
Применение углеродных нанотрубок в биомедицине 1 / Mattson, M. P., Haddon, R. C., & Rao, A. M. (2000). Molecular Functionalization of Carbon Nanotubes and Use as Substrates for Neuronal Growth. Journal of Molecular Neuroscience, 14(3), 175–182. doi10.1385jmn143175
.pdfJournal of Molecular Neuroscience
Copyright © 2000 Humana Press Inc.
All rights of any nature whatsoever reserved.
ISSN0895-8696/00/14:175–182/$12.00
Molecular Functionalization of Carbon Nanotubes and Use as Substrates for Neuronal Growth
Mark P. Mattson,*,1,2 Robert C. Haddon,3 and Apparao M. Rao 4
1Sanders-Brown Research Center on Aging and Department of Anatomy and Neurobiology, University of Kentucky, Lexington, KY 40536; *,2Laboratory of Neurosciences, National Institute on Aging, 5600 Nathan Shock Boulevard, Baltimore, MD 21224; 3Department of Chemistry and Physics, University of Kentucky, Lexington, KY 40506; 4Center for Applied Energy Research and Department of Physics and Astronomy, University of Kentucky, Lexington, KY 40511
Received December 17, 1999; Accepted March 8, 2000
Abstract
Carbon nanotubes are strong, flexible, conduct electrical current, and can be functionalized with different molecules, properties that may be useful in basic and applied neuroscience research. We report the first application of carbon nanotube technology to neuroscience research. Methods were developed for growing embryonic rat-brain neurons on multiwalled carbon nanotubes. On unmodified nanotubes, neurons extend only one or two neurites, which exhibit very few branches. In contrast, neurons grown on nanotubes coated with the bioactive molecule 4-hydroxynonenal elaborate multiple neurites, which exhibit extensive branching. These findings establish the feasability of using nanotubes as substrates for nerve cell growth and as probes of neuronal function at the nanometer scale.
Index Entries: Brain; growth cones, hippocampus; hydroxynonenal; nanotechnology.
Introduction
Carbon nanotubes are strong, flexible, conduct electrical current (Rao et al., 1997; Wong et al., 1997; Tans et al., 1998), and can be functionalized with different molecules (Chen et al., 1998; Wong et al., 1998), properties that may be useful in basic and applied neuroscience research. The mammalian
nervous system is the most complex cellular communication network known, containing over 1011 nerve cells (neurons), each of which has an elaborate morphology extending neurites (axons and dendrites) over long distances. The growth of neurites and the formation of synapses during development and regeneration is controlled by a highly motile structural specialization at the tip of the neu-
*Author to whom all correspondence and reprint requests should be addressed. E-mail: mattsonm@grc.nia.nih.gov
Journal of Molecular Neuroscience |
175 |
Volume 14, 2000 |
176
rite called the growth cone (Goodman, 1996; Kater et al., 1998). One commonly used approach to studying mechanisms that regulate neurite outgrowth employs cultures of dissociated neurons from brains or spinal cords of embryonic rodents. In such cultures the neurons are seeded into dishes in which the culture surface has been coated with uniform layer of adhesive molecules that promotes neurite outgrowth. Although studies of such cultures have led to the identification of a number of molecules that promote or inhibit neurite outgrowth (e.g., neurotrophic factors and neurotransmitters) (Mattson, 1988; Song and Poo, 1999), a large gap in our understanding of the regulation of neurite outgrowth exists because of the lack of methods for manipulating the growth environment at the nanometer scale.
Methods
Production, Dispersion, and Modification
of Multi-Walled Carbon Nanotubes
Multiwalled nanotubes were synthesized via the catalytic decomposition of a ferrocene-xylene mixture as described previously (Andrews et al., 1999). Nanotubes were dispersed by bath sonication for 5 min in 100% ethanol. The dispersed nanotubes were applied to 22 mm2 glass coverslips that had been coated with a thin layer of polyethyleneimine. The ethanol was allowed to evaporate under ambient conditions, resulting in firm adherance of the nanotubes to the coverslips. The coverslips were then placed in 35-mm diameter plastic culture dishes, sterilized by a 5-min exposure to UV light, and culture medium was added to the dishes. The next day dissociated embryonic rat hippocampal neurons were seeded into the cultures. Adhesion of 4-hydroxynonenal (4-HNE) to nanotubes was accomplished by first dispersing the nanotubes in 100% ethanol, and then incubating the dispersed nanotubes in an acidic solution, pH 5.0, of 50% ethanol (4 mg nano-tubes/mL) containing 200 M 4-HNE (Cayman Chemical Co.). The mixture was then sonicated for 20 min in a bath sonicator, and nanotubes were applied to polyethyleneiminecoated glass coverslips. The coverslips were then washed extensively with phosphate-buffered saline (PBS), sterilized by exposure to UV light for 5 min, and placed in culture dishes containing medium.
