients. Spinal Cord, 2001, 39:26–30.
17.Schlager TA et al. Effect of cranberry juice on bacteriuria in children with neurogenic bladder receiving intermittent catheterization. Journal of Pediatrics, 1999, 135:698–702.
18.Sobota AE. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. Journal of Urology, 1984, 131:1013–1016.
19.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infections in women. Canadian Journal of Urology, 2002, 9:1558–1562.
20.Walker EB et al. Cranberry concentrate: UTI prophylaxis. Journal of Family Practice, 1997, 45:167–168.
21.Rogers J. Pass the cranberry juice. Nursing Times, 1991, 87:36–37.
22.Hrastinger A et al. Is there clinical evidence supporting the use of botanical dietary supplements in children? Journal of Pediatrics, 2005, 146:311–317.
164

Fructus Macrocarponii
23.Mahady GB, Fong HHS, Farnsworth NR. Cranberry. In: Botanical dietary supplements: quality, safety and efficacy. Lisse, Swets & Zeitlinger, 2001.
24.Cavanagh HMA, Hipwell M, Wilkinson JM. Antibacterial activity of berry fruits used for culinary purposes. Journal of Medicinal Food, 2003, 6:57–61.
25.Ahuja S, Kaack B, Roberts J. Loss of fimbrial adhesion with the addition of Vaccinum macrocarpon to the growth medium of P-fimbriated Escherichia coli. Journal of Urology, 1998, 159:559–562.
26.Howell AB et al. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial cell surfaces by proanthocyanidin extracts from cranberries.
New England Journal of Medicine, 1998, 339:1085–1086.
27.Ofek I et al. Anti-Escherichia adhesin activity of cranberry and blueberry juices. New England Journal of Medicine, 1991, 324:1599.
28.Zafriri D et al. Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eucaryotic cells. Antimicrobial Agents Chemotherapy, 1989, 33:92–98.
29.Foo LY et al. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry, 2000, 54:173–181.
30.Howell AB et al. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry, 2005, 66:2281–2291.
31.Burger O et al. A high molecular mass constituent of cranberry juice inhibits
Helicobacter pylori adhesion to human gastric mucus. FEMS Immunology and Medical Microbiology, 2000, 29:295–301.
32.Howell AB, Foxman B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. Journal of the American Medical Association, 2002, 287:3082–3083.
33.Habash MB et al. The effect of water, ascorbic acid, and cranberry derived supplementation on human urine and uropathogen adhesion to silicone rubber. Canadian Journal of Microbiology, 1999, 45:691–694.
34.Zheng W, Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. Journal of Agricultural and Food Chemistry, 2003, 51:502–509.
35.Yan X et al. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). Journal of Agricultural and Food Chemistry, 2002, 50:5844–5849.
36.Youdim KA et al. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. Journal of Nutritional Biochemistry, 2002, 13:282–288.
37.McMurdo MET et al. Does ingestion of cranberry reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebocontrolled trial. Age and Ageing, 2005, 34:256–261.
38.Avorn J et al. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. Journal of the American Medical Association, 1994, 271:751–754.
39.Waites KB et al. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. Journal of Spinal Cord Medicine, 2004, 27:35–40.
165

WHO monographs on selected medicinal plants
40.Tsukada K et al. Cranberry juice and its impact on peri-stomal skin conditions for urostomy patients. Ostomy/Wound Management, 1994, 40:60–62, 64, 66–67.
41.Morris NS, Stickler DJ. Does drinking cranberry juice produce urine inhibitory to the development of crystalline, catheter-blocking Proteus mirabilis biofilms? British Journal of Urology International, 2001, 88:192–197.
42.McHarg T, Rodgers A, Charlton K. Influence of cranberry juice on the urinary risk factors for calcium oxalate kidney stone formation. British Journal of Urology International, 2003, 92:765–768.
43.Terris MK, Issa MM, Tacker JR. Dietary supplementation with cranberry concentrate tablets may increase the risk of nephrolithiasis. Urology, 2001, 57:26–29.
44.Kessler T, Jansen B, Hesse A. Effect of blackcurrant-, cranberryand plum juice consumption on risk factors associated with kidney stone formation.
European Journal of Clinical Nutrition, 2002, 56:1020–1023.
45.Davies JK, Ahktar N, Ranasinge E. A juicy problem. The Lancet, 2001, 358:216.
46.Garcia-Calatayud S, Larreina Córdoba JJ, Lozano de la Torre MJ. Intoxicación grave por zumo de arándanos [Cranberry intoxication in a 4-month- old infant]. Anales Espanoles de Pediatriá, 2002, 56:72–73 [in Spanish].
47.Nowack R. Die amerikanische Cranberry (Vaccinum macrocarpon Aiton).
Zeitschrift für Phytotherapie, 2003, 24:40–46.
48.Suvarna R, Pirmohamed M, Henderson L. Possible interaction between warfarin and cranberry juice. British Medical Journal, 2003, 327:20–27.
49.Grant P. Warfarin and cranberry: an interaction? Journal of Heart Valve Disease, 2004, 13:25–26.
166

