
2 курс / Нормальная физиология / Учебное_пособие_по_физиологии_крови_Авдеева_Е_В_,_Репалова_Н_В_
.pdf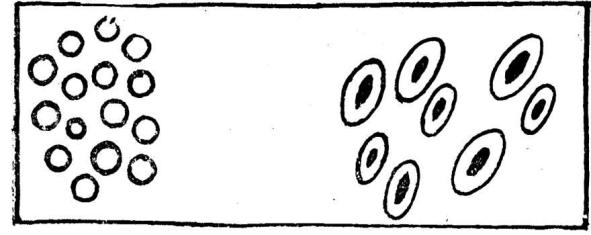
2.Write down the formula for calculating the quantity of erythrocytes and explain the meaning of each element.
3.During an analysis of a woman’s blood, the following were found: erythrocytes
– 4.2 x 1012 l, Hb – 125 g/l. Determine the color index. Are these normal values?
4.Absolute content of hemoglobin in investigated blood is 14,7g%. Determine the relative content of hemoglobin.
5.Calculate the total number of erythrocytes in 1 liter of blood if the sum of erythrocytes within 5 big squares is 300. Is the result normal? Explain your answer.
6.Calculate the color index if the relative content of hemoglobin in the blood
is 12% and the number of erythrocytes is 5000000. Is the result normal? Explain your answer.
7. What differentiates mature frog’s erythrocytes from man erythrocytes?
Fig. 11. Erythrocytes of frog and man.
8.It is known that people constantly living in the mountains, the blood contains more red blood cell count than those living at sea level. What is the mechanism of increasing the number of erythrocytes and how those that have physiological significance?
9.What is the physiological significance that the haemoglobin is within the erythrocyte rather than the plasma?
10.In the study of blood it was found, shown in hematocrit (formed elements and plasma) in one patients plasma is 40% and elements – 60% in another patient plasma – 60% and formed elements – 40%.
a)Are these figures normal or not?
b)Specify the possible changes in hematocrits index.
11.What are the properties of blood plasma relating to the ability of blood to participate in the process of thermoregulation ?
12.During the period of thirst in a person, 1mm blood contained 5million red blood cells and the total volume of hematocrit was 45% of the volume of the whole blood, thirst quencher after the number of red blood cell reduces to 4.5 million, and the volume of their hematocrit rose to 49%.
a)Determine the volume of the red blood cells in both cases.
31
b) With the change of the red blood cell define the direction and intensity of changes in osmotic pressure of blood plasma after drinking water?
13. Intrudcing rabbit serum previously anemic bloodletting, another rabbit eritropoety encourages the latter.
a)What shows this experiment?
b)What is erythropoetyn , where is it produced, what acts (point of applicatins) that stimulates its production?
RECOMMENDED LITERATURE
1.Guyton, A.C. Textbook of medical physiology / A.C. Guyton, J.E. Hall. – 11 th ed. - Philadelphia: Elsevier Saunder. – 2006. - Ch. 32.
2.Ganong, W.F. Review of medical physiology / W.F. Ganong. – 22 th ed. - New York : Lange Medical Books / McGraw-Hill. - 2005. – Section VI, Ch. 27.
3.Lecture material.
4.Avdeyeva, E.V. Supplement for laboratory classes in normal physiology (for
students of medical, stomatological and pharmaceutical faculties) / E.V. Avdeyeva., L.I. Khokhlova. - Kursk, 2004. - P. 6-7.
5. Zavyalov, A.V. Methodical handbook in normal physiology. Tasks for selfcontrol: physiology of blood system, cardiovascular physiology / A.V. Zavyalov, E.V. Avdeyeva, L.I. Khokhlova. – Kursk, 2000.
SUPPLEMENTARY LITERATURE
1.Gutnik, K.B. Physiology for “lazy” students / K.B. Gutnik, V. Kobrin, D. Nash.
– M. : Логосфера, 2009. – 200 p.
2.Widmaier, E. Vander’s human physiology. The mechanisms of body function / E. Widmaier, H. Raff, K. Strang. – Boston : Mc. Graw-Hill, Higher Education, 2008. – 770 p.
PHYSICO-CHEMICAL PROPERTIES OF BLOOD.
PHYSIOLOGY OF LEUKOCYTES
TOPIC MOTIVATION
Relative constancy of a blood parameters series is necessary for normal functioning of organs and tissues. Some of them, such as osmotic resistance, erythrocyte sedimentation rate, leucocytes quantity, can deviate from physiological bounds into pathological conditions. That is why a good knowledge of these parameters and skills of their determination have a great clinical meaning.
PURPOSE OF SELF-PREPARATION
After working over this material, the student has to know: the osmotic and the oncotic pressure of blood, osmotic resistance of erythrocytes, erythrocyte sedimentation rate, characteristic of leukocytes, their types and functions and also the physiological fluctuations of leukocyte quantity in blood, method of calculation of
32
leukocytes quantity. Also, the student should be familiarized with methods of determining erythrocyte osmotic resistance, erythrocyte sedimentation rate.
