
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
356
hypodense homogeneous fluid collections, at times multiple; some obstruct a bile duct. An elevated amylase level in aspirated fluid confirms the diagnosis. If necessary, percutaneous drainage is performed.
Hepatic Artery Aneurysm
With gray-scale US, some aneurysms simulate a cyst; the two are distinguishable with Doppler US unless the aneurysm contains a thrombus.
Benign Neoplasms
The vast majority of intrahepatic tumors found to be isoor hyperintense on T1-weighted MR sequences are of liver parenchymal origin (this finding applies only to high field strength magnets).
A common feature of benign liver neoplasms is the presence of fat within the tumor, either within hepatocytes or as discrete lipomatous tissue.
Adenoma
Clinical
ADVANCED IMAGING OF THE ABDOMEN
enlarges, and she presents with an acute abdomen due to tumor rupture and hemoperitoneum.
An adenoma has a potential for malignant transformation, although the risk of malignancy is unknown. a-Fetoprotein levels are not elevated with an adenoma; an elevated level in a setting of a known adenoma suggests carcinomatous transformation.
These tumors are supplied mostly from hepatic artery branches and an acute hemorrhage is amenable to therapeutic arterial embolization. Suspected adenomas are usually resected rather than biopsied because of possible hemorrhage and their potential for carcinomatous transformation. In addition, as discussed below, in a number of these tumors a clear-cut differentiation from hepatocellular carcinoma is not feasible by imaging alone.
Pathology
Adenomas are well-marginated, hypervascular benign neoplasms, at times containing regions of hemorrhage or necrosis. These tumors consist of hepatocytes containing increased glycogen and lipid interspersed by dilated sinusoids, but a normal acinar arrangement is missing, and supporting bile ductule and portal
Ahepatocellular adenoma is a wellstructures are lacking. Varying amounts of
circumscribed, hypervascular, and usually solitary neoplasm. Most occur in women between the ages of 20 and 40 years who are on oral contraceptive therapy. In some, an adenoma regresses after cessation of therapy. Less often adenomas develop in women taking estrogen or in the presence of estrogen-producing neoplasms and in men on anabolic steroids. Adenomas do develop in infants. They are more prevalent than expected in patients with glycogen storage diseases types I and III, and in these patients multiple adenomas (adenomatosis) are encountered. An occasional one becomes pedunculated.
Some adenomas are discovered as incidental findings. Symptoms, if present, range from dull pain, to repeated episodes of severe pain due to hemorrhage into the tumor or adjacent parenchyma, to sudden onset of pain and shock due to spontaneous intraperitoneal hemorrhage. A not atypical scenario is a woman taking oral contraceptives, imaging and biopsy reveal an incidental hepatic adenoma, the tumor
Kupffer cells are present. Only an inconstant capsule is evident, and thus hemorrhage, common with these tumors, readily spreads to surrounding liver parenchyma.
Imaging
Adenomas tend to contain fat and glycogen, with the fat being relatively uniform in distribution. Focal calcifications are identified in some. An occasional adenoma is pedunculated. In distinction to focal nodular hyperplasia, adenomas do not have a central stellate scar. The presence of a scar implies prior necrosis.
Precontrast CT reveals an isoto hypodense tumor, the latter presumably secondary to increased fat or prior necrosis. Acute hemorrhage appears hyperdense on noncontrast CT, a condition usually found in patients with acute symptoms. Adenomas enhance during the arterial phase. Small feeding arteries accentuate an arterial phase peripheral enhancement, followed by a centripetal pattern. The lesion

357
LIVER
becomes isodense or even hypodense on delayed images. Most adenomas are homogeneous, but larger ones tend toward heterogeneous enhancement due to hemorrhage and necrosis.
Precontrast CT detected 86% of 44 adenomas, 100% on arterial phase, 82% on portal venous phase, and 88% of 24 on delayed phase (88); contrast enhancement was homogeneous except for regions of fat, hemorrhage, and necrosis. An occasional adenoma contains calcifications.
Adenomas tend to be hyperechoic due to their fat and glycogen content. Hemorrhage also modifies their US appearance, with recent hemorrhage being hyperechoic and old blood hypoechoic, thus mimicking a cyst. Any prominent subcapsular vessels are detected by color Doppler imaging. Often the US and Doppler appearance is similar to focal nodular hyperplasia.
