
Морф. изменения в почках при плазмоцитоме.-1
.pdf
Renal Lesions Associated With Plasma Cell Dyscrasias
Practical Approach to Diagnosis, New Concepts, and Challenges
Guillermo A. Herrera, MD
● Context.—Patients with plasma cell dyscrasias (myeloma) may exhibit a variety of renal manifestations as a result of damage from circulating lightand heavy-chain immunoglobulin components produced by the neoplastic plasma cells. The renal alterations can occur in any of the renal compartments, and in a significant number of the cases more than one compartment is affected. Research in the laboratory has helped considerably in providing a solid conceptual understanding of how renal damage occurs.
Objectives.—To detail advances that have been made in the diagnosis of these conditions and to provide an account of research accomplishments that have solidified diagnostic criteria. The new knowledge that has been acquired serves to provide a solid platform for the future design of new therapeutic interventions aimed at ameliorating or abolishing the progressive renal damage that typically takes place.
Patients with circulating monoclonal proteins may or may not have systemic manifestations with associated organ damage. Those patients without recognizable organ involvement are considered to exhibit a monoclonal gammopathy of unknown significance (MGUS). Monoclonal gammopathy of unknown significance occurs in approximately 0.15% of the population.1 Conceptually, MGUS is considered to be an asymptomatic premalignant disorder. Patients with MGUS are administered no therapy but are closely followed, as a percentage of them progress to develop organ damage with time and transform into having a full-blown plasma cell dyscrasia (myeloma). For a diagnosis of MGUS, the serum monoclonal protein levels should be less than 3 mg/dL and plasma cells in the bone marrow aspirate/biopsy less than 10%, and there should be an absence of end organ damage, including lytic bone lesions, anemia, hypercalcemia, or renal failure. Monoclonal gammopathy of unknown significance is found in 3% of individuals aged 50 years, 5% among those 70 years or
older, and 7.5% among those 85 years or older.2
Patients with MGUS have an overall 1% lifelong risk per year of progression to multiple myeloma or related
Accepted for publication June 11, 2008.
From the Pathology Department, Nephrocor Laboratory, Tempe, Ariz. The author has no relevant financial interest in the products or com-
panies described in this article.
Reprints: Guillermo A. Herrera, MD, Pathology Department, Nephrocor Laboratory, 1700 N Desert Dr, Tempe, AZ 85281 (e-mail: gherrera@bostwicklaboratories.com).
Arch Pathol Lab Med—Vol 133, February 2009
Data Sources.—Translational efforts have substantially contributed to elucidate mechanistically the molecular events responsible for the renal damage. The spectrum of renal manifestations associated with plasma cell dyscrasias has expanded significantly in the last 10 years. Diagnostic criteria have also been refined. This information has been summarized from work done at several institutions.
Conclusions.—A number of significant challenges remain in the diagnosis of these conditions, some of which will be discussed in this article. Dealing with these challenges will require additional translational efforts and close cooperation between basic researchers, clinicians, and pathologists in order to improve the diagnostic tools available to renal pathologists and to acquire a more complete understanding of clinical and pathologic manifestations associated with these conditions.
(Arch Pathol Lab Med. 2009;133:249–267)
conditions.3,4 To change a patient from the MGUS category into a diagnosis of plasma cell dyscrasia and initiate appropriate treatment for the malignancy, the burden of proof rests essentially on the demonstration of organ involvement or overt abnormal plasmacytic proliferation in the bone marrow. Bone marrow aspiration and biopsy may not show definitive findings to support a morphologic diagnosis of plasma cell dyscrasia and trigger therapy. In part, this is due to the misconception that a significant increase of plasma cells, atypical cellular changes, and sheets of plasma cells are absolute requirements for a diagnosis of plasma cell dyscrasia/myeloma. The use of ancillary diagnostic techniques to typify plasma cells and demonstrate a clonal population in the bone marrow, even when the percentage of plasma cells is within acceptable or so-called normal limits, is not a widespread practice.
Because renal damage is the most common, and in most cases the earliest expression of systemic involvement in these patients, proper evaluation of renal biopsies is very important.