PLAN OF THE TOPIC STUDY
1.Osmotic pressure of blood.
a)Factors which influence the osmotic blood pressure of blood.
b)Cryoscopic method for measuring osmotic pressure.
c)Size of osmotic pressure in human blood.
d)Notion about iso-, hyperand hypotonic solutions.
e)Importance of osmotic blood pressure constancy.
2.Types of hemolysis.
3.Determination of erythrocytes osmotic resistance. Factors which influence the osmotic resistance of erythrocytes.
4.Oncotic blood pressure.
a)Significance.
b)Plasma proteins and its functions
5.Blood as a suspension. Erythrocyte sedimentation rate.
6.Buffer systems of the blood.
7.Respiratory acidosis and alkalosis.
8.Metabolic acidosis and alkalosis.
9.Innate and acquired immunity.
10.General characteristics of leukocytes.
a)Defensive properties of neutrophils and macrophages.
b)Eosinophils.
c)Basophils.
d)B-lymphocyte system.
e)T-lymphocyte system.
11.Leukogram and it s medical importance.
12.Physiological and reactive leukocytosis. Leukopenia.
THE QUESTIONS YOU SHOULD PAY ATTENTION TO
1. Osmotic blood pressure.
The main function of blood plasma minerals is the creation of osmotic pressure. Posm = 7.5 atm (0.3 osmole, 745 kPa, 5600 mm Hg). Osmotic pressure is the force that causes the passage of solvent through semi-permeable membrane. Osmosis is the flow of solvent molecules through a semipermeable membrane from a region of low to high solute concentration, until equilibrium is established. 96% of the osmotic pressure is made by Na+ and Cl- ions, because the molecular mass of NaCl is small.
Cryoscopic method is a physicochemical research method based on measurement of the lowering of the freezing point of a solution relative to the freezing point of the pure solvent. Raoult’s law states that, for infinitely dilute solutions (in the absence of electrolytic dissociation), the dependence
33
tc = Ec n
exists, where: tc is the lowering of the freezing point of the solution in degrees Celsius;
n is the concentration of the solution.
Ec is called the cryoscopic constant of the solvent (for water, 1.86).
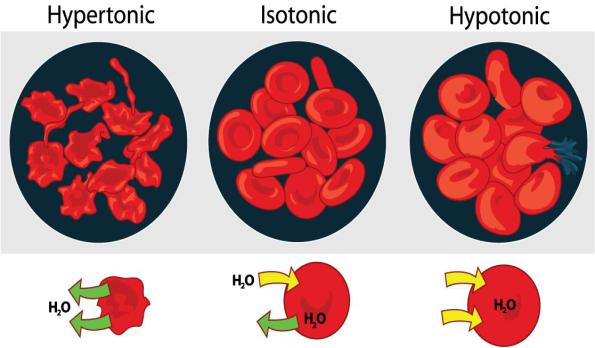
Solutions, the osmotic pressure of which is the same as blood plasma has, are called isotonic. For example, 0.9% solution of NaCl, Ringer’s solution, RingerLock’s solution, Tirode, 5% glucose solution, haemodesis.
Solutions with the osmotic pressure of which is higher than the plasma is called hypertonic, if it is lower it is called hypotonic.
In hypertonic solution water leaves the cells, cells firm, their normal turgor is disbalanced. It is called plasmolysis. In clinics it is used for counting the erythrocytes: blood is diluted by 3% solution of NaCl, erythrocytes shrink in this solution and they are easy to count.
In hypotonic solution water penetrates cells, cells swell, oedema and destruction of the cells occurs, which is called haemolysis (fig. 12).
Types of hemolysis see in the previous topic.
Fig. 12. Erythrocytes in the solutions of different NaCl concentrations.
2. Plasma proteins.
Plasma proteins can be divided into several fractions:
1. Albumins (35-50 g/l). They are mostly small proteins. Synthesized in the liver. The main functions of albumins are transport and trophic. Due to high hydrophilic, small sizes and high concentration in blood plasma albumins play important role in the
34
creation of oncotic pressure. Decrease in albumin concentration to 30 g/l and lower can lead to the decrease of oncotic pressure and the development of oedemas.
2.Globulins (20-40 g/l): α1, α2, β, γ. Synthesized in the liver and reticuloendothelial cells as well as plasma cells present in the lymph nodes, spleen, bone marrow. The main functions of globulins are transport and protective.
3.Fibrinogen (2-4 g/l). It is the largest protein of blood plasma. Synthesized by the
liver. Fibrinogen plays a role in processes of blood clotting and fibrin plag formation.