Some adenomas have MR characteristics departing only slightly from normal liver parenchyma, others vary in their MRI appearance and mimic other tumors, including focal nodular hyperplasia (Fig. 7.26). T1-weighted SE MRI reveals most to be hyperintense and a minority isointense or hypointense to liver
parenchyma. Most are hyperintense on T2weighted sequences; they vary in heterogeneity, presumably due to their lipid content, hemorrhage, and necrosis. The degree of T1-weighted hyperintensity is a measure of a lesion’s fat content. As expected, gadolinium-enhanced MRI shows most adenomas to have early arterial enhancement, fading to become isointense during later phases.
Hemorrhage and surrounding iron deposition is evident in some adenomas. An adenoma rich in iron (called a siderotic adenoma) is hyperintense on T1-weighted images and hypointense T2-weighted images. More common are focal iron deposits, resulting in a heterogeneous MR appearance.
Three adenomas showed a mean of –7% (±24%) decrease in signal intensity on ferumoxides-enhanced T2-weighted MRI (81), presumably reflecting reticuloendothelial uptake.
The presence of hepatocytes results in tumor take-up of hepatocyte-specific scintigraphic agents such as Mn-DPDP.
Most adenomas do not have Tc-99m–sulfur colloid uptake, although a minority contain Kupffer cells. Tagged red blood cell imaging
A B
Figure 7.26. Hepatic adenoma (arrows). It is hypointense on T1- |
|
(A) and hyperintense on T2-weighted images (B). C: It shows inho- |
|
mogeneous contrast enhancement. (Source: Burgener FA, Meyers |
|
SP, Tan RK, Zaunbauer W. Differential Diagnosis in Magnetic Reso- |
|
nance Imaging. Stuttgart: Thieme, 2002, with permission.) |
C |

358
shows early uptake and a cold defect on delayed scans.
Arteriography reveals a hypervascular tumor, at times containing hypovascular foci due to hemorrhage or necrosis. Arteriography generally does not aid in differentiating adenomas from other hepatic tumors, but arterial embolization is occasionally helpful in their preoperative management.
Many of the imaging findings of an adenoma are similar to those of a hypervascular metastasis. Imaging can usually suggest a liver adenoma, but atypical findings make differentiation from other focal tumors difficult.
Adenomatosis
Adenomas range from one, to several, to numerous scattered throughout the liver, with the latter termed adenomatosis. Adenomatosis occurs equally in both sexes and is not related to medication.
In a collection of 15 adults with >10 hepatic adenomas each and no evidence of glycogen storage disease or anabolic steroid use, 73% presented with abdominal pain, hepatomegaly was found in 67%, and abnormal liver function in 91% (89); resection showed common intratumoral hemorrhage, but only 27% evidenced clinical and imaging hemorrhage. Computed tomography and MR revealed hypervascularity in 63% and intratumoral fat in 50% of patients with CT and 80% with MR; these non– steroid-dependent adenomas grew over time, and two patients developed hepatocellular carcinomas.
Lipoma
Hepatic lipomas are rare. An association appears to exist with renal angiomyolipomas and tuberous sclerosis. An occasional patient has multiple liver lipomas.
Precontrast CT readily reveals their lipomatous nature. Postcontrast, tumor density is variable depending on the overall vascularity and the amount of other tissue present. Ultrasonography shows a hyperechoic, well-marginated tumor with posterior attenuation. It mimics focal fat or a hemangioma. A typical lipoma is hyperintense on T1-weighted images. With all sequences, including fat suppression and post-
ADVANCED IMAGING OF THE ABDOMEN
contrast, a lipoma mimics other fat-containing structures.
Occasionally seen is an encapsulated fatty nodule on the liver surface, called a pseudolipoma by some. These nodules have a fibrous capsule and contain necrotic mature fat. Computed tomography reveals subcapsular fat. Some of these tumors develop focal calcifications.
Not all lesions containing fat are lipomas. Both adenomas and angiomyolipomas contain fat. A rare hepatocellular carcinoma, especially in a setting of cirrhosis, contains sufficient fat to be detectable with imaging. A rare liver xanthoma develops in a patient with hyperlipidemia. Most myelolipomas occur in the adrenal glands; hepatic myelolipomas are rare focal, heterogeneous tumors having varied MR signal intensities, reflecting a mix of fat, marrow, and occasional calcifications.
