
Книги по МРТ КТ на английском языке / Neurosurgery Fundamentals Agarval 1 ed 2019
.pdf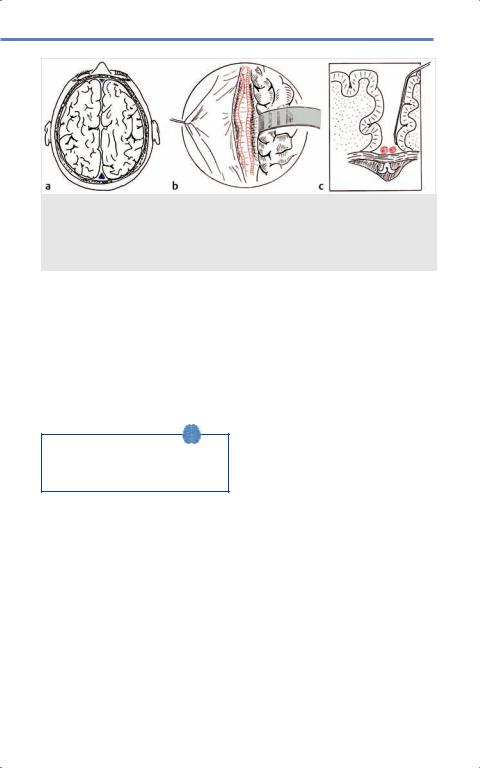
15.2 Epilepsy
Fig. 15.10 Corpus callosotomy. (a) View of operative site at vertex of field with (b) corpus callosum exposed following interhemispheric dissection and (c) coronal view with paired pericallosal vessels superior to body of corpus callosum. (Reproduced from Starr P, Barbaro N, Larson P, Functional Neurosurgery, 2nd edition, ©2008, Thieme Publishers, New York.)36
The vagus nerve has greater than 80% afferent visceral fibers with a vast number of cortical, subcortical, deep nuclei, and brainstem projections involved in epileptogenesis. Although the exact mechanism is not understood, VNS uses electrical stimulation at the left vagus nerve that is transmitted rostrally throughout the CNS, leading to positive effects in seizure rate reduction.
Studies show that many patients will have seizure frequency reductions of 50% in about 50% of patients.37
VNS placement is not first-line treatment, and is reserved for patients who failed multiple treatments, EZ in eloquent cortex, and/or failed prior epilepsy surgery with no restrictions on seizure type (e.g.,
comparable efficacy in generalized and partial seizures).
Vagus nerve is approached on the left side and located in the carotid sheath between the carotid and jugular vein below the carotid bifurcation, and is exposed over a distance of 3 cm. Helical electrodes are placed around the vagus nerve and are connected to an infraclavicular or axillary pulse generator in a fashion similar to DBS ( Fig. 15.11).37,38
Adverse events with use of VNS are usually transient with patients experienc- ing voice alteration (20–30%), cough (6%), or paresthesias (10%), which diminish over time. Surgical complications are rare including infection requiring removal of system (1%), vocal cord injury (< 1%), and lower facial weakness (1%), with the most common reason for repeat surgery being replacement of a depleted pulse generator.38
309
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

Movement Disorders and Epilepsy
Fig. 15.11 Vagus nerve stimulation. (a–c) Typical positioning and anatomical landmarks noted with neck and axillary markings. (d) Technique for placement of helical electrodes around vagus nerve. (Reproduced from Starr P, Barbaro N, Larson P, Functional Neurosurgery, 2nd edition, ©2008, Thieme Publishers, New York.)38
Pearls
• Common DBS Targets:
◦◦PD: STN, GPi
◦◦Essential Tremor: VIM
◦◦Dystonia Target: GPi
• Engel Outcome Classification: class I, seizure free; class II, rare disabling seizures (> 2/yr); class III, worthwhile and variable; class IV, no worthwhile change.
•Mesial temporal lobe epilepsy with Engel class I outcomes of approximately 70% following anterior temporal lobectomy.
