
- •Task №1
- •1. Present your arguments (logical and numerical) on the assessment
- •3. Comparison of the quantum energy with the binding energy of clusters in water. Calculation of the water heating temperature necessary for the destruction of its cluster structure:
- •4. Comparison of quantum energy with the energy of chemical bonding of atoms in a water molecule:
- •5. Comparison of the quantum energy from a microwave oven with the thermal energy characteristic of protein denaturation:
- •2. Calculation of the number of quanta for heating:
- •Task №3
- •3. Compare numerically 2 typical devices: vacuum and semiconductor according to the following parameters:
- •1. Numerical comparison of the maximum velocities of charged particles:
- •2. Numerical comparison of the length of the interaction region for the span angle of π - radians:
- •3. Numerical comparison of the bulk charge density:
- •4. Calculation of the microperviance and «plasma» frequency for a vacuum device:
- •5. Calculation of the Debye length and plasma frequency for a semiconductor device:
- •2 Балл task №4
- •4. Is it possible to provide high-speed modulation and grouping of charged particles in semiconductor devices using the initial part of the field-velocity characteristic?
- •2 Балл task №5
- •Task №6
- •6. Determine the noise factor of the amplifier in dB if its effective noise temperature is 115 k.
- •2. Calculation of the effective noise temperature of two such devices connected in a cascade:
- •3. Analyze the result:
- •0.75 Балл
- •1. Балл
MINISTRY OF EDUCATION AND SCIENCE OF RUSSIA
SAINT PETERSBURG STATE ELECTROTECHNICAL UNIVERSITY
«LETI» N.A. V.I. ULYANOVA (LENINA)
9.5+0.5а=10а балл Приятно осознавать, что есть такие студенты!
REPORT
on individual homework №1
in the discipline «Microwave electronics»
Topic: Theoretical foundations of microwave electronics
Student gr. 0207 _________________ Malikov B.I.
Teacher _________________ Ivanov V.A.
Saint Petersburg
2023
CONTENT
Task №1 …………………………………………………………………………... 3
Task №2 …………………………………………………………………………... 7
Task №3 …………………………………………………………………………. 11
Task №4 …………………………………………………………………………. 16
Task №5 …………………………………………………………………………. 19
Task №6 …………………………………………………………………………. 21
Task №7 …………………………………………………………………………. 23
List of used literature ……………………………………………………………. 25
Task №1
1. Present your arguments (logical and numerical) on the assessment
of the nature and features of the effects of microwave radiation on
humans. To do this, use the results of the following calculations.
1.1 Calculate the quantum energy of microwave radiation with a frequency of: 8,725 [GHz] and 34,5 [GHz]
1.2
Compare this energy with the energy of thermal vibrations of
molecules at Т
= 42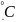 .
.
1.3 Compare the quantum energy with the binding energy of clusters in water.
To what temperature should water be heated to destroy its cluster structure?
1.4 Compare the quantum energy with the energy of chemical bonding of atoms in a water molecule.
1.5 Compare the quantum energy from a microwave oven with the thermal energy characteristic of protein denaturation.
Given:
GSM [1]: f0 = 1725 МHz => fGSM = 1,725 + Ngroup = 1,725 + 7 = 8,725 GHz
5G [2]: f0 = 27,5 GHz => f5G = 27,5 + Ngroup = 27,5 + 7 = 34,5 GHz
T = 42 = 315 K
Solving:
1. Calculation of the quantum energy of microwave radiation:
The quantum energy is calculated by the formula:
E
= h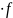 (1)
(1)
where
h = 6,626 10-26
J
s
– Planck 's constant; f – radiation frequency.
10-26
J
s
– Planck 's constant; f – radiation frequency.
Then, according to the formula (1), for the GSM standard:
EGSM = h fGSM = 6,626 10-34 8,725 109 = 5,781 10-24 J
For the 5G standard:
E5G = h f5G = 6,626 10-34 34,5 109 = 2,286 10-23 J
Thus, the energy of the microwave radiation quantum for the 5G standard is 2,286 10-23/5,781 10-24 = 4 times more energy for the GSM standard.
2. Calculation of the energy of thermal vibrations of molecules at Т=42 , comparison with the quantum energy of microwave radiation:
The energy of thermal vibrations is calculated by the formula:
ET = k T (2)
where
k =
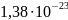 J/K – Boltzmann constant, T – temperature.
J/K – Boltzmann constant, T – temperature.
Then, the energy of thermal vibrations of molecules according to the formula (2):
ET
= k
T
=
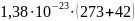 = 4,347
10-21
J
= 4,347
10-21
J
Thus, the energy of thermal vibrations of molecules is 4,347 10-21 /5,781 10-24 = 752 times more energy at the GSM frequency and 4,347 10-21/2,286 10-23 = 190 times more energy at the 5G frequency.
3. Comparison of the quantum energy with the binding energy of clusters in water. Calculation of the water heating temperature necessary for the destruction of its cluster structure:
Binding energy of clusters in water [3]:
EH20 = 0,24 eV = 3,845 10-20 J
That is, the binding energy of clusters in water is 3,845 10-20/5,781 10-24 = 6651 times more energy at GSM frequency, and 3,845 10-20/2,286 10-23 = 1682 times more energy at 5G frequency.
Let's calculate the water heating temperature, which is necessary for the destruction of its cluster structure:
EH2O
= k
T
=> T =
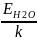 =
=
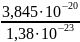 =
2786 K
=
2786 K
4. Comparison of quantum energy with the energy of chemical bonding of atoms in a water molecule:
The energy of the chemical bond of atoms in a water molecule [3]:
Echem = 4,8 eV = 7,69 10-19 J
Thus, the energy of chemical bonding of atoms in a water molecule in
7,69 10-19/5,781 10-24 = 133022 times more energy of the GSM standard and in 7,69 10-19/5,781 10-24 = 33640 times more energy of the 5G standard.
Thus, based on the data obtained, we conclude that microwave radiation cannot destroy the chemical bonds of atoms in a water molecule.