Functions of plasma proteins:
1.Transport function. There are special spots in the molecule of protein, which are able to bind inorganic substances (for example, ions, water) and organic substances (for example, hormones, biologically active substances) and to transport them.
2.Trophic function. Proteins are the source of amino acids,
which with peripheral tissues, are used for the formation of proper, specified for the organ proteins. Proteins are the source of energy. When breaking down 1 g of protein in the organism is 4,1 kkal.
3.Enzymatic function. There are a lot of protein-enzymes into the plasma. There are secretory and indicatory (cellular) enzymes. Typical representatives of secretory enzymes are protein-enzymes of blood clotting. Indicatory enzymes penetrate the blood from other organs.
4.Hemostatic function. Proteins comprise biochemical systems of blood plasma, which provides hemostasis, namely blood clotting system, anticoagulatory system, fibrinolytic system, kallikrein-kinine system.
5.Participating in maintaining pH of blood. Proteins form protein buffer. In acidic medium, they work as bases, binding acids, in base, they react like acids, binding bases. This property of proteins is called amphoteric. Plasma proteins are responsible for 15% of the buffering capacity of blood.
6.Proteins are the source of biologically active substances.
For example, kinins and angiotensin.
7.Protective function. Proteins participate in non-specific and specific protection of the organism. Non-specific protection is represented by complement system proteins, interferons, orosomucoid and viruses’ inhibitors. Specific is realized by antibodies: congenital (agglutinins) and acquired.
8.Making creative connections. Proteins participate in transferring of the information, which affects genetic apparatus of cells, provides growth, development and differentiation of tissues. For example, proteins are growth factor of nervous tissue, erythropoietin etc.
9.Creation of oncotic (colloid-osmotic) pressure.
Ponc= 25-30 mmHg. 80% of oncotic pressure is made by albumins (molecule of albumin has small size and in volume of plasma, its quantity is the highest).
Significance of oncotic pressure constancy.
The protein concentration decrease in blood plasma will lead to the decrease of the reabsorbtion, delay of the water in intercellular medium and development of the intercellular oedema. This can happen during starvation (cachexy oedemas); in pathological processes in kidneys, in the consequence of which proteinuria and loss
35

of proteins can occur (nephrotic oedemas); in disorder in albumin liver synthesis (hepatic oedemas) and other.
3. pH of blood.
pH is a measure of the acidity of an aqueous solution.
It is the negative decimal logarithm of hydrogen concentration
Normal pH of blood is: 7.45 (arterial blood) – 7.35 (venous blood)
If pH is lesser than 7.3, it is acidosis.
If pH is more than 7.5, it is alkalosis (fig. 13).
The pH level of blood is important in ensuring the proper functionality of biological systems. The optimal pH level of blood is 7.4, which is maintained by different types of buffer systems of the body. The addition of an acid or a base to a substance changes its pH level. A buffer is a solution (or a substance) that has the ability to maintain pH and bring it back to its optimal value. It does this by the additional or removal of hydrogen ions. Buffers working in the body fluid adjust the pH level of blood and contribute to lower pH if its level rises above 7.4 by making the blood slightly more acidic. If the pH of blood falls below 7.4, buffers act to take up hydrogen atoms and decrease the acidity of blood.
Fig. 13. pH of blood.
The human body has four native buffer systems:
bicarbonate,
hemoglobin,
protein,
phosphate.
Protein buffer system.
Proteins are the most important and widely operating buffers in the body fluid.
The protein buffer system is an integral component of body’s pH controlling mechanism. Protein buffers are either intracellular or extracellular. Plasma proteins function as buffers but their amount is small in comparison with the intracellular
36
protein buffers. Protein buffers include basic group, and acidic protein buffer groups, that act as hydrogen ion donors to maintain the pH level at 7.4.
Hemoglobin buffer system.
The protein hemoglobin makes an excellent buffer. It can bind to small amounts of acid in blood, helping to remove that acid before it changes the blood's pH. It is localized inside erythrocytes. It accounts for about 75% of buffer-blood volume.
It consists of: HHb (acid) and KHb (base).
HHb + KOH → KHb + H2O KHb + HCl → KCl + HHb
Phosphate Buffer System
The phosphate buffer system is comprised of two ions: hydrogen phosphate ions and dihydrogen phosphate ions. The pH level of blood drops is below 7.4 when the H+ ions in the bloodstream increase. Hydrogen phosphate ions accept all additional H+ ions to reestablish the equilibrium between the hydroxide and hydrogen ions in the blood. When the pH level of blood increases above 7.4, the dihydrogen phosphate ions release additional hydrogen ions to reinstate the pH level of blood to its optimal 7.4. The concentration of phosphate is low in the extracellular fluid but the phosphate buffer system is an important urinary buffer.
It consists of : H2NaPO4 (acid) and HNa2PO4 (base).