•Extratemporal lobe epilepsy can be treated via multiple approaches
including:
◦◦Cortical resections (can be curative). ◦◦Multiple subpial transections
(Engel I to III outcomes up to 80%).
◦◦Hemispherectomy (Engel I outcomes up to 74–90%).
◦◦Corpus callosotomy (80–100% reduction in drop attaches).
◦◦Vagus nerve stimulation (seizure frequency reductions of 50% in about 50% of patients).
15.3 Top Hits
15.3.1 Questions
1.What are the main targets for PD, essential tremor and dystonia? a) PD: VIM, ET: VIM, Dystonia: GPi. b) PD: GPi, ET: GPi, Dystonia: STN. c) PD: GPi/STN, ET: VIM, Dystonia:
GPi.
d) PD: STN, ET: VIM, Dystonia: STN.
310
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

15.3 Top Hits
2.What are the stereotactic coordinates for STN, GPi, and VIM?
3.What are the cardinal motor symptoms of PD?
a) Resting tremor, bradykinesia, shuffled gait, rigidity.
b) Masked facies, on/off fluctuations, bradykinesia.
c) Rigidity, bradykinesia, on/off fluctuations.
d) Action tremor, rigidity, on/off fluctuations.
4.What is the main difference between STN and GPi-DBS in PD?
a) STN has improved outcomes in cardinal motor symptoms over GPi.
b) GPi has improved reduction in on/ off fluctuations compared to STN. c) They are both equivalent in every
outcome measure.
d) STN noted greater reduction in medication requirements and more cognitive and behavioral side effects over GPi.
5.Dystonia can be classified as which of the following:
a) Location of body affected (general- ized, focal, multifocal, segmental, hemidystonia), etiology (primary or idiopathic, secondary or symp-
tomatic), or age of onset (early < 26 years or late > 26 years).
b) Location of body affected (general- ized, focal, multifocal, segmental, hemidystonia), etiology (primary or idiopathic, secondary or symptomatic), or age of onset (early
< 9 years or late > 9 years). c) Tremor type (resting, postural,
kinetic), etiology (primary or idiopathic, secondary or symptomatic), or age of onset (early < 26 years or late > 26 years).
d) Location of body affected (general- ized, focal, multifocal, segmental, hemidystonia), etiology (primary
or idiopathic, secondary or symptomatic, tertiary), or age of onset (early < 26 years or late
>26 years).
6.Which of the following is first-line treatment for ET:
a) Benzodiazepines, botulinum toxin A. b) Alcohol, primidone,
benzodiazepines. c) Propranolol, primidone. d) Primidone, botulinum toxin A.
7.What are the main components of phase 1 investigations?
a) Seizure semiology, imaging studies, EEG, neuropsychological assessment.
b) Seizure semiology, intracranial electrodes, fMRI.
c) EEG, imaging studies.
d) Seizure semiology, EEG, intracranial electrodes, functional studies.
8.Phase 2 investigations include which of the following?
a) Subdural grids and strips electrodes, sEEG intracerebral electrodes, foramen ovale electrodes.
b) Neuropsychological assessments, Wada, MEG, fMRI.
c) Scalp EEG, Wada, fMRI, MEG. d) Seizures semiology, imaging stud-
ies, EEG, intracerebral electrodes.
9.What are typical measurements for an anterior temporal lobectomy?
a) A 6 and 6 cm from anterior tip of the temporal lobe of neocortex in both dominant and nondominant cases.
b) A 6 or 4 cm from anterior tip of the temporal lobe of neocortex in dominant or nondominant cases.
c) A 4 or 6 cm from anterior tip of the temporal lobe of neocortex in dominant or nondominant cases, respectively.
d) No typical standard is used.
311
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

Movement Disorders and Epilepsy
10.Which technique is based on the idea that the functional unit in the cortex is a vertically oriented column such that disruption of the horizontal fibers does not eliminate function but will control conduction of epileptic discharges, thus decreasing synchronized cell discharge necessary for an epileptic?
a) Anterior temporal lobectomy. b) Hemispherectomy.
c) Vagus nerve stimulation. d) Multiple subpial transection.