Mattson, Haddon, and Rao
Methods for Primary Neuronal
Culture and Microscopy
Primary hippocampal cell cultures were established from E18 rat embryos using methods described previously (Mattson et al., 1988). Dissociated cells were seeded onto polyethyleneiminecoated 22 mm2 glass coverslips, and incubated in Neurobasal medium containing B-27 supplements (Gibco-BRL) plus 2 mM L-glutamine, 1 mM HEPES, and 0.001% gentamicin sulfate (Sigma). Methods for confocal laser-scanning microscope analysis of immunostained nanotubes were similar to those described previously (Mattson and Partin, 1999). Briefly, cells were visualized under visible light and then scanned with the laser (488 nm excitation and 510 nm emission). Transmitted light images were acquired by scanning the sample with the laser turned off. For SEM analysis cells were fixed in a solution of 2% glutaraldehyde in PBS, dehydrated in solutions of increasing ethanol concentration, and critical-point dried. Samples were then coated with a thin layer of gold particles and examined and photographed using a S900FE Hitachi SEM.
Immunochemistry
Nanotubes on coverslips were incubated for 10 min in PBS containing 4% normal horse serum, followed by a 3-h incubation in PBS containing 4% horse serum plus a 1 100 dilution of mouse monoclonal antibody (MAb) against 4-HNE (Mattson and Kater, 1988; Mattson et al., 1997). For confocal microscopy, samples were then incubated for 1 h in PBS containing a 1 200 dilution of biotinylated horse antimouse IgG, followed by a 30-min incubation in PBS containing 4 g/mL fluoresceinavidin conjugate (Vector Laboratories). For SEM analysis samples were incubated for 2 h in a secondary (sheep–antimouse) antibody conjugated to 5 nm gold particles.
Results and Discussion
Technologies have recently been developed that allow large-scale production of highly purified carbon nanotubes under conditions in which tube diameters and lengths, and tube composition (e.g., single-walled and multiwalled) can be reliably controlled (Thess et al., 1996; Journet et al., 1997; Ren et al., 1998; Fan et al., 1999). Our studies employed
Journal of Molecular Neuroscience |
Volume 14, 2000 |
Nanotubes and Neurite Growth |
177 |
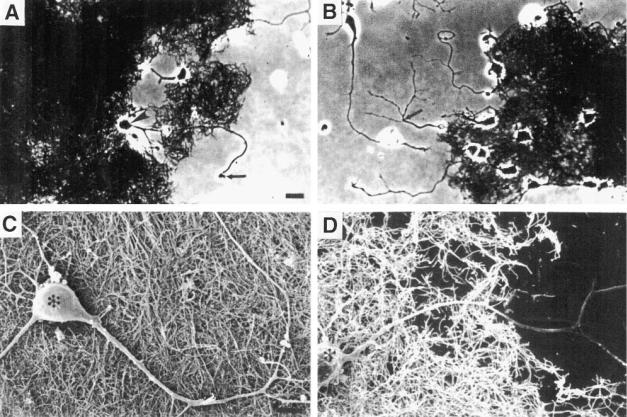
Fig. 1. Multiwalled carbon nanotubes support neuronal survival and provide a permissive substrate for neurite outgrowth. Phase-contrast (A) and (B) and scanning electron (C) and (D) micrographs showing embryonic hippocampal neurons growing on dispersed nanotubes and adjacent culture substrate. Axons extend from neuronal cell bodies across nanotubes and onto the polyethyleneimine-coated glass surface. Arrows point to a growth cone (panel A) and a branchpoint of an axon (panel B). Asterisks mark neuronal cell bodies (panels C and D). Scale bars: (A) and (B), 10 m; (C) and (D), 5 m.