In order to link the renal biopsy findings with an underlying plasma cell dyscrasia, pathologists have to be aware of the vast and heterogeneous spectrum of renal pathologic manifestations.5–11 Subtle and early cases can be truly challenging and require use of a variety of diagnostic techniques.12,13 Unfortunately, in some instances doubt still remains even after a thorough evaluation of the renal biopsy is completed because of existing diagnostic limitations.
Lesions Associated With Plasma Cell Dyscrasias—Herrera 249

Figure 1. A through C, Myeloma cast nephropathy. D, Tubular casts in a patient with end-stage renal disease. Typical myeloma casts exhibit fracture planes (A) and are associated with reactive tubular cells (B). Also, multinucleated giant cells may be present (C). D, Tubular casts from a patient with end-stage renal disease showing similar internal morphology; note the fracture planes, but also that surrounding tubular cells are flattened and do not appear reactive (hematoxylin-eosin, original magnifications 250 [A and D] and 350 [B and C]).
Challenges that are inherent to the interpretation of the renal biopsies from these patients will be discussed in this article. One of the confounding factors that may affect the accurate interpretation of renal samples is that these patients may have other concurrent renal diseases that may mask the proper identification of alterations that may be associated with monoclonal deposition of immunoglobulin components and related renal damage.
A GENERAL APPROACH TO THE DIAGNOSIS OF THESE CONDITIONS: ESSENTIAL DIAGNOSTIC CRITERIA
To link any renal alterations with an underlying plasma cell dyscrasia, there is a need to demonstrate the association of the particular lesion detected with deposition of monoclonal immunoglobulin components and to establish a cause-effect relationship. Therefore, immunofluorescence evaluation becomes extremely important in these cases. The routine use of antibodies to and light chains in the evaluation of renal biopsies is essential so that unusual, early, and subtle manifestations can be detected. Once routine testing for light chains became the norm, these conditions were detected with much more frequency, and
the spectrum of morphologic manifestations expanded. It is also crucial that the staining for immunoglobulins be carefully evaluated, as the confirmation of the deposition of heavy-chain components is key to diagnosing heavy- chain–associated disorders. Commercially available antibodies to and light chains and heavy chains are routinely used to evaluate these cases.
Ultrastructural evaluation is also very important, as the specific morphologic correlates of these conditions must be carefully assessed to either support the suspected diagnosis or to provide additional evidence.
Another technique that can provide extremely useful information in some cases is immunogold labeling at the ultrastructural level.12 There are cases that lack definitive morphologic findings by light or electron microscopy, and the immunofluorescence data are inconclusive; the presence of monoclonal immunoglobulin components in the kidney can be confirmed objectively using immunogold labeling. This is particularly useful in early cases and in circumstances where immunomorphologic parameters are doubtful, suboptimal, or fulfill less than diagnostic criteria. If the immunofluorescence data are definitive (ie, de-
250 Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera |

Figure 2. Myeloma cast nephropathy. Numerous casts in medullary ducts (A) staining for light chains (B). No staining for light chains was present (hematoxylin-eosin stain [A] and direct fluorescence for light chains, fluorescein; original magnifications 150).
Figure 3. Myeloma cast nephropathy. Transmission electron microscopy. A, A rather nondescript cast found in a patient with light chain cast nephropathy. B, Another cast shows contents gold labeled for light chains. There was no labeling for light chains (uranyl acetate and lead citrate stain [A and B] and immunogold labeling, 10-nm gold particles [B]; original magnifications7500 [A] and 6500 [B]).
fine a pattern characteristic of monoclonal light chain deposition disease), this finding essentially suffices to make an unequivocal diagnosis.
Because morphologic features encountered in cases associated with plasma cell dyscrasias overlap with those seen in a variety of other conditions, and a generic morphologic diagnosis can be made in virtually every case, the association of a particular case with a plasma cell dyscrasia can be easily missed. Unfortunately, this important association is often ignored, delaying proper patient management.
CLINICAL INFORMATION/LABORATORY TESTING:
IMPORTANT CONSIDERATIONS
Although in some cases there is documented clinical evidence that the patient has a plasma cell dyscrasia, in a significant number of the cases the clinical information available at the time of the renal biopsy is incomplete. Occasionally, the pertinent laboratory tests to explore whether a patient may have a plasma cell dyscrasia have been requested, but the results are not available at the time the biopsy is interpreted. In other cases, the clinician does not suspect a plasma cell dyscrasia, and therefore has not requested the appropriate tests to be performed.