11._______ is a disconnective technique that decreases the risk of delayed hydrocephalus and superficial cerebral hemosiderosis compared to
________.
a) Functional hemispherectomy, anatomical hemispherectomy. b) Anatomical hemispherectomy, functional hemispherectomy. c) Anatomical hemispherectomy,
multiple subpial transection. d) Multiple subpial transection, functional hemispherectomy.
Answers
1.c. The main targets for the major movement disorders are as follows: PD targets both the STN and GPi likely due to overactivity following net loss of dopaminergic input. Essential tremor targets VIM likely due to mediation by a neuronal loop involving the cerebellothalamocortical fibers with tremors cells in the VIM. Dystonia targets the GPi likely due to complex mechanisms involving loss of motor inhibitory function leading to excessive contraction of agonist and antagonist muscles, abnormal somatosensory input, motor cortex overexcitability and loss of intracortical inhibition with imbalance of direct and indirect pathways in the basal ganglia.
312
2.See Table 15.1. The stereotactic coordinates for the major movement disorders targets are: STN: 11–13 mm lateral to MCP, 4–5 mm ventral to AC/ PC line, and 3–4 mm posterior to the MCP; GPi: 19–21 mm lateral to MCP, 4–5 mm ventral to AC/PC line, and 2–3 mm anterior to MCP; VIM: 11 mm lateral to 3rd ventricular wall, at the level of the AC/PC line, and 5–6 mm anterior to the PC.
3.a. The cardinal motor symptoms of PD are resting tremor, bradykinesia, shuffled gait, and rigidity due to imbalances in the basal ganglia direct and indirect pathways including STN and GPi overactivity following net loss of dopaminergic input.
4.d. The main difference between STN and GPi DBS for the treatment of PD is that STN is noted to have a greater reduction in medication requirements (>50% at 12 months) but with more cognitive and behavioral side effects, making it the preferred target.
5.a. Dystonia can be understood based on several classification schemes to help guide clinicians in their treatment of disease and symptoms management, which include: location of body affected (generalized, focal, multifocal, segmental, hemidystonia); etiology (primary or idiopathic, secondary or symptomatic); or age of onset (early > 26 years or late > 26 years).
6.c. First line treatments alone or in combination for Essential Tremor include b-blockers like propranolol and anticonvulsants like primidone with class I evidence noting tremor reduction up to 60% in 50% of patients.
7.a. The main components of phase 1, or noninvasive investigations in the work up of epilepsy include seizure semiology, imaging studies (e.g., MRI, PET, SPECT), EEG (video), and neuropsychological assessment (memory tests,
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

15.3 Top Hits
language evaluation including fMRI, Wada test, and/or MEG). These tools are geared to help clinicians both better localize the epileptogenic zone and assess the risk of postoperative deficits in planning for epilepsy surgery.
8.a. Phase 2 investigations should only be performed if noninvasive studies do not enable the clinician to proceed to surgery with confidence, and they include: subdural grids and strips electrodes, sEEG intracerebral electrodes, foramen ovale electrodes. Phase 2 investigations are most in patients with simple or complex partial seizures (with or without secondary generalization), without structural lesion(s) on imaging, bilateral ictal and interictal activity, discordant data between seizures, EEG and imaging, and EZ localization near or involving eloquent areas.
9.c. Typical measurements for an anterior temporal lobectomy are: a 4 or 6 cm from anterior tip of the temporal lobe of neocortex in dominant or nondominant cases respectively, with resection of neocortical portion followed by mesial structures without significant impact on memory, cognitive function, or language. A more conservative technique developed by Spencer et al minimizes lateral resection and maximizes mesial resection, with sparing most of the STG, removal of 3 to 3.5 cm of the MTG, ITG, most of the amygdala, and 3 to 4 cm of the hippocampus and parahippocampal gyrus.