Angiomyolipoma
A benign mixed mesenchymal tumor, an angiomyolipoma was previously classified as a hamartoma, although a rare metastasis argues for neoplastic consideration. It is a rare tumor in the liver except in patients with tuberous sclerosis who also have renal angiomyolipomas. Occasionally they are multiple. Histologically, liver angiomyolipomas are similar to those found in kidneys. Some exhibit extramedullary hematopoiesis.
The overall imaging appearance is variable depending on the amount of fat present, which ranges from minimal to mimicry of a lipoma. In fact, presence of fat is what distinguishes this tumor from other, more ominous tumors. Imaging typically reveals an inhomogeneous fatty component within a tumor.
Most liver angiomyolipomas are hypervascular.
A typical CT appearance is that of a hypodense tumor showing delayed enhancement during the portal venous phase (90); central vessels are evident in some of these tumors. They are hyperechoic by US and hyperintense on T1-weighted MRI. Fat-suppression techniques show a decreasing signal intensity.
Nevertheless, in some patients CT, US, and angiography findings are atypical and mimic focal nodular hyperplasia, hepatocellular carcinoma, or even a rare myelolipoma. Thus an occasional angiomyolipoma contains no visible

359
LIVER
fat, US revealed these to be mainly hypoechoic, and with MR they range from hypoto isointense on T1-weighted sequences and hyperintense on T2-weighted sequences.
Malignant Neoplasms
Hepatocellular Carcinoma
Clinical
In the United States and Western Europe the most common liver malignancy is a metastasis. In most other parts of the world hepatocellular carcinoma predominates. The incidence is gradually increasing in the United States, with a disproportionate increase in a younger patient population. While a hepatocellular carcinoma is often considered an adult tumor, it is the second most common liver malignancy in children (after hepatoblastoma).
Different presentations are seen in different countries. In North America, cirrhotic patients developing a hepatocellular carcinoma are significantly older than those without cirrhosis; survival is longer in patients without cirrhosis. Underlying chronic liver disease makes hepatocellular carcinoma detection both more difficult and also provides a clue to its presence.
Serum a-fetoprotein levels are not always elevated with these tumors, especially smaller ones, and with fibrolamellar variant carcinomas (discussed in a later section). On the other hand, diffuse hepatocellular carcinoma, infiltrating extensively, is invariably associated with high levels. Elevated a-fetoprotein levels, however, are also found in cirrhosis and some other chronic liver diseases, fulminant hepatitis, and some metastases. Hypoglycemia is common. Most often jaundice at the initial presentation is due to underlying liver failure, either secondary to parenchymal replacement by tumor or superimposed cirrhosis. Jaundice due to bile duct obstruction by tumor is unusual except late in the course. A rare hepatocellular carcinoma erodes into the bile ducts and sheds tumor fragments, and jaundice ensues secondary to tumor thrombus. This subset tends to mimic a cholangiocarcinoma in their appearance.
Most hepatocellular carcinomas are poorly differentiated and have an aggressive growth pattern. Portal vein, hepatic vein, and inferior vena cava invasion are common.
Associated Conditions
A number of underlying conditions are associated with the subsequent development of hepatocellular carcinoma (Table 7.11). Underlying chronic liver disease is common. In North America, cirrhosis is often present. In China, HBV infection in younger patients is associated with subsequent hepatocellular carcinomas without underlying cirrhosis, a finding consistent with a direct viral effect on liver cell transformation. These younger patients tend to have larger tumors than older patients, and their tumors are more advanced when first discovered. Excess liver iron also appears to predispose to cancer. In cirrhotics, risk of hepatocellular carcinoma is increased in those with iron deposits in regenerative nodules compared to those without such iron; iron in regenerative nodules tends to parallel parenchymal iron deposits.
Hepatocellular carcinomas have developed after long-term anabolic steroid administration, mostly for hematologic disorders, and in women on long-term oral contraceptives. Nevertheless, no conclusive evidence exists of a relationship between oral contraceptives and hepatocellular carcinoma. In Sweden, with
Table 7.11. Conditions associated with hepatocellular carcinoma
Mostly in adults
Viral hepatitis
Cirrhosis
Hepatic adenoma
Adenomatous hyperplasia
Hemochromatosis
Hepatotoxin exposure
Androgen use
Porphyria
Acute intermittent
Porphyria cutanea tarda
Polyvinyl chloride exposure
Diabetes mellitus
Hepatic vein thrombosis
Mostly in children Biliary atresia
Glycogen storage disease type I Hereditary tyrosinemia Congenital hepatic fibrosis a1-Antitrypsin deficiency Alagille syndrome

360
extensive oral contraceptive use, hepatocellular carcinoma incidence in women parallels that of men; on the other hand, in Japan, with negligible oral contraceptive use, incidence and mortality is gradually rising.