multi-walled nanotubes with diameters of approx 20 nm and lengths of 20–100 m, which are produced as sheets in which the nanotubes are arranged in parallel arrays using a method based on a controlled decomposition of a ferrocene– xylene mixture using a two-stage quartz reactor (Andrews et al., 1999). We found that nanotubes in such sheets can be dispersed to varying extents by suspension in ethanol followed by sonication. We applied the dispersed nanotubes to glass coverslips that had been coated with polyethyleneimine, a substrate for neuronal growth, and then seeded dissociated embryonic rat hippocampal neurons onto the coverslips in culture dishes. Cultures were examined and photographed using a light micro-
scope with phase-contrast optics, and some of the cultures were prepared for examination with a scanning electron microscope (SEM). Examples of light and SEM micrographs of neurons growth for 3 d on nanotubes are shown in Fig. 1.
Neurons attached to the nanotubes and extended one or two neurites, which grew to distances of approximately 70–90 m within 3 d (Fig. 1). Neurons on nanotubes survived and continued to grow through at least 8 d in culture indicating that the nanotubes support long-term cell survival. SEM examination of neurons growing on nanotubes revealed that, although the nanotubes permitted neurite outgrowth, they did not appear to influence the direction of growth (Fig. 1). Thus, neurites
Journal of Molecular Neuroscience |
Volume 14, 2000 |
178 |
Mattson, Haddon, and Rao |
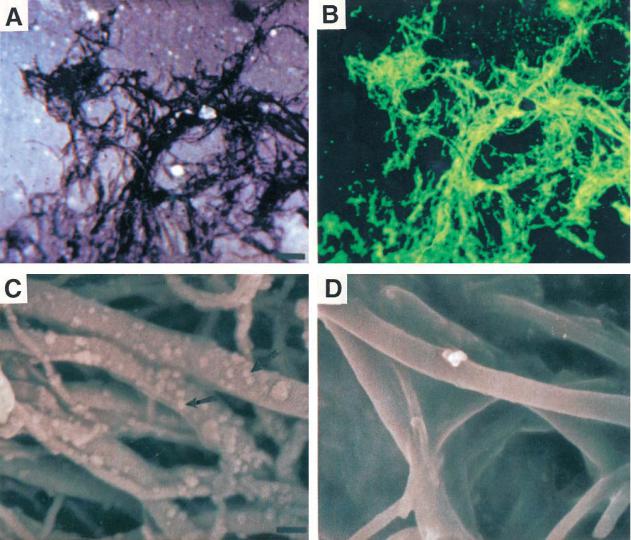
Fig. 2. Multiwalled nanotubes can be coated with 4-hydroxynonenal (4-HNE). (A) and (B) Confocal laser scanning microscope images of nanotubes that had been reacted with 4-HNE and then processed for immunofluores- cence-based detection of 4-HNE. Panel (A) is a transmitted light micrograph showing the distribution of the nanotubes on the glass culture substrate. Panel (B) is a fluorescence image of the same microscope field showing localization of 4-HNE (green). (C) SEM micrograph showing nanotubes reacted with 4-HNE and then processed for immuno-gold based detection of 4-HNE. The arrows point to gold particles that decorate sites of binding of the 4-HNE antibody. (D) Nanotubes reacted with a solution containing 4-HNE that had been preadsorbed with a 100-fold molar excess of the amino acid histidine and then processed for immuno-gold based detection of 4-HNE. Note that essentially no gold particles are associated with the nanotubes. Scale bars: (A) and (B), 10 m; (C) and
(D) 20 nm.