Cortex Magnoliae
Definition
Cortex Magnoliae consists of the dried stem, trunk or root bark of Magnolia officinalis Rehder and Wilson, M. officinalis Rehder and Wilson var. biloba Rehder and Wilson (1–3), or of M. obovata Thunberg. (1, 3, 4) (Magnoliaceae).12
Synonyms
Magnolia hypoleuca Diels, non Sieb. et Zucc. (6, 7).
Selected vernacular names
Chung-pu, danghoobak, hou-po, hou-pu, hòupò, hsin-j, koboku, magnolia bark, mubak (8–11).
Geographical distribution
Native to China (7, 9, 11).
Description
Deciduous tree, large, up to 22 m in height. Bark smooth, light rusty-ash grey colour and aromatic. Branchlets light-green or yellowish. Leaves entire, very large, elliptic-obovate, up to 35 cm long and 10–20 cm wide. Large creamy-white, fragrant flowers, bisexual, 15–20 cm in diameter; 9–15 petals, 8–10 cm in length by 3 cm in width, with the outer 3 being pale green tinged pink on the edge and the inner 6–12 being creamy white. Fruit oblong-ovoid, 10–12 cm in length, apex truncate and base rounded. Carpels rounded at the base. Seeds single (7, 9).
1The flowers of Magnolia officinalis Rehder and Wilson and M. officinalis Rehder and Wilson var. biloba Rehder and Wilson are also official in the Chinese Pharmacopoeia and the Japanese Standards for Herbal Medicines (2, 3, 5). However, there is currently insufficient information available to warrant the preparation of a monograph on this material.
167

WHO monographs on selected medicinal plants
Plant material of interest: dried stem, trunk or root bark
General appearance
Trunk bark: rough, 2–7 mm thick, plate-like or semi-tubular bark rolled into large, tight cylinders. The outer surface is greyish white to greyish brown, and rough, sometimes cork layer removed, and externally brown. Inner surface smooth, light brown to purplish brown. Cut surface extremely fibrous, light reddish brown to purplish brown (1, 3, 4). Stem bark: quilled or double-quilled, 30–35 cm long, 2–7 mm thick, commonly known as tongpu. Root bark: quilled singly or irregular pieces some curved like chicken intestine, commonly known as jichangpo (2).
Organoleptic properties
Odour: aromatic; taste: pungent and slightly bitter (1–4).
Microscopic characteristics
Transverse section reveals a thick cork layer or several thin cork layers. The outer surface of the cortex shows a ring of stone cells and scattered on the inner surface are numerous oil cells and groups of stone cells. Phloem rays 1–3 cells wide; fibre groups, mostly several in bundles, scattered. Bast fibre groups lined stepwise between the medullary rays in the secondary cortex. Most of the oil cells are scattered in the primary cortex, with small numbers scattered in the secondary cortex, but some can also be observed in the narrow medullary rays (1–4).
Powdered plant material
Yellow-brown powder consisting of a yellowish to red-brown cork layer; stone cells of various sizes or groups; numerous fibres, 12–32 μm in diameter, walls strongly thickened, sometimes undulate or serrate on one side, 1ignified, pit canals indistinct; oil cells containing a yellowish-brown to red-brown substance; single starch grains of about 10 μm in diameter and 2- to 4-com- pound starch grains, and parenchyma cells containing starch grains (1, 2, 4).
General identity tests
Macroscopic and microscopic examinations (1–4), microchemical test (4) and thin-layer chromatography (1–3).
Purity tests
Microbiological
Tests for specific microorganisms and microbial contamination limits are as described in the WHO guidelines on assessing quality of herbal medicines with reference to contaminants and residues (12).
168