In cirrhotic patients, creation of a portosystemic shunt does not appear to increase risk for hepatocellular carcinoma.
Some evidence suggests that patients with chronic hepatic vein thrombosis are at risk for hepatocellular carcinoma even without any evidence for underlying cirrhosis.
An occasional patient develops a preceding paraneoplastic syndrome.
Rupture/Bleeding
A hepatocellular carcinoma is prone to spontaneous rupture and bleeding, generally into the peritoneal cavity. A rare hepatocellular carcinoma first manifests with a spontaneous rupture and the patient presents with hemoperitoneum. Some patients initially develop a subcapsular hematoma, followed by capsular rupture and hemoperitoneum. In general, larger tumors and left lobe tumors are more prone to rupture, compared to the right lobe. Peritoneal tumor spread and omental seeding are uncommon. Some tumors invade adjacent colon and result in lower gastrointestinal hemorrhage. Transcatheter selective arterial embolization can arrest such bleeding, but it is, of course only palliative.
These patients are also prone to portal hypertension, esophageal varices, and subsequent variceal bleeding. Prophylactic variceal sclerotherapy is feasible, thus decreasing the risk of subsequent variceal bleeding. Transjugular intrahepatic portosystemic shunting also helps correct portal hypertension.
Screening
In spite of its high prevalent in the Orient and Africa, with the exception of a few Asian countries few populations practice hepatocellular carcinoma screening, which is usually based on liver US and serum a-fetoprotein levels. Any patients with suspicious findings then undergo further confirmatory diagnoses.A Hong Kong US screening study of hepatitis B virus carriers with elevated a-fetoprotein levels achieved a sensitivity and specificity for hepa-
ADVANCED IMAGING OF THE ABDOMEN
tocellular carcinoma detection of 86% and 82%, respectively (91). Nevertheless, little evidence suggests that screening leads to any significant decrease in mortality. The few screening studies for hepatocellular carcinoma in patients with cirrhosis, generally consisting of US and serum a-fetoprotein levels every six months, have detected a cancer incidence of roughly 5% per follow-up year; yet in spite of such screening, cure rates for patients with a detected cancer amenable to definitive therapy are low and validity for screening cirrhosis patients can be questioned.
Pathology
Adenomatous hyperplasia is a poorly understood condition developing in some patients with chronic liver disease. Patients with surgically resected adenomatous hyperplasia nodules that contain cellular atypia and focal malignancy tend to develop hepatocellular carcinomas within a few years, while those with adenomatous hyperplasia but without cellular atypia do not. A sequence of regenerating nodules, adenomatous hyperplasia, and atypia appears to be a pathway for hepatocellular carcinoma development. Atypia leads to low-grade malignancy and presumably to an advanced hepatocellular carcinoma.
Whether hepatocellular carcinomas are multicentric in origin is debatable. The frequent occurrence of hepatocellular carcinomas after resection of adenomatous hyperplasia implies a multicentric origin. Direct injection of radiopaque material into resected tumor specimens shows that spread is by capsule invasion, intrahepatic invasion, and portal vein.
Small hepatocellular carcinomas tend to be of a low-grade malignancy and, in fact, macroscopic features of early hepatocellular carcinomas often resemble those of hyperplasia or adenoma rather than more advanced carcinomas. A not uncommon pattern is that of well-differentiated cells mimicking benign hepatocytes in connective tissue, factors related to patient survival; these cells became less differentiated with growth. Nevertheless, welldifferentiated cancers do have a potential for metastasis.
In North America, hepatocellular carcinomas developing in a setting of cirrhosis tend to be less well-differentiated than in those without

361
LIVER
cirrhosis and are more prone to invade the portal vein. Encapsulated tumors are more common in patients without cirrhosis.
Pathologists divide hepatocellular carcinomas into clear-cell and non–clear-cell types, but no significant prognostic differences are evident between the two.
Histologically, some metastatic carcinomas are very similar in appearance to hepatocellular carcinomas. This differentiation is especially difficult with small needle biopsy specimens; immunohistochemical staining for human albumin messenger RNA (mRNA) is helpful in some. Albumin mRNA is detected in a majority of primary and metastatic hepatocellular carcinomas, but not in nonhepatocellular tumors.