10.d. Multiple subpial transections are most commonly used to treat EZ located in eloquent cortex with Engel I to III outcomes of up to 80%. This technique is based on the idea that the functional unit in the cortex is a vertically oriented column such that disruption of the horizontal fibers does not eliminate function but will
control conduction of epileptic discharges, thus decreasing synchronized cell discharge necessary for an epileptic spike.
11.a. Functional hemispherectomy (or hemispherotomy) is a disconnective technique introduced in the late 20th century decreasing the risks associated with anatomical hemispherectomies, including delayed hydrocephalus and superficial cerebral hemosiderosis. Outcomes for functional hemispherectomy are highly dependent on pathology with Engel class I outcomes in 81% of patients with a postischemic etiology compared to 40% in patients with hemimegalencephaly.
References
[1]Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;
79(4):368–376
[2]Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017; 3:17013
[3]Chokshi FH. Imaging of deep brain stimulation. In: Kanekar S, ed. Imaging of Neurodegenerative Disorders. Thieme; 2015:424
[4]De la Cruz P, Plakas C, Zamora AR, Pilitsis JG. Deep brain stimulation for Parkinson’s disease. In: Harbaugh RS, Christopher I, Couldwell William T, Berger Mitchel S, eds. Neurosurgery Knowledge Update: A Comprehensive Review. 2015:984
[5]Kopell BH, Horenstein Craig I, Rezai, Ali R. Deep Brain Stimulation for Dystonia. Movement Disorder Surgery, New York, NY: Thieme; 2008
[6]Taghva AS, Oluigbo CO, Rezai AR. Basic Principles of Deep Brain Stimulation for Movement Disorders, Neuropsychiatric Disorders, and New Frontiers, Principles of Neurological Surgery, Philadelphia, PA: Elsevier; 2012
[7]Whitworth LA, Burchiel KJ. Deep Brain Stimulation in Movement Disorders: Parkinson's Disease, Essential Tremor, Dystonia, Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[8]Starr PA. Pallidal Interventions for Parkinson’s Disease, Youmans Neurological Surgery, Philadelphia, PA: Elsevier; 2011
[9]Machado AG, Deogaonkar M, Cooper S. Deep brain stimulation for movement disorders: patient selection and technical options. Cleve Clin J Med. 2012 Jul;79 Suppl 2:S19-24. doi: 10.3949/ccjm.79.s2a.04
[10]Kobayashi K, Kim JH, Anderson WS, Lenz FA. Surgi- cal Management of Tremor, Youmans Neurological Surgery, Philadelphia, PA: Elsevier; 2011
313
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

Movement Disorders and Epilepsy
[11]Na YC, Chang WS, Jung HH, Kweon EJ, Chang JW. Unilateral magnetic resonance-guided focused ultrasound pallidotomy for Parkinson disease. Neu- rology. 2015; 85(6):549–551
[12]Hamani C, Schwalb JM, Hutchison WD, Lozano A.
Microelectrode-Guided Pallidotomy. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[13]Benito-León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol.
2006; 2(12):666–678, quiz 2p, 691
[14]Louis ED. Clinical practice. Essential tremor. N Engl J Med. 2001; 345(12):887–891
[15]Elias WJ, Lipsman N, Ondo WG, et al. A rand- omized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016; 375(8):
730–739
[16]Schwalb JM, Hamani C, Lozano A. Thalamic deep brain stimulation for the control of tremor. In: Starr
PA, Barbaro NM, Larson PS, eds. Functional Neuro- surgery. 2nd ed. Thieme; 2008
[17]Isaias IU, Tagliati M. Patient Selection Criteria for Deep Brain Stimulation in Movement Disorders, Youmans Neurological Surgery, Philadelphia, PA: Elsevier; 2011
[18]Geyer HL, Bressman SB. The diagnosis of dystonia. Lancet Neurol. 2006; 5(9):780–790
[19]Phukan J, Albanese A, Gasser T, Warner T. Primary dystonia and dystonia-plus syndromes: clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol. 2011; 10(12):1074–1085
[20]Jetté N, Sander JW, Keezer MR. Surgical treatment for epilepsy: the potential gap between evi- dence and practice. Lancet Neurol. 2016; 15(9): 982–994
[21] Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014; 13(11):1114–1126
[22]Velasco AL, Velasco F, Boleaga B, Nunez JM, Trejo
D. Presurgical Evaluation for Epilepsy Including Intracranial Electrodes, Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[23]Smith JR, Fountas KN. Subdural and stereotactic depth electrode implantation in the evaluation of ablative epilepsy surgery candidates. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[24]Ulm III AJ, Tanriover N, Rhoton Jr AL, Roper SN.