Renal manifestations associated with these disorders are quite variable depending on the pattern of renal damage. If cast nephropathy or acute proximal tubular damage is present, acute renal failure is a common manifestation. If a glomerulopathy (ie, associated with light chain deposition disease or amyloidosis) is the main lesion, proteinuria with or without nephrotic syndrome is the most common clinical presentation.5–11 In both situations the clinical presentation may be characterized by slowly progressive renal dysfunction.
A rather common scenario is that a patient who is being followed after a diagnosis of MGUS (presence of monoclonal spike on serum protein electrophoresis) has been established, or the patient has been diagnosed recently with MGUS and develops renal manifestations. In these settings, the most common clinical finding that leads to a renal biopsy is proteinuria with or without associated nephrotic syndrome. In patients with renal dysfunction, a kidney biopsy is performed to assess whether the renal problem is related to the paraproteinemia, thus requiring the treatment of the plasma cell dyscrasia.
It is also important to understand that some of the patients with plasma cell dyscrasia do not exhibit a monoclonal spike in the serum due to the fact that they have
Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera 251 |

Figure 4. Proximal tubulopathy associated with (A) Fanconi and (B) non-Fanconi syndrome. Note subtle needle-shaped empty spaces within proximal tubular cells (A) and prominent granular eosinophilic cytoplasm and a mitotic figure in tubular cells (B) (hematoxylin-eosin, original magnifications 500).
Figure 5. Proximal tubulopathy. Transmission electron microscopy. Desquamation, fragmentation, and segmental loss of microvillous borders of proximal tubular cells associated with the prominence of lysosomes (A). Markedly atypical lysosomes may be present, such as depicted in B (uranyl acetate and lead citrate stain, original magnifications 5600 [A] and 7500 [B]).
circulating light chains. Because of their low molecular weight (generally 25 kd or less), light chains are freely filtered and appear in the urine. In these cases it is very important to detect the presence of abnormal light chains (Bence-Jones proteins) in the urine, where the monoclonal spike can be detected. To do this effectively, there is a need to concentrate urine in instances when the amounts of light chains are small. This is particularly important in cases with glomerulopathies, because the light chains become entrapped in the mesangium, and only a small amount appears in the urine.
Immunofixation was until recently the most reliable methodology available to detect small amounts of light chains. Determination of free light chains in the serum has become a common method to diagnose and follow patients with plasma cell dyscrasias. A similar test to detect abnormal circulating heavy chains in the serum is being developed. A recent comparison of immunofixation and serum-free light chains showed advantages and disadvantages of each technique.14 Free light-chain assays are sensitive and very specific in detecting serum monoclonal light chains. Serum-free light-chain assays combined with
252 Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera |

Figure 6. Proximal tubulopathy associated with Fanconi syndrome. Transmission electron microscopy. A, Loss of microvillous border in proximal tubular cell with intracytoplasmic needle-shaped inclusion. In B and C, cytoplasmic inclusions are labeled for light chains with gold particles. C, Image manipulation to better appreciate gold particles labeling the cytoplasmic inclusions (uranyl acetate and lead citrate stain [A through C] and immunogold labeling, 10-nm gold particles [B and C]; original magnifications 7500 [A and C] and 5400 [B]).
immunofixation virtually eliminate the need for urine screening for monoclonal gammopathies.15
LIGHT MICROSCOPIC PATTERNS IN
PLASMA CELL–ASSOCIATED RENAL DISEASES:
AN OVERVIEW OF CHALLENGES AND
CONTRIBUTIONS OF ANCILLARY DIAGNOSTIC
TECHNIQUES
Any of the renal compartments can be affected. In some cases, more than one compartment is involved.5–11 There are some morphologic patterns that are readily recognizable at the light microscopic level, especially when the manifestations are florid, including that of myeloma cast nephropathy, nodular glomerulosclerosis associated with light or heavy chain deposition disease, and light and heavy chain (AL and AH) amyloidosis. Subtle and early manifestations of the above-mentioned conditions and other less well-recognized patterns can be missed easily if the data available emanating from light, electron, and immunofluorescence microscopies are not properly collated and evaluated. This article will focus on these lesscommon manifestations, and will relate them to the more readily recognizable ones in an effort to provide a conceptual framework that can help in the understanding of the heterogeneity of these conditions.