grew straight across the surfaces of nanotubes arranged in various orientations with respect to the direction of neurite outgrowth. In cases where neurites grew across nanotubes and then onto the
polyethyleneimine-coated glass coverslips, the neurites typically did not form branches on the nanotubes, but did form branches on the coverslips (Fig. 1B,D), suggesting that unmodified nanotubes
Journal of Molecular Neuroscience |
Volume 14, 2000 |
Nanotubes and Neurite Growth |
179 |
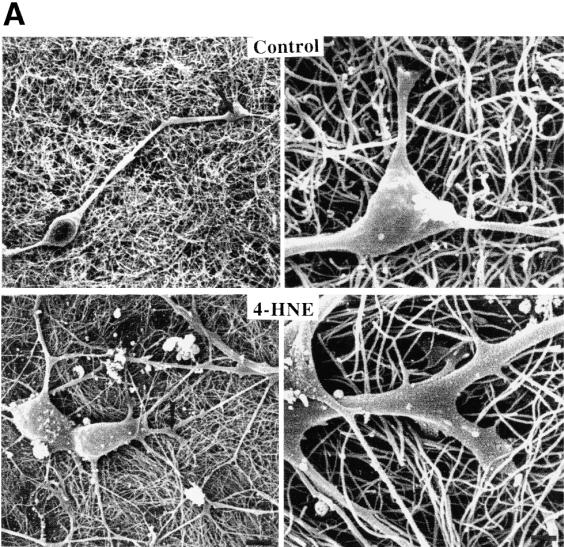
Fig. 3A. Neurite outgrowth and branching is enhanced in neurons growing on nanotubes coated with 4-HNE.
(A) SEM micrographs showing neurons that had grown for 3 d on unmodified control nanotubes (upper) and nanotubes coated with 4-HNE (lower). The right panel is a high magnification view of the region of the neurite designated by the arrow in the left panel. Scale bars: left panels, 5 m; right panels, 100 nm.
do not provide a substrate that is conducive for branch formation.
Recent studies have demonstrated that nanotubes can be modified with a variety of molecules including carboxyl and amine groups (Chen et al., 1998; Wong et al., 1998; Hamon et al., 1999). In order to determine whether molecules that effect neurite growth can be coupled to nanotubes, and to study the influence of such modified nanotubes on neu-
rite outgrowth, we established methods for coupling the aldehyde 4-hydroxynonenal (4-HNE) to nanotubes. 4-HNE is a lipid peroxidation product that covalently modifies proteins on cystine, histidine, and lysine residues (Esterbauer et al., 1991; Uchida and Stadtman, 1992). Previous studies had shown that 4-HNE can induce increases of intracellular Ca2+ levels (Mark et al., 1997) and modify cytoskeletal proteins (Mattson et al., 1997), signal-
Journal of Molecular Neuroscience |
Volume 14, 2000 |
180
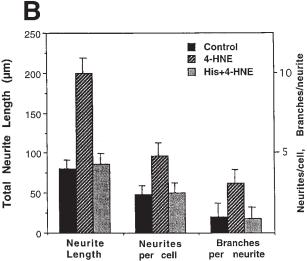
Fig. 3B. Neurons were grown for 3 d on unmodified nanotubes, nanotubes reacted with 4-HNE, or nanotubes that had been preadsorbed with a 100-fold molar excess of the amino acid histidine. The indicated parameters of neurite outgrowth were then quantified. Values are the mean and SD of measurements made in eight separate cultures (10–15 neurons assessed/culture). For each outgrowth parameter the value for neurons grown on nanotubes coated with 4-HNE was significantly greater than the value for each of the other two conditions ( p < 0.001 in each case; ANOVA with Scheffe’s post-hoc tests).
ing mechanisms that regulate neurite outgrowth in many types of neurons including cultured embryonic hippocampal neurons (Mattson and Kater, 1987; Waeg et al., 1996). Coupling of 4-HNE to nanotubes was accomplished by sonicating the nanotubes in an acidic solution containing 200 M 4-HNE. The nanotubes were then applied to glass coverslips and were either prepared for immunochemical analyses or cell culture. In order to determine whether 4-HNE was bound to the nanotubes, an antibody against 4-HNE (Waeg et al., 1996; Mattson et al., 1997) was employed. Examination of the nanotubes using confocal laser-scanning microscopy and SEM showed that 4-HNE antibody bound strongly to the nanotubes that had been reacted with 4-HNE, but not to untreated nanotubes or to nanotubes reacted with 4-HNE that had been preincubated with a 100-fold molar excess of histidine (Fig. 2). 4-HNE was present along the length of the nanotubes. These findings demonstrate a
Mattson, Haddon, and Rao
physical association of 4-HNE with the nanotubes under the conditions employed.