A rare extrahepatic bile duct hepatocellular carcinoma is reported. Whether these represent hepatoid differentiation of a cholangiocarcinoma or are indeed primary extrahepatic hepatocellular carcinomas is speculation. An occasional such tumor contains both hepatocyte and bile duct cell features, and an immature progenitor cell origin appears reasonable.
Because current imaging detects smaller and smaller lesions, the pathologist is now faced with the task of differentiating between lowgrade malignancy and atypical hyperplasia.
Arteriovenous Shunting
Hepatocellular carcinomas are fed mostly by arterial blood, although from a hemodynamic viewpoint the situation is complex. Many of these tumors drain, in part, into a portal venule and thus mimic an arterioportal vein fistula. Shunting is more common into the portal vein than hepatic vein.
Ideally, arterioportal shunting is identified as early portal vein filling after arterial contrast injection. Shunting is often considered to be present if arterial phase CT shows wedgeshaped enhancement peripheral to a tumor and if this region becomes isoor hyperattenuating during the portal phase. Extensive shunting leads to hepatic artery enlargement. Arterioportal shunting affects liver perfusion and decreases tumor enhancement, resulting in heterogeneous enhancement and, at times, even a hypovascular CT appearance. Such shunting is unusual with hypovascular metastases, but
nontumor-associated arterioportal shunts do exist in a cirrhotic liver and can mimic a hypervascular tumor.
Arteriovenous shunting is detected with hepatic arteriography or scintigraphy by injecting Tc-99m–macroaggregated albumin (MAA) into the tumor feeding artery (angioscintigraphy). The latter technique is very sensitive in detecting small shunts. Angioscintigraphy can estimate lung shunting in patients with hepatocellular carcinomas (lung shunting is calculated as the total count over the lungs divided by the total count over both the lungs and liver) and correlates with tumor size (estimated by CT or US) and vascularity.
The presence of arteriovenous shunting has a bearing on prognosis and is considered a contraindication to arterial chemoembolization. Transcatheter arterial embolization with steel coils in some of these patients with severe arterioportal shunting results in shunt resolution and resumption of hepatopetal portal blood flow and an improved quality of life, although it has little effect on survival.
A word of caution. Non–tumor-associated arterioportal shunts do exist in a cirrhotic liver and can mimic a hypervascular tumor. Postcontrast CT reveals such nontumorous shunts as wedge-shaped and homogeneously enhancing regions, at times containing an internal linear branching pattern during the arterial phase.
Detection
Comparison Studies
The imaging technique yielding the highest tumor nodule detection rate is not established, although a trend is evident in favor of more complex contrast-enhanced MR imaging. Both superparamagnetic and hepatobiliary MR contrast agents show promise. A number of studies, mostly by Japanese investigators, show that arterial-phase MR is superior to arterialphase CT or any other CT or MR phase in detecting and evaluating small hepatocellular carcinomas. Ferumoxides-enhanced MRI achieved a 93% sensitivity and 99% specificity in tumor detection, while combined CT arterial portography and hepatic arteriography achieved a 91% sensitivity and 94% specificity (92), and the authors suggest that MRI can replace CT for preoperative evaluation of these patients.

362
Tumor detection using a combined unenhanced, gadolinium-enhanced, and ferumoxides-enhanced MRI was similar to that obtained with a combined CT arterial portography and biphasic CT hepatic arteriography (93); an advantage of the MRI approach is that invasive CT angiography is not necessary.
Detection accuracy of malignant hepatic tumors (a mix of hepatocellular carcinomas and metastases) by SPIO-enhanced MR imaging is superior to unenhanced MRI and is similar to CT arterial portography (94). Relative tumor detection sensitivities depend on tumor size; CT and US detect more smaller tumors than DSA or even possibly iodized oil CT; but the latter are more sensitive with a larger tumor volume.
Magnetic resonance is probably more sensitive than CT in showing that a capsule is present and in identifying vascular involvement.
General Imaging Findings
A differentiation of hemangiomas from hepatocellular carcinomas (and metastases) was discussed above (see Differential Diagnosis of Focal Tumors).
About half of hepatocellular carcinomas occur as solitary, discrete tumors, with the other half consisting of several to multiple focal lesions or diffuse liver infiltration (Fig. 7.27). A rare one is extrahepatic, connected to a lobe by a pedicle. In children, signs pointing toward a hepatocellular carcinoma are the presence of underlying liver disease and significant venous
ADVANCED IMAGING OF THE ABDOMEN
Figure 7.27. Multifocal hepatocellular carcinoma.
invasion, findings somewhat uncommon with a hepatoblastoma. Imaging findings in children tend to mimic those seen with a hepatoblastoma (Fig. 7.28).