Surgical anatomy of the temporal lobe. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[25]Wyler AR. Temporal lobectomy. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[26]Sheth SA, Mian MK, Eskandar EN, Cosgrove GR.
Temporal Lobe Operations in Intractable Epilepsy,
Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[27]Abson-Kraemer DL, Spencer DD. Temporal lobec- tomy under general anesthesia. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[28]Englot DJ, Birk H, Chang EF. Seizure outcomes in nonresective epilepsy surgery: an update. Neuro- surg Rev. 2017; 40(2):181–194
[29]Pereira EAC, Green AL. Surgical Management of Extratemporal Lobe Epilepsy, Schmidek and Sweet
Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[30]Smitherman S, Guthikonda B, Yoshor D. Surgical treatment of extratemporal epilepsy. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[31]Whisler WW. Multiple subpial transection. In: Starr
PA, Barbaro NM, Larson PS, eds. Functional Neuro- surgery. 2nd ed. Thieme; 2008
[32]Cossu M, Cardinale F, Castana L, Lo Russo G. Multi- lobar Resection and Hemispherectomy in Epilepsy Surgery, Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[33]Schramm J. Functional hemispherectomy. In: Starr
PA, Barbaro NM, Larson PS, eds. Functional Neuro- surgery. 2nd ed. Thieme; 2008
[34]Boongird A, Bingaman WE. Anatomical hemi- spherectomy. In: Starr PA, Barbaro NM, Larson PS, eds. Functional Neurosurgery. 2nd ed. Thieme; 2008
[35]Chang EF, Rowland NC, Barbaro NM. Corpus Callosotomy: Indications and Techniques, Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[36]Guthikonda B, Smitherman S, Yoshor D. Sectioning of the corpus callosum for epilepsy. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
[37]Madsen JR. Treatment of Intractable Epilepsy by Electrical Stimulation of the Vagus Nerve, Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, Philadelphia, PA: Elsevier; 2012
[38]Liu CY, Amar AP, Levy ML, Apuzzo MLJ. Vagus nerve stimulation for intractable epilepsy. In: Starr PA,
Barbaro NM, Larson PS, eds. Functional Neurosur- gery. 2nd ed. Thieme; 2008
314
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

16 Stereotactic Radiosurgery
Rachel Jacobs, Daniel Tonetti, L Dade Lunsford
16.1 Introduction
The use of ionizing radiation in the treatment of malignancy is well established across medical specialties. In the field of neurological surgery, external beam radiation therapy (XRT) has broad application in treating vascular abnormalities and neoplasms. Dr. Lars Leksell developed radiosurgery with the use of a stereotactic frame at the Karolinska Institute in Stockholm, Sweden ( Fig. 16.1). The initial North American Gamma Knife Surgery instrument experience of 207 patients from the University of Pittsburgh was published in 1990, demonstrating that the treatment of brain tumors and arteriovenous malformations (AVMs) was associated with no patient mortality and very little morbidity within the first 6 months after surgery.1 Furthermore, the average length of hospital stay and hospital charges were both significantly lower for Gamma Knife than the average length of hospital stay and hospital charges for traditional operative measures (craniotomy).1 Throughout this chapter, the
Fig. 16.1 Lars Leksell at the console surrounded by colleagues with the prototype noninvasive Leksell’s Gamma Knife. (Reproduced from Lunsford L, Sheehan J, Intracranial Stereotactic Radiosurgery, 2nd edition, ©2015, Thieme Publishers, New York.)
achievements associated with the clinical implementation of stereotactic radiosurgery (SRS) will be described.