We next cultured embryonic hippocampal neurons on nanotubes coated with 4-HNE, and on control nanotubes that were either untreated or reacted with 4-HNE that had been preadsorbed with excess histidine. SEM examination suggested that neurons grown on nanotubes modified with 4-HNE had more elaborate neuritic arbors than did neurons grown on unmodified nanotubes (Fig. 3A). We therefore quantified numbers of neurites/neuron, total neurite length/neuron, and numbers of branches/ neurite in neurons grown on nanotubes coated with 4-HNE, and neurons grown on control nanotubes (Fig. 3B). Whereas neurons grown on control nanotubes possessed only one or two neurites, those grown on 4-HNE-modified nanotubes elaborated 4–6 neurites. Total neurite length was increased more than twofold, and number of branches/neurite were increased approximately threefold, in neurons grown on 4-HNE-modified nanotubes. These findings demonstrate a striking effect of nanotubebound 4-HNE on neurite outgrowth, and establish the feasibility of using chemically modified carbon nanotubes as a tool for studying and manipulating neurite outgrowth.
Our findings show, for the first time, that neurons can attach to and grow across the surfaces of carbon nanotubes. Previous studies have demonstrated that substrate “adhesiveness” plays an important role in the process of growth cone motility and neurite branching (Lustgarten et al., 1991). We found that surfaces of unmodified nanotubes were permissive for neurite outgrowth, but did not promote neurite branching, suggesting that adhesion of growth cones to the carbon surface was relatively weak. However, when nanotubes were coated with 4-HNE neurite branching and total neurite outgrowth were greatly enhanced. These findings suggest the possibility that 4-HNE enhances adhesion of growth cones to the nanotubes. Previous studies have shown that Ca2+ influx can regulate growth cone motility and neurite elongation (Kater et al., 1988), and that 4-HNE can modulate intracellular Ca2+ levels in cultured hippocampal neurons (Mark et al., 1997), as well as in nonneuronal cells (Carini et al., 1996). It is therefore possible that that the neurite outgrowth-enhancing effect of 4-HNE involves changes in intracellular Ca2+ levels, although this remains to be established.
Journal of Molecular Neuroscience |
Volume 14, 2000 |
Nanotubes and Neurite Growth
The convergence of two previously distinct fields, nanotube technology and neurobiology, paves the way for future studies in which nanotubes are used to study the formation and function of nerve cell networks, and possibly to restore function of damaged neuronal circuits. Nanotubes of defined diameters ranging from 1 nm (singlewalled nanotubes) to 10–100 nm (multiwalled nanotubes) can now be routinely produced. These diameters are similar to those of small nerve fibers, growth cone filopodia, and synaptic contacts. Functionalization of such nanotubes with one or more specific bioactive molecules will thus allow a dissection of the molecular control of neuronal architecture at focal microdomains similar to those that occur in vivo. This contrasts with current culture methods in which neurons are grown on a uniform flat substrate that bears little resemblance to the cell surfaces and extracellular matrix structures that neurons encounter in vivo. Because nanotubes are excellent conductors, they will also prove valuable for electrophysiological analyses of neuronal microcircuitry. Moreover, the ability to functionalize nanotubes with molecules that promote neurite outgrowth suggests clinical applications of this technology, in light of the limited success of attempts to promote the regeneration of nerves in the spinal cord and brain by the use of crude synthetic “bridges” (Lustgarten et al., 1991). In contrast to the artificial growth substrates employed thus far, nanotubes have diameters similar to those of neurites and may therefore allow for the kinds of localized molecular interactions that may be required for formation of functional neuronal circuits.
Acknowledgments
We thank D. Jacques and R. Gonzalez for providing the multiwalled nanotube samples and for help with electron microscopy. Supported by the National Institute on Aging and the National Science Foundation.
References
Andrews R., Jacques D., Rao A. M., Derbyshire F., Qian D., Fan X., et al. (1999) Continuous production of aligned carbon nanotubes: a step closer to commercial realization. Chem. Phys. Lett. 303, 467.
Carini R., Bellomo G., Paradisi L., Dianzani M. U., and Albano E. (1996) 4-Hydroxynonenal triggers
181
Ca2+ influx in isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 18, 772–776.
Chen J., Hamon M. A., Hu H., Chen Y., Rao A. M., Eklund P. C., and Haddon R. C. (1998) Solution properties of single-walled carbon nanotubes.
Science 282, 95–98.
Esterbauer H., Schaur R. J., and Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad. Biol. Med. 11, 81–128.