Cortex Magnoliae
Chemical
To be established in accordance with national requirements.
Foreign organic matter
To be established in accordance with national requirements.
Total ash
Not more than 6% (1, 3–5).
Acid-insoluble ash
To be established in accordance with national requirements.
Water-soluble extractive
To be established in accordance with national requirements.
Alcohol -soluble extractive
Not less than 12% (1).
Loss on drying
To be established in accordance with national requirements.
Pesticide residues
The recommended maximum limit of aldrin and dieldrin is not more than 0.05 mg/kg (13). For other pesticides, see the European pharmacopoeia (13), and the WHO guidelines on assessing quality of herbal medicines with reference to contaminants and residues (12) and pesticide residues (14).
Heavy metals
For maximum limits and analysis of heavy metals, consult the WHO guidelines on assessing quality of herbal medicines with reference to contaminants and residues (12).
Radioactive residues
Where applicable, consult the WHO guidelines on assessing quality of herbal medicines with reference to contaminants and residues (12).
Chemical assay
Total magnolol and honokiol not less than 2.0% by high-performance liquid chromatography (2).
Major chemical constituents
Magnolol (0.01–16%) and honokiol (0.005–9%) are bioactive major constituents belonging to the lignan group of compounds. Another major
169
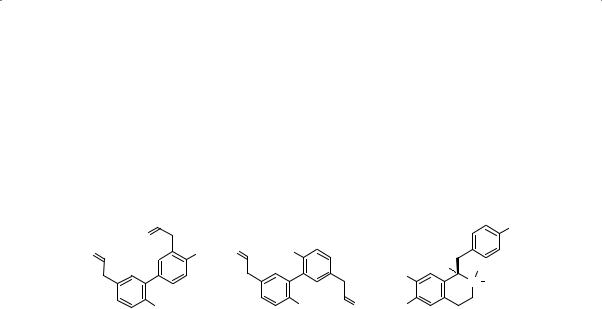
WHO monographs on selected medicinal plants
constituent is the isoquinoline alkaloid, magnocurarine (0.15–0.23%). The presence of liriodendrine has also been reported. The bark also contains an essential oil, the major constituents of which are cadinol (14%), 1,4-cineole (6%), p-cymene (8%), and Β-eudesmol (17%) and geraniol (9%), among others (8, 15–18). The structures of magnolol, honokiol and magnocurarine are presented below.
Honokiol |
Magnolol |
|
Magnocurarine |
OH |
H2C |
|
|
|
|
H C |
OH H2C |
HO |
|
|
2 |
|
|
|
|
|
|
|
H |
CH3 |
|
|
|
HO |
N+ CH3 |
|
|
|
|
|
OH |
|
OH |
CH2 H3CO |
|
Medicinal uses
Uses supported by clinical data
No information was found.
Uses described in pharmacopoeias and well established documents
Used orally for the treatment of gastrointestinal disorders such as constipation, dyspepsia, gastritis, nausea and vomiting. Also used orally to treat anxiety, coughs and shortness of breath (2).
Uses described in traditional medicine
Treatment of allergic rhinitis, headache, lack of appetite, respiratory congestion, neurosis and fever, and as a uterine stimulant (6, 8, 19, 20).
Pharmacology
Experimental pharmacology
Since no clinical studies have directly evaluated Cortex Magnoliae for any therapeutic condition and very few pharmacological studies have been conducted on extracts of the bark, most of this section describes the pharmacology and clinical studies of the major chemical constituents, particularly magnolol. The correlation of these data to the crude drug or its extracts requires further investigation.
Anti-allergic activity
Oral administration of 0.01–1.0 g/kg body weight (bw) of an aqueous extract of the bark dose-dependently inhibited compound 48/80-induced systemic anaphylaxis in rats (21). At the same dose, the aqueous extract
170