Most hepatocellular carcinomas are rather vascular, supplied primarily by hepatic artery branches.Vascular invasion more often involves portal vein branches rather than hepatic veins. Arterioportal shunting and hemorrhage are common. The less common well-differentiated hepatocellular carcinomas tend toward hypovascularity.
Computed tomography is not reliable in detecting bile duct invasion. Cholangiography
A 
 B
B
Figure 7.28. Hepatocellular carcinoma in a 5-year old girl. A,B: Arterial phase CT reveals a tumor replacing most of the left lobe and nodules scattered in the right lobe. A hepatoblastoma was in the differential diagnosis. (Courtesy of Luann Teschmacher, M.D., University of Rochester.)
363
LIVER
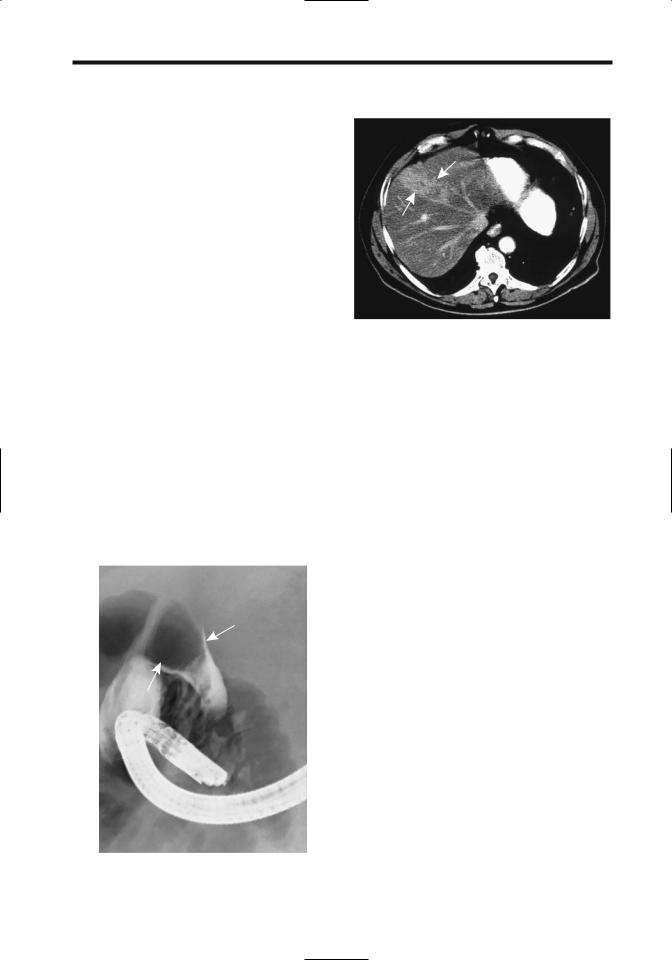
can identify an intraductal tumor (Fig. 7.29), at times mimicking a stone. Also, a tumor involving the liver hilum may obstruct bile ducts and result in intrahepatic biliary dilation without direct bile duct invasion.
Aside from fibrolamellar carcinomas, gross calcifications in a hepatocellular carcinoma are uncommon. An occasional one contains a central scar, which is more often detected in a carcinoma developing in a noncirrhotic liver than in a cirrhotic liver.
Computed Tomography
A characteristic unenhanced CT appearance of hepatocellular carcinomas is that of a hypodense to isodense tumor. Some isodense tumors contain a thin hypodense rim, but the margin between tumor and adjacent parenchyma tends to be poorly defined. Typical postcontrast findings consist of marked neovascularity, enlarged feeding arteries, a dense tumor vascular blush, normal or delayed contrast washout, and arterioportal shunting. Nevertheless, considerable CT variability exists and an occasional one is even hypovascular (Fig. 7.30). Some reveal an enhancing rim during the portal
Figure 7.29. Hepatocellular carcinoma growing within hepatic duct (arrow), an uncommon presentation.
Figure 7.30. Hepatocellular carcinoma. Contrast-enhanced CT identifies a tumor replacing part of the right lobe (arrows). (Courtesy of Patrick Fultz, M.D., University of Rochester.)
phase. In general, CT contrast-enhancing regions represent viable tumor tissue, while hypodense regions are either necrotic or contain fibrosis and hemorrhage.