Another complicating factor is that these conditions may be found in combination. For example, a given patient may have light chain deposition disease and light chain cast nephropathy, or AL/AH amyloidosis and light/heavy chain deposition disease. Other combinations also have been reported.16–19 Light chain cast nephropathy has been traditionally considered the most common pattern of renal damage in patients with plasma cell dyscrasias. This information emerged primarily from autopsy studies.20–22 However, AL amyloidosis has been found to be more common in a recent renal biopsy series.23
LIGHT CHAIN (MYELOMA) CAST NEPHROPATHY
The typical clinical presentation is acute renal deterioration or frank renal failure. There may be precipitating factors, such as dehydration, hypercalcemia, hyperurice-
Figure 7. Inflammatory tubular interstitial nephritis associated with monoclonal light chains. Note interstitial inflammatory infiltrate associated with tubulitis (A) and linear fluorescence staining of tubular basement membranes for light chains (B) (hematoxy- lin-eosin [A] and direct fluorescence staining, fluorescein [B]; original magnifications 250 [A] and 350 [B]).
Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera 253 |
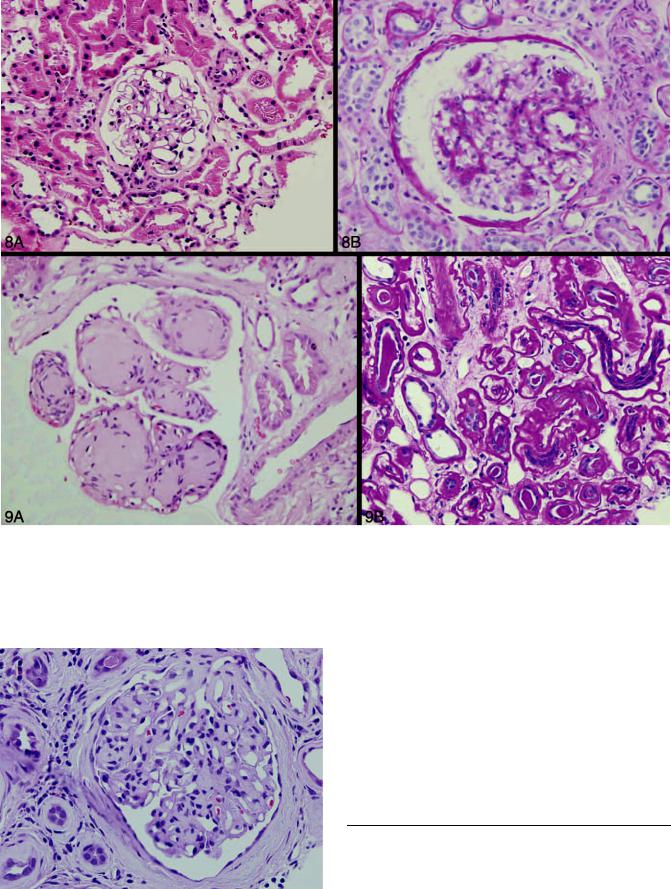
Figure 8. Light chain deposition disease. In A, note essentially normal glomerulus (minimal-change glomerulopathy) and a mesangial proliferative pattern in B; both patterns associated with monoclonal light chain deposition disease (hematoxylin-eosin, original magnifications 250).
Figure 9. Light chain deposition disease. A, Classical pattern of nodular glomerulosclerosis associated with light and heavy chain deposition disease, and (B) peculiar thickening of tubular basement membranes associated with light chain deposition disease (hematoxylin-eosin, original magnifications 500 [A] and 250 [B]).
mia, infections, or exposure to contrast media, nonsteroidal anti-inflammatory drugs, nephrotoxins, or loop diuretics, such as furosemide.