H2NaPO4 + NaOH → HNa2PO4 + H2O HNa2PO4 + HCl → NaCl + H2NaPO4
Bicarbonate Buffer System
The bicarbonate buffer system functions to maintain the pH level in blood of mammals. It also plays a major role in the formation of acid in the stomach, and to neutralize the pH of chyme that enters the small intestine from the stomach. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi-product of cellular respiration.
It consists of: H2CO3 (acid) and NaHCO3 (base)
Acid–base imbalances that overcome the buffer system can be compensated in the short term by changing the rate of ventilation.
37

The kidneys are slower to compensate, but renal physiology has several powerful mechanisms to control pH by the excretion of excess acid or base (fig. 14). In response to acidosis, tubular cells reabsorb more bicarbonate from the tubular fluid, collecting duct cells secrete more hydrogen and generate more bicarbonate, and ammonia genesis leads to increased formation of the NH3 buffer. In responses to alkalosis, the kidneys may excrete more bicarbonate by decreasing hydrogen ion secretion from the tubular epithelial cells, and lowering rates of glutamine metabolism and ammonium excretion.
Fig. 14. Buffers of body.
Respiratory and metabolic acidosis and alkalosis. (Guyton, A.C. Textbook of medical physiology / A.C. Guyton, J.E. Hall. – 11 th ed. – Philadelphia: Elsevier Saunder. – 2006. – Ch. 30).
Fig. 15. Regulation of pH.
38

4. Leukocyte Physiology.
Fig. 16. Leukocytes.
4.1. Shape.
White blood cells are different from red cells in the fact that they are usually larger in size 10-14 micrometers in diameter. White blood cells do not contain hemoglobin which in turn makes them translucent. Many times in diagrams or pictures white blood cells are represented in blue color, mainly because blue is the color of the stain used to see the cells. White blood cells also have nuclei, that are somewhat segmented and are surrounded by electrons inside the membrane (fig. 16).
4.2.Functions.
1. Protective
2. Transport
3. Metabolic
4. Regenerator
4.3.Classified by phenotype.
White blood cells (leukocytes) are also known as "WBC's". White blood cells are made in the bone marrow but they also divide in the blood and lymphatic systems. They are commonly amoeboid (cells that move or feed by means of temporary projections, called pseudopods and escape the circulatory system through the capillary beds. WBC are classified by phenotype which can be identified by looking at WBC under a microscope (tab.1). The granular phenotype are able to stain blue. The agranular phenotype are able to stain red. Neutrophils make up 50-70% of granular cells, eosinophils make up 2-4%, and basophils 0-1%. monocytes make up 2-8% of agranular cells. B and T lymphocytes make up 20-30%. As you can see, there is a great deal of differentiation between WBC. These special cells help our bodies defend themselves against pathogens. Not only they do help our immune system but they remove toxins, wastes, and abnormal or damaged cells.
39
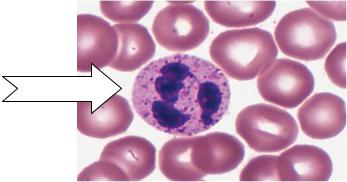
Table 1
Classification and normal value of leukocyte
Absolute Value |
(×109/L) |
Percentage (%) |
|
|
|
Total numbers of leukocytes |
4.0~9.0 |
|
Neutrophil (juvenile and band) |
0.04~0.5 |
1~5 |
Neutrophil (segmented) |
2.0~7.0 |
50~70 |
Eosinophil |
0.02~0.5 |
0.5~5 |
Basophil |
0.0~0.2 |
0~1 |
Monocyte |
0.12~0.8 |
3~8 |
Lymphocyte |
0.8~4.0 |
20~40 |
|
|
|
Normal of leukocytes is 4∙109 - 9∙109 / liter of blood.
The increased quantity of leukocytes is called leukocytosis − physiological: digestive, myogenic, emotional, in pregnancy and pathological (leukosis).
The decreased quantity of leukocytes is called leukopenia (usually in pathology). Newborn: number is higher 15×109/L, after birth 3 or 4 days to 3 months, being
about 10×109/L, mainly, neutrophil, 70%; secondarily, lymphocyte.
Circadian changes: number of WBC is more in the afternoon than in the morning.
Food taking, ache and mood excitation: number of WBC is remarkably higher. Heavy exercise and laboring: increasing numbers, about 35×109/L, return to
original level after the activity has been stopped.
Terminal pregnancy of female: numbers changes in 12~17×109/L, and during parturition, 34×109/L, and after parturition 2~5 days, number return to original level.
Neutrophils.
Another name, polymorphonuclear, PMN, 6~8 h in the vessels.
Functions: It plays a very important role in nonspecific cellular immunity system which is against pathogenic microorganism, such as bacteria, virus, parasite, etc.
Fig. 17. Neutrophil.
40