What is the relative value of arterial phase, portal venous phase, and delayed phase CT in detecting these tumors? Arterial phase imaging yields the highest tumor-to-parenchyma contrast and detects more tumors than the other phases. During the arterial phase they tend toward intense homogeneous enhancement, a finding also seen with other hypervascular tumors, both benign and malignant. Larger tumors have heterogeneous arterial-phase enhancement, probably due to interspersed necrosis. Tumors missed on the arterial phase tended to be well differentiated, do not have a fibrous capsule, and are relatively hypovascular. Although different publications provide different tumor detection rates, relative tumor detection between various imaging phases is remarkably constant. A combination of arterial and portal venous phase images detects significantly more tumors than a combination of unenhanced and portal venous phase images. Any combination of two phases that includes the arterial phase is superior to a combination of portal and delayed phases. Or, for maximum tumor detection, all three phases should be obtained because a small minority of tumors are detected only on unenhanced images.
A novel technique consists of both early and late arterial-phase images obtained serially

364
during a single breath-hold multidetector CT study. Such double arterial-phase imaging yielded detection sensitivities of 54% for the early arterial phase, 78% for the late arterial phase, and 86% for the double arterial phase (95); the number of false-positive tumors was also reduced with double arterial-phase imaging.
The place of 3D liver CT reconstruction in evaluating hepatocellular carcinomas is not yet clear in spite of some obvious advantages. It is useful to the surgeon in defining vascular and tumor anatomy in specific segments when planning resection, yet at present 3D reconstruction is not widely practiced; considerable operator time and experience is necessary to achieve meaningful results.
One technique consists of reconstructing maximum intensity projection images of intrahepatic portal venous branches and hepatic veins, plus shaded surface display images of hepatic tumors from postcontrast CT data; when superimposed, these two sets of images provided a 3D relationship between vessels and tumors, aiding tumor localization prior to resection.
Do CT arterial portography and CT hepatic arteriography add significant information beyond what is obtained with triple-phase CT during preoperative evaluation of hepatocellular carcinomas? Adding CT arterial portography to triple-phase CT increased sensitivity from 94% to 96% and the further addition of CT hepatic arteriography increased it further to 97% (96); a disadvantage is that CT arterial portography and CT hepatic arteriography increase the false-positive rates without a corresponding gain in sensitivity.
Computed tomography hepatic arteriography aids, in part, in differentiating dysplastic nodules from hepatocellular carcinomas. Thus CT hepatic arteriography reveals that although high-grade dysplastic nodules range from hypoto hyperdense, a majority of low-grade dysplastic nodules are isodense (97); carcinomas, on the other hand, are mostly hyperdense, especially moderately to poorly differentiated ones.
A limitation of CT hepatic arteriography is that in some patients the entire liver cannot be studied because of an aberrant blood supply; examples include the presence of an aberrant
ADVANCED IMAGING OF THE ABDOMEN
left hepatic artery originating from the left gastric artery and occlusion of a right hepatic artery; injection of contrast into the hepatic artery does not opacify these segments and thus misses tumors there.
Computed tomography arterial portography involves placing an angiographic catheter into the splenic artery or superior mesenteric artery distal to the origin of any hepatic arteries. During portal-phase imaging the normal liver is markedly enhanced, with little enhancement of neoplasms receiving their blood supply from the hepatic artery, thus accentuating contrast differences between normal parenchyma and a neoplasm. Potentially this technique allows detection of smaller tumors. Similarly to CT hepatic arteriography, CT arterial portography aids in differentiating dysplastic nodules from hepatocellular carcinomas. Thus 77% of low-grade dysplastic nodules were isodense, while only 32% of high-grade dysplastic nodules were isodense (the rest ranged from slightly to markedly hypodense) (97); all welldifferentiated carcinomas ranged from slightly to markedly hypodense and moderately to poorly differentiated carcinomas all were markedly hypodense. Practical application of such CT correlation with histologic findings is yet to be established.