Manifestations at the light microscopic level diagnostic of light chain (myeloma) cast nephropathy may be difficult to assess with certainty. Cast nephropathy has not been described as associated with heavy-chain disease, except in very rare instances (ie, associated with Waldenstro¨m macroglobulinemia, which could be conceptualized as a peculiar heavy-chain [immunoglobulin M]–associated disorder).24 The pathology of Waldestro¨m macroglobulinemia is different from that of other gammopathies and will not be discussed in this article. Therefore, for all practical pur-
←
Figure 10. Light chain deposition disease, early manifestations. Segmental thickening of glomerular capillary walls, expanded mesangial areas, and beginning of mesangial nodule formation as early glomerular manifestations of light chain deposition disease (hematoxylin-eo- sin, original magnification 500).
254 Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera |

Figure 11. Light (A) and heavy (B) chain deposition disease. Note similar findings in light and heavy chain deposition disease with mesangial nodularity. Note more prominent hypercellularity associated with heavy chain deposition disease compared with light chain deposition disease (hematoxylin-eosin, original magnifications 350).
Figure 12. Light chain deposition disease. Note linear staining along peripheral capillary walls in glomeruli and tubular basement membranes in A and predominantly granular mesangial staining for light chains in B (direct fluorescence for light chains, fluorescein; original magnifications500).
poses cast nephropathy is only associated with abnormal light chains.
On light microscopic examination, typical myeloma casts show fracture planes (Figure 1, A) and polymorphonuclear cells. In addition, there is generally a reaction of surrounding tubular cells (Figure 1, B), which become enlarged and may acquire prominent nucleoli. Sometimes, the casts are associated with multinucleated giant cells of histiocytic origin, even when the tubular basement membranes appear intact and the casts’ contents have not extravasated into the interstitium (Figure 1, C).25 Not all casts in patients with myeloma exhibit the entire spectrum of
morphologic features noted above. The casts also may break through the tubular basement membrane and elicit an interstitial reaction, which may include multinucleated giant cells. They are usually in the distal nephron and are formed as a result of interactions of the pathologic light chain and Tamm-Horsfall protein.26,27 Large casts may also be found in proximal tubules and even in the urinary space, arriving there by retrograde extension. An interstitial inflammatory infiltrate, which may contain eosinophils, is commonly associated with the tubular casts.
One of the most important unsettled points is that there are no guidelines as to how many tubular casts should be
Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera 255 |

Figure 13. Light chain deposition disease. Transmission electron microscopy. Appearance of light-chain deposits can be variable. The light-chain deposits can be very subtle, as noted in A, or rather distinct with punctate electron densities in a subendothelial location along peripheral capillary walls, as shown in B (uranyl acetate and lead citrate stain, original magnifications 8500 [A] and7500 [B]).
present to make such a diagnosis. The number of casts may be quite variable in a biopsy specimen due to sampling, and even in kidneys sampled extensively at autopsy. The morphologic findings associated with the casts may also pose some diagnostic problems. Occasionally, when tubular casts are present in other conditions, primarily in patients with end-stage renal disease (Figure 1, D) or in those patients taking certain drugs, such as rifampin28 and tacrolimus/rapamycin,29 they may mimic those seen in association with myeloma. The cells lining the tubules associated with casts in these 2 situations are generally flattened and do not reveal reactive features. Furthermore, multinucleated giant cells are not present.
Because there is relatively little change in the structure of the light chains that are delivered to the distal nephrons, there are usually no difficulties in identifying these light chains using the commercially available antibodies. However, the immunofluorescence findings associated with the tubular casts may be confusing, difficult to interpret, or nondiagnostic. Casts that have formed relatively rapidly and generally a short time before the renal biopsy was performed may reveal light-chain restriction (Figure 2) or distinct preponderance of one light chain over the other, findings that can be helpful to establish a definitive diagnosis or to support a diagnostic impression.5 However, in most cases, both and light chains can be found in the casts, making it impossible to establish monoclonality. This occurs because the nonpertinent light chain becomes trapped in the casts. Casts with crystalline material may be diagnostic in the proper clinicopathologic setting,5,13 especially when monoclonality can be demonstrated in the crystals using routine fluorescence microscopy, immunohistochemistry, or ultrastructural immunolabeling.