Fan S., Chapline M. G., Franklin N. R., Tombler T. W., Cassell A. M., and Dai H. (1999) Self-oriented regular arrays of carbon nanotubes and their field emission properties. Science 283, 512–514.
Goodman C. S. (1996) Mechanisms and molecules that control growth cone guidance. Annu. Rev. Neurosci. 19, 341–377.
Hamon M. A., Chen J., Hu H., Chen Y., Rao A. M., Eklund P. C., and Haddon R. C. (1999) Dissolution of single-walled carbon nanotubes. Adv. Mater. 11, 834–840.
Journet C., Maser W. K., Bernier P., Loiseau A., Lamy de la Chapelle M., Lefrant S., et al. (1997) Large scale production of single wall carbon nanotubes by the electric arc technique. Nature 388, 756–758.
Kater S. B., Mattson M. P., Cohan C., and Connor J. (1988) Calcium regulation of the neuronal growth cone. Trends Neurosci. 11, 315–321.
Lustgarten J. H., Proctor M., Haroun R. I., Avellino A. M., Pindzola A. A., and Kliot M. (1991) Semipermeable polymer tubes provide a microenvironment for in vivo analysis of dorsal root regeneration. J. Biomech. Eng. 113, 184–188.
Mark R. J., Lovell M. A., Markesbery W. R., Uchida K., and Mattson M. P. (1997) A role for 4-hydroxy- nonenal in disruption of ion homeostasis and neuronal death induced by amyloid `-peptide.
J. Neurochem. 68, 255–264.
Mattson M. P. (1988) Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. Rev. 13, 179–212.
Mattson M. P. and Kater S. B. (1987) Calcium regulation of neurite elongation and growth cone motility. J. Neurosci. 7, 4034–4043.
Mattson M. P. and Kater S. B. (1988) Intracellular messengers in the generation and degeneration of hippo-campal neuroarchitecture. J. Neurosci. Res. 21, 447–464.
Mattson M. P., Dou P., and Kater S. B. (1988) Out- growth-regulating actions of glutamate in isolated hippo-campal pyramidal neurons. J. Neurosci. 8, 2087–2100.
Journal of Molecular Neuroscience |
Volume 14, 2000 |
182
Mattson M. P., Fu W., Waeg G., and Uchida K. (1997) 4-hydroxynonenal, a product of lipid peroxidation, inhibits dephosphorylation of the microtubuleassociated protein tau. NeuroReport 8, 2275–2281.
Mattson M. P. and Partin J. (1999) Evidence for mitochondrial control of neuronal polarity. J. Neurosci. Res. 56, 8–20.
Rao A. M., Richter E., Bandow S., Chase B., Eklund P. C., Williams K. A., et al. (1997) Diameter-selec- tive Raman scattering from vibrational modes in carbon nanotubes. Science 275, 187–191.
Ren Z. F., Huang Z. P., Xu J. W., Wang J. H., Bush P., Siegal M. P., and Provencio P. N. (1998) Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 282, 1105–1107.
Song H. J. and Poo M. M. (1999) Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 9, 355–363.
Suter D. M. and Forscher P. (1998) An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr. Opin. Neurobiol. 8, 106–116.
Mattson, Haddon, and Rao
Tans S. J., Vershueren A. R. M., and Dekker C. (1998) Room temperature transistor based on a single carbon nanotube. Nature 393, 49–52.
Thess A., Lee R., Nikolaev P., Dai H., Petit P., Robert J., et al. (1996) Crystalline ropes of metallic carbon nanotubes. Science 273, 483–487.
Uchida K. and Stadtman E. R. (1992) Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc. Natl. Acad. Sci. USA 89,
4544–4548.
Waeg G., Dimsity G., and Esterbauer H. (1996) Monoclonal antibodies for detection of 4-hydroxy- nonenal modified proteins. Free Rad. Res. 25, 149–159.
Wong E. W., Sheehan P. E., and Lieber C. M. (1997) Nanobeam mechanics: elasticity, strength, and toughness of nanorods and nanotubes. Science 277, 1971–1975.
Wong S. S., Joselevich E., Woolley A. T., Caung C. L., and Lieber C. M. (1998) Covalently functionalized nanotubes as nanometre-sized probes in chemistry and biology. Nature 394, 52–55.
Journal of Molecular Neuroscience |
Volume 14, 2000 |