While CT arterial portography is rather sensitive, its specificity is rather low. Hemangiomas, focal nodular hyperplasia, regenerating nodules, and liver parenchyma being fed by aberrant vessels are detected, and in many instances these false-positive lesions cannot be distinguished from neoplasms. Follow-up double-phase CT arteriography appears useful in differentiating hepatic tumors, especially malignancies, from nonspecific perfusion abnormalities (called pseudolesions by some). These nonneoplastic perfusion abnormalities tend to be more common adjacent to the falciform ligament, gallbladder, and posterior edge of the medial segment. For unknown reasons these abnormalities occur less often adjacent to the falciform ligament in patients with cirrhosis.
Hepatocellular carcinomas not identified by CT arterial portography (i.e., false negative) tend to be central in location or associated with segmental portal vein thromboses obstructing the flow of contrast to the liver periphery.

365
LIVER
Computed tomography arterial portography is of limited value in a setting of portal hypertension, regardless of the etiology; insufficient iodine is delivered via the portal vein to the liver to achieve adequate contrast differences for tumor visualization. Enhancement is also decreased if portosystemic shunting has developed. In these settings CT arteriography is a better choice.
Malignant tumor size tends to be overestimated with CT arterial portography. Reasons are due to parenchymal compression, portal vein obstruction, or possibly a siphoning effect by these hypervascular tumors.
Although an iodized oil CT study (also called Lipiodol CT) is often used as a gold standard for tumor detection, it has distinct limitations. It is generally assumed that iodized oil is retained only by malignant cells, although evidence suggests that oil is also retained by other tumors. Iodized oil can accumulate in a hemangioma. Iodized oil uptake tends to be homogeneous in tumors smaller than 2cm in diameter, becoming inhomogeneous in larger ones, presumably secondary to necrosis.
It would be naive to think that iodized oil CT detects all tumors. Iodized oil CT appears to be a tarnished gold standard when examining tumors in explanted livers, with pretransplantation iodized oil CT sensitivity being rather low. Nevertheless, contrary to some studies, it often detects more tumors than other imaging modalities.
Occasionally spasm is encountered during arterial injection of Lipiodol, resulting in proximal Lipiodol reflux. Intraarterial buflomedil has been used to decrease arterial spasm.
Ultrasonography
Sonographic findings of hepatocellular carcinomas vary and are nonspecific. Sonography detects only a minority of hepatocellular carcinomas in patients with advanced cirrhosis, in one study identifying only 27% of tumors prior to liver transplantation (98). In the West, even small tumors range from (mostly) hypoechoic to (less often) hyperechoic, while in parts of Africa multinodular, hyperechoic tumors are more common. A thin hypoechoic rim with a hyperechoic center is evident in some; such a
target sign is nonspecific and is also seen with metastases and some benign tumors. A complex pattern develops with growth, and these tumors range from heterogeneous, hyperechoic, hypoechoic, and nodular, to diffuse infiltration. An occasional one mimics an abscess.
Ultrasonography depicts “hemangioma-like” lesions in some cirrhotic patients (99); followup revealed that half were hyperechoic hepatocellular carcinomas.
Color Doppler signals are detected both at the periphery and within vascular tumors; they are detected in a majority of hepatocellular carcinomas and focal nodular hyperplasias. Power Doppler US is superior to color Doppler US in visualizing hepatocellular carcinoma blood flow and identifying tortuous intratumoral vessels. Numerous studies have confirmed that contrast-enhanced power Doppler US is superior to unenhanced power Doppler US in detecting hepatocellular carcinoma tumor vascularity. Contrast-enhanced color Doppler US of most hepatocellular carcinomas reveals intratumoral signals, afferent vessels, and peripheral vascularity.
Doppler US–generated hepatic artery velocity histograms show hepatocellular carcinoma artery flow tending toward turbulent. Occasionally color Doppler US detects reversed portal blood flow adjacent to a hepatocellular carcinoma; such flow reversal is nonspecific and is also identified with metastases, abscesses, and even subcapsular hematomas. Color Doppler US detects a feeding artery in hepatocellular carcinomas more often after contrast; a feeding artery, however, is not pathognomonic of a hepatocellular carcinoma.
Carbon dioxide–enhanced US is used to study blood flow patterns. In some hands US enhanced with intraarterial carbon dioxide microbubbles detects more hepatocellular carcinomas than contrast-enhanced CT or even iodized oil-CT. After carbon dioxide microbubble injection into the hepatic artery, real-time B-mode US will identify blood drainage into a portal vein branch through a hepatocellular carcinoma, thus confirming arterioportal shunting.
Pulse-inversion harmonic US with preand serial postintravenous microbubble contrast injection reveals dense tumor staining, enhancement ranging from homogeneous to