Electron microscopy is usually not very helpful in this condition, as the casts may vary substantially in their appearance30 and not uncommonly exhibit a rather nonspecific appearance (Figure 3, A). Rarely, the casts may contain crystalline structures.5,20 In a small subset of these patients the casts stain with Congo red, are apple green birefringent, and contain fibrillary material with characteristics most consistent with amyloid fibrils.31 Ultrastructural immunogold labeling may be of value in assessing light-chain monoclonality associated with the cast con-
tents (Figure 3, B),12,13 even when immunofluorescence has failed.
ACUTE PROXIMAL TUBULAR DAMAGE
(PROXIMAL TUBULOPATHY)
The typical clinical presentation is progressive renal insufficiency or acute renal failure. In cases associated with Fanconi syndrome, the most classical presentation includes tubular dysfunction with aminoaciduria, phosphaturia, and glucosuria. They may also have nonnephrotic-range proteinuria, uricosuria and, at times, renal tubular acidosis.
Acute tubular damage (acute tubulopathy) mimics acute tubular necrosis by light microscopy.5,13,32 At the light microscopic level the alterations in the proximal tu-
Figure 14. Light chain deposition disease. Transmission electron microscopy. Powdery, electron-dense light-chain deposits are present along peripheral capillary wall and in the mesangium (uranyl acetate and lead citrate stain, original magnification 7500).
256 Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera |

Figure 15. Light chain deposition disease. Transmission electron microscopy. Lightchain deposits in mesangial areas (A) are gold labeled for light chains in B (uranyl acetate and lead citrate stain [A and B] and immunogold labeling, 10-nm gold particles [B]; original magnifications 9500 [A] and10 500 [B]).
bules can be quite subtle or overt, and the degree of tubular damage does not correlate directly with the functional abnormalities. Two morphologic patterns can be seen in these cases that correlate with the presence or absence of Fanconi syndrome.
On the hematoxylin-eosin stain, needle-shaped empty spaces can be noted in the cytoplasm of proximal tubular cells (Figure 4, A) in those cases associated with Fanconi syndrome. In the non-Fanconi type, the cytoplasm of the proximal tubular cells may be markedly eosinophilic and granular due to an abundance of lysosomes (Figure 4, B). Mitoses can be seen in tubular cells. Localization of monoclonal light-chain components within the damaged proximal tubular cells becomes important in making this diagnosis. Light-chain monoclonality can be demonstrated in the cytoplasm of proximal tubules using immunofluorescence or immunogold labeling at the ultrastructural level.13,32
At the ultrastructural level there is prominence of the lysosomal system with variably sized and shaped lysosomes and, in some instances, markedly atypical lysosomes are identified (Figure 5) in the non-Fanconi type of acute tubular damage. The monotypic light chains are localized to the lysosomes using ultrastructural labeling techniques,13,32 and may be or . Vacuolization, apical blebbing, fragmentation, and desquamation of proximal tubular cells can be present. This pattern of proximal tubular damage has been reproduced in the laboratory, and the molecular events that take place have been elucidat-
ed.33,34
One of the unique clinical and pathologic manifestations associated with proximal tubular damage is represented by Fanconi syndrome.35 In this particular type of proximal tubular injury, needle-shaped, often described as crystal- line-like, inclusions are virtually always present in the cytoplasm of proximal tubular cells, which show evidence of damage. These inclusions are difficult to detect at the light microscopic level in many of the cases, and are much easier to identify ultrastructurally (Figure 6). The number of these peculiar inclusions per tubular cell can be quite variable.36 All but one of the reported cases associated with Fanconi syndrome have been light chain restricted. The pathologic light chains in these cases belong to the 1 subgroup.37,38 Biochemical analysis of the primary structure of these light chains has demonstrated substitutions in residues 28 and 38 of the complementarity-determining region 1 (CDR 1), which results in a resistance to normal
catabolism of these light chains in the proximal tubules. As a result, intracytoplasmic inclusions form. In a minority of the cases, the aforementioned cytoplasmic inclusions are highlighted and easily detectable on the immunofluorescence stain for light chains.10 Ultrastructurally, the inclusions may at times exhibit a somewhat fibrillary appearance, and their shape and electron density can be variable from case to case (Figure 6). The cytoplasmic inclusions are rather characteristic in most cases, and allow a definitive diagnosis. Immunogold labeling at the ultrastructural level is extremely helpful in demonstrating
Figure 16. Light chain deposition disease. Transmission electron microscopy. Light-chain deposits located on top of glomerular basement membranes, mimicking dense deposit disease (uranyl acetate and lead citrate, original magnification 5600).
Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera 257 |

Figure 17. Light chain deposition disease. Transmission electron microscopy. In A, lightchain deposits are noted on top of the lamina densa of the glomerular basement membrane, obliterating the lamina densa, and are in subendothelial and subepithelial locations in B (uranyl acetate and lead citrate stain, original magnifications 10 500 [A] and 9500 [B]).
light-chain monoclonality associated with the inclusions (Figure 6, B and C), solidifying the diagnosis.12,13,36
Renal Fanconi syndrome associated with plasma cell dyscrasia has also been reproduced in the research laboratory, resulting in insightful observations that have revolutionized our overall understanding of this entity. This translational effort has provided a solid platform for the future development of novel therapeutic interventions for this disorder.37–39
ACUTE TUBULAR INTERSTITIAL NEPHRITIS
Patients with this pattern of renal injury are usually older than 50 years and present with acute renal failure.40,41 Nonnephrotic-range proteinuria may be found, and serum creatinine is generally increased.
This interstitial manifestation of plasma cell–associated renal disease mimics acute tubular interstitial nephritis by light microscopy.39 Interstitial inflammatory infiltrates associated with variable degrees of tubulitis represent the typical light microscopic finding associated with this condition (Figure 7, A). Eosinophils may be present in the interstitial inflammatory infiltrates, and although tubulitis is a constant finding, the inflammatory process may be focal.40,41
Demonstration of an association with a monoclonal plasma cell process is crucial to make a definitive diagnosis; therefore, demonstrating light-chain deposition and restriction associated with the interstitial inflammatory process is key (Figure 7, B). There are no tubular casts.
Immunofluorescence and electron microscopy individually may or may not be helpful, but both combined should provide sufficient evidence to support the diagnosis in most cases.40
Ultrastructural labeling has also been used to assess light chain monoclonality associated with the tubular interstitial manifestations with excellent results, even in cases when other techniques have failed.40 Most of these cases are light chain restricted.
Glomeruli and vasculature are entirely normal in this pattern of light-chain–associated interstitial disease. This pattern of interstitial disease could be conceptualized as an interstitial form of light chain deposition disease without manifestations in other renal compartments.41
LIGHT AND HEAVY CHAIN DEPOSITION DISEASE
The average age of patients with these disorders is 55 to 60 years. At the time of diagnosis, acute renal failure is present in 30% of patients, and a similar percentage is already dialysis dependent. More than 90% of the patients exhibit proteinuria, with nephrotic syndrome only occurring in a minority. A high percentage are hypertensive, and most also have hematuria and/or varying degrees of renal insufficiency.
The spectrum of glomerular manifestations in light and heavy chain deposition disease is broad. Light and heavy chain deposition disease can coexist, albeit uncommon- ly.9–11,42,43 Our understanding of light chain deposition disease is far more advanced than that of heavy chain deposition disease.
Recently, an in vitro mesangial cell culture model of light-chain–associated disease reproduced in the laboratory the sequence of events that likely occur in vivo, and the sequential molecular mechanisms involved have been dissected.44 This disease represents a good example of the important contribution of basic research to the understanding of pathologic morphologic expressions of this disorder. Such translational efforts have contributed much to our progress in conceptualizing mechanisms involved45 and understanding of the key events that take place and are amenable to therapeutic intervention.
Proper interpretation of some of the light morphologic expressions in these disorders and linking the pathology findings to an underlying plasma cell dyscrasia require a high degree of sophistication, use of ancillary diagnostic techniques, and careful integration of all of the data obtained from the renal biopsy.
258 Arch Pathol Lab Med—Vol 133, February 2009 |
Lesions Associated With Plasma Cell Dyscrasias—Herrera |