16.2 Radiation Background
Fractioned radiotherapy refers to the daily administration of small radiation doses to a large treatment target. This process of fractioning the total dose increases the killing of tumor cells and lessens damage to healthy tissues by allowing time for repair of damage to DNA.2 SRS, by contrast, is the delivery of a single or limited number of doses to a small, precisely defined treatment target via an array of nonparallel radiation beams.2,3 Damage via photons in electromagnetic waves is initiated via the Compton effect in which the initial pho- ton–atom interaction discharges an electron, which then ionizes other atoms and breaks chemical bonds.4
The two most critical clinical considerations in the use of radiosurgery are a sharp dose gradient and accurate target positioning.
A margin is included around the target lesion to account for positioning uncertainties.
16.3 Types of Radiation
16.3.1 Conventional Radiotherapy
Conventional radiotherapy involves delivering radiation in one or two beams without highly conformal treatment techniques,
315
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

Stereotactic Radiosurgery
unlike SRS.5 This is of particular use in the spine, where most tumors are metastases, and many cases may involve spinal cord impingement or effacement. Conventional radiotherapy is a widely accepted treatment for spinal metastatic disease. The goals of this therapy are effective pain palliation, preservation of neurologic function and ambulation, spinal stability to prevent progression of disease, and improved quality of life.6
16.3.2 Stereotactic Radiosurgery
SRS and its derivative stereotactic radiotherapy (SRT) are the main focuses of this chapter. SRS refers to the delivery of a single large dose of radiation, while radiotherapy generally refers to fractionated radiation therapy, delivering several small doses of radiation across several treatment sessions. While these strict definitions may be of academic interest, the following practical definition of SRS is utilized: “Stereotactic radiosurgery typically is performed in a single session, using a rigidly attached stereotactic guiding device, other immobilization technology, and/or a stereotactic image-guided system, but can be performed in a limited number of sessions, up to a maximum of five.7” Therefore, the
more general term SRS will be used in this review.
16.4 Main SRS Modalities
16.4.1 Gamma Knife Surgery
Gamma Knife radiosurgery (GKRS) contains 192 to 201 individual cobalt-60 radiation sources in a donut ring array in a heavily shielded assembly ( Fig. 16.2).8 A stereotactic frame is surgically fixed to the patient’s skull, allowing for precise positioning within the source array. While each individual source is not biologically active on its own, the intersection of these sources produces an ablative dose of radiation capable of inactivating or damaging the cells within the target. Volumetrically accurate dose calculation tools allow the clinician to control the specific three-dimensional shape of the ablative area while minimizing the dose of radiation delivered to surrounding tissues (sharp dose gradients). The newest Gamma Knife model is the Icon, a model that contains 192 cobalt-60 sources and does not require a frame for treatment ( Fig. 16.3).
Fig. 16.2 Gamma Unit 5, installed at Presbyterian University Hospital, began clinical operation on August 14, 1987. (Reproduced from Lunsford
L, Sheehan J, Intracranial Stereotactic Radiosurgery, 2nd edition, ©2015, Thieme Publishers, New York.)
316
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

16.5 Dosing
Linear Accelerators
The principle underlying a linear accelerator (LINAC) system is similar to that of Gamma Knife; a small volume of tissue receives an ablative dose of radiation as a result of the intersection of multiple X-ray beams.9 While the Gamma Knife uses multiple cobalt-60 sources emitting gamma rays, LINAC generate X-rays and rotates about the patient’s head, delivering high-energy photon beams to a precise location throughout the rotation. Each beam passes through other brain tissues only momentarily. CyberKnife is a mobile LINAC affixed to an image-guided robotic arm. When this device is used, the use of a stereotactic head frame is no longer required. Other versions include the Varian True Beam linear accelerator and the Novalis linear accelerator adapter for various LINACs.