Cortex Magnoliae
also significantly inhibited local immunoglobulin E (IgE)-mediated passive cutaneous anaphylactic reaction and reduced the levels of plasma histamine in a dose-dependent manner (p < 0.05). In vitro, the extract (at concentrations of 0.001–1.0 mg/ml) concentration-dependently inhibited the histamine release from rat peritoneal mast cells activated by compound 48/80 or anti-dinitrophenyl IgE (21).
Anti-asthmatic activity
Magnolol stimulates calcium channel activity in tracheal smooth muscle cells as assessed by the patch clamp technique (22). In whole-cell current recordings, magnolol reversibly increased the amplitude of potassium outward currents. The increase in outward current caused by magnolol was sensitive to inhibition by iberiotoxin (200 nM) or paxilline (1 μM), but not by glibenclamide (10 μM). In inside-out patches, addition of magnolol to the bath did not modify single channel conductance, but effectively enhanced the activity of large conductance calcium (Ca) activated potassium BK (Ca) channels. Magnolol increased the probability of these channel openings in a concentration-dependent manner with a median effective concentration (EC50) value of 1.5 μM. The direct stimulation of these BK (Ca) channels by magnolol may explain the mechanism by which it acts as an anti-asthmatic compound (22).
Antibacterial activity
An ethanol extract of the bark inhibited the growth of Actinomyces viscosus ATCC 19246, Streptococcus mutans Ingbritt and Streptococcus sobrinus 6715, with a minimum bactericidal concentration of 0.488, 0.488 and 1.250 g/l, respectively (23). The antimicrobial activities of honokiol and magnolol were assayed using the agar dilution method and the minimum inhibitory concentration (MIC) was determined for each compound using a twofold serial dilution assay. The results showed that honokiol and magnolol have a marked antimicrobial effect (MIC, 25.0 μg/ml) against
Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Micrococcus luteus and Bacillus subtilis, but were not active against Shigella flexneii, Staphylococcus epidermidis, Enterobacter aerogenes, Proteus vulgaris, Escherichia coli and Pseudomonas aeruginosa
(24). An extract of the bark and magnolol inhibited the growth of Helicobacter pylori in vitro (25).
Anti-gastric ulcer activity
Intragastric administration of aqueous and methanol extract of the crude drug (400 mg/kg bw) reduced gastric juice secretion and increased the pH of gastric secretions in mice pretreated with indometacin (26). Indometa-
171

WHO monographs on selected medicinal plants
cin-induced gastric ulceration was reduced in animals treated with 400 mg/ kg bw of the methanol extract (26). Intragastric administration of an ethanol extract of the crude drug at a dose of 5.0 or 15.0 g/kg bw inhibited hydrochloric acid-induced gastric ulceration in mice (27).
Anti-inflammatory activity
Magnolol, isolated and purified from the crude drug, inhibited mouse hind-paw oedema induced by carrageenan, compound 48/80 and polymyxin B and reversed passive Arthus reaction when administered orally at a dose of 30 mg/kg bw (28).
Antioxidant activity
The accumulation of oxygen-free radicals and activation of neutrophils are implicated in the pathophysiological mechanisms that mediate myocardial ischaemia/reperfusion injury. Thus, antioxidants are purported to have cardioprotective activity. The antioxidant effect of magnolol was evaluated in an open-chest anaesthetized rat model of myocardial ischaemia/reperfusion injury (29). Intravenous pretreatment with magnolol, at a dose of 0.2 and 0.5 μg/kg bw at 10 minutes prior to 45 minutes of left coronary artery occlusion, reduced the incidence and duration of ventricular fibrillation and reduced mortality when compared with the control group. After 1 hour of reperfusion, pretreatment with magnolol reduced infarct size. In addition, magnolol, at a dose of 0.2 μg/kg bw, reduced superoxide anion production and myeloperoxidase activity, an index of neutrophil infiltration in the ischaemic myocardium (29).
Restenosis, a common complication after balloon angioplasty, involves a number of cytokines, chemotactic factors and growth factors. Antioxidants have been shown to inhibit intimal thickening after balloon injury in hyperlipidaemic animals. The effects of magnolol on the expression of monocyte chemotactic protein-1 and on intimal response in balloon-injured aorta of cholesterol-fed rabbits were investigated. The animals were fed a 2% highcholesterol diet together with daily intramuscular injection of either 1 μg/kg bw of magnolol or vehicle solvent for a total of 6 weeks, while 10 rabbits fed a regular diet served as a control group. A balloon denudation of abdominal aorta was performed in each group at the end of the third week, and aortas were harvested at the end of 6 weeks. Treatment with magnolol significantly inhibited copper-induced low-density lipoprotein oxidation in cholesterolfed rabbits and reduced atheroma formation (p < 0.05) in thoracic aortas without lowering serum cholesterol. The intimal response was significantly attenuated in magnolol-treated rabbits receiving high cholesterol when compared to those of the control high-cholesterol group (p < 0.05) (30).
172