Fig. 16.3 Leksell Gamma Knife® IconTM. (Image is provided courtesy of Elekta, Inc. 2018.)
of GKRS and LINAC systems. Specifically, the dose slowly increases followed by a rapid dose increase to the Bragg peak, beyond which the dose falls to zero.10 While the energy is delivered by heavy charged particles, the ability to induce ionization is similar to that of the electromagnetic waves employed in other SRS modalities.
16.5 Dosing
Biologically effective dose (BED) is a measure of the true biological effect of any radiotherapy treatment,11 and is often modeled by the following equation:
BED (Gy) = n × d × [1 + |
d |
] |
α /β |
Where n = the number of doses, d = dose per fraction, and the factors α and β describe the cell response to radiation.4
Proton Beam SRS
Proton beam SRS uses a magnet to accelerate a proton through a magnetic field, targeting the beam to a specific region of interest. The dose distribution affected by proton beam therapy is different from that
16.5.1 Tolerance
Based on early Radiation Therapy Oncology Group (RTOG) guidelines, 24 Gy is recommended for tumors less than or equal to 2 cm, 18 Gy for tumors 2.1 to 3 cm, and 15 Gy for tumors greater than 3 cm in size.12
317
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.

Stereotactic Radiosurgery
Subsequent experience has found that such doses are not necessary for malignant tumor control, and much smaller doses are used for benign tumors. For the brainstem, typical practice is to limit the lateral brainstem to no more than 12 Gy.13 For the optic nerves and chiasm, most studies suggest that the maximum point dose should be 8 Gy in a single fraction.14 Most of the data determining tolerance to the cochlea arises from the vestibular schwannoma literature, and average cochlear doses of 3.5 to 5 Gy can be utilized to improve hearing preservation rates.15 At our institution, we use 4.2 Gy. Regarding the spinal cord, Sahgal et al documented radiation-induced myelopathy following SRS and found that no more than 12.4 Gy is recommended in a single fraction.16
16.5.2 Dose Limitations
Special sensory nerves, such as the optic and vestibulocochlear nerves, are the most radiosensitive. This is in contrast to motor nerves including those of the parasellar region and the lower cranial nerves, which are able to tolerate higher doses, though the exact dosage tolerance of cranial nerves is uncertain.4 The brainstem is also considered radiosensitive because of the critical neural structures and pathways. It is particularly susceptible to edema from SRS treatment in conjunction with its clinical importance. In general, the most critical radiation-sensitive regions include the optic nerve, optic chiasm, the brainstem, pituitary gland, and the cochlea.
16.5.3 Compressive Tumors of the Spinal Cord, Brainstem, or Optic Structures
Surgical removal should be emphasized in these situations, especially for benign lesions
318
in a younger population or those lesions symptomatic due to mass effect, as substantial risk of neurologic injury may result from edema following SRS delivered within a few millimeters of the margins of the isocenter in these regions.4 Furthermore, the latency interval after SRS may allow for some interval growth before tumor response.
16.6 Occupational Exposure
The U.S. Nuclear Regulatory Commission advises taking every reasonable effort to keep the radiation dose as far below the limits as possible, as is consistent with the purpose of the licensed activity, with recommendations to keep exposure less than or equal to 2 rem (roentgen equivalent man)/y averaged over 5 years.4 Steps to reduce occupational radiation dose during surgery include increasing the distance from the radiation source, shielding, and keeping “boost” mode usage, which can double radiation output, to a minimum. In general, medical personnel are monitored and are not physically present during radiation delivery.
16.7 Adverse Reactions
The mechanism by which XRT causes side effects is not confirmed, however, the etiology may include immune system effects, damage to vascular endothelium and resultant breakdown of the blood–brain barrier (BBB), and glial injury.
16.7.1 Radiation Vasculopathy
Vascular endothelium and oligodendroglial cells are the most susceptible to radiation necrosis, as radiation is selectively toxic to more rapidly dividing cells.4 Radiation
Agarwal, Neurosurgery Fundamentals (ISBN 978-1-62623-822-0), copyright © 2019 Thieme Medical Publishers. All rights reserved. Usage subject to terms and conditions of license.
