
Биоинженерия / ТИ_ССС / PIIS0085253815507424
.pdf
Kidney International, Vol. 67 (2005), pp. 2488–2493
TECHNICAL NOTE
Antibodies against macrophages that overlap in specificity with fibroblasts
TSUTOMU INOUE, DAVID PLIETH, CHRISTO D. VENKOV, CAROL XU, and ERIC G. NEILSON
Department of Medicine and Department of Cell and Developmental Biology, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, Tennessee
Antibodies against macrophages that overlap in specificity with fibroblasts.
Background. Fibroblasts can be misidentified as macrophages because both cell types share antigens that are associated with popular antibodies targeting the monocyte/ macrophage lineage. With the recent description of fibroblastspecific protein 1 (FSP1), we revisited the specificity of antibodies directed against macrophages to determine systematically which antibodies best distinguish both cell types in fi- brotic tissues.
Methods. Tissue fibrosis was produced in mice carrying the GFP transgene encoding green fluorescent protein under the control of the FSP1 promoter. Single cell suspensions from these marked tissues were submitted for flow cytometry using antibodies against Mac-1, Mac-2, Mac-3, F4/80, CD68, major histocompatibility complex (MHC) class II, and CD45, and cDNA amplification of mRNA encoding the above target antigens was performed using specific primer sets in sorted pools of cells. Fibrotic tissues were also stained by immunohistochemistry with the same antibodies and examined under confocal microscopy.
Results. Comparison overlap between FSP1+ fibroblasts with each of the macrophage markers demonstrated that all antimacrophage antibodies (Mac-1, Mac-2, Mac-3, CD68, MHC class II, and CD45) except one (F4/80) recognize both cell types.
Conclusion. Antibodies directed against F4/80 clearly distinguish macrophages from FSP1+ fibroblasts in fibrotic tissues and is the preferred antibody in mice.
Macrophages and fibroblasts frequently appear together in fibrotic tissue following inflammation. A number of antibodies are available to identify monocytes or macrophages, although their specificity for fibroblasts has not been studied systematically. Until the characterization of fibroblast-specific protein 1 (FSP1) a few years ago [1], fibroblasts were bereft of specific markers. FSP1, also known as S100A4, is only expressed in fibroblasts [1–
Key words: macrophage, fibroblast, FSP1, S100A4, F4/80, Mac-1, Mac- 2, Mac-3, CD68, CD45.
Received for publication October 15, 2004 and in revised form December 11, 2004 Accepted for publication December 20, 2004
C 2005 by the International Society of Nephrology
3], in epithelia undergoing epithelial-mesenchymal transition (EMT) to form fibroblasts [3, 4] or in metastatic tumor cells [5]. Although FSP1+ fibroblasts in fibrotic tissue typically express vimentin and HSP47, or occasionally a - smooth muscle actin (a -SMA) [6], none of these proteins are specific for fibroblasts. Primary mesenchymal cells also do not express FSP1, since the gene encoding FSP1 is only active after embryonic day (E) 8.5 in the mouse [1]. FSP1 is expressed during murine development only in fibroblasts derived from secondary epithelium following EMT [3], or briefly in very early granulocytic lineages from the bone marrow that disappears quickly with maturation (unpublished observations). About 12% of tissue fibroblasts come from the blood stream after they are released by the bone marrow [4]. FSP1+ fibroblasts from bone marrow and peripheral blood are Mac-1+ and Gr- 1low−negative in preliminary data using flow cytometry with a lineage panel including Mac-1, Gr-1, B220, CD3, and TER119 (data not shown). The present study was undertaken because of concern that fibroblasts might be regularly misidentified as monocytes or macrophages if care is not taken in choosing a reliable cell-specific marker.
METHODS
Transgenic mice and the fibrosis model of unilateral ureteral obstruction (UUO)
Three-month-old BALB/c FSP1.GFP (green fluorescent protein) transgenic mice and GFP− littermates were used in all experiments. FSP1.GFP transgenic mice express GFP in fibroblasts under the control of the FSP1 promoter [5]. UUOs were created in single kidneys and harvested at 21 days as previously described [7]. Institutional Animal Care and Use Committee at Vanderbilt University approved all animal studies.
Flow cytometry
Three weeks after operation, anesthetized mice were systemically perfused with 50 mL ice-cold phosphate buffered saline (PBS) before the kidneys were removed. Diced tissue specimens were incubated for 30 minutes
2488
Inoue et al: Fibroblast and macrophage identification
|
|
A |
|
|
|
B |
|
C |
|
4 |
|
Whole cell suspension |
|
Interstitial cell gate |
|||
|
|
|
|
|
4 |
|
|
|
scatterSide |
10 |
|
|
|
F4/80 |
10 |
|
countCell |
10 |
|
|
|
10 |
|
|||
|
|
|
|
|
|
Mac |
|
|
|
2 |
|
|
|
|
2 |
Fib |
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
0 |
|
100 |
|
10 |
0 |
500 |
1000 |
|
10 |
102 |
104 |
|
|
|
100 |
|||||
|
|
|
Forward scatter |
|
|
GFP (FSP1) |
|
|
2489
Fibroblast
3.6 ± 1.8
102 104 F4/80
D
Fibroblast |
Cell count |
100
J
Macrophage |
Cell count |
100
Mac-1
|
|
E |
70.2 ± 7.2 |
Cell count |
|
|
|
|
102 |
104 |
100 |
|
|
K |
99.4 ± 0.5 |
Cell count |
|
|
|
|
102 |
104 |
100 |
Mac-2
|
|
F |
70.7 ± 5.2 |
Cell count |
|
|
|
|
102 |
104 |
100 |
|
|
L |
20.1 ± 7.5 |
Cell count |
|
|
|
|
102 |
104 |
100 |
Mac-3
|
|
G |
56.6 ± 4.1 |
Cell count |
|
|
|
|
102 |
104 |
100 |
|
|
M |
99.0 ± 0.9 |
Cell count |
|
|
|
|
102 |
104 |
100 |
CD68
|
|
H |
35.5 ± 7.4 |
Cell count |
|
|
|
|
102 |
104 |
100 |
|
|
N |
94.9 ± 4.7 |
Cell count |
|
|
|
|
102 |
104 |
100 |
MHCII
|
|
I |
66.1 ± 7.3 |
Cell count |
|
|
|
|
102 |
104 |
100 |
|
|
O |
98.8 ± 0.8 |
Cell count |
|
|
|
|
102 |
104 |
100 |
CD45
89.6 ± 6.9
102 104
94.2 ± 3.8
102 104
A
Whole cell suspension
|
4 |
|
|
|
scatterSide |
10 |
|
|
|
10 |
|
|
|
|
|
2 |
|
|
|
|
0 |
|
|
|
|
10 |
0 |
500 |
1000 |
|
|
Forward scatter
B |
|
|
|
FSP1 |
F4/80 |
Mac-1 |
Mac-2 |
CD68 |
MHCII |
CD45 |
GAPDH CollagenI |
100bp ladder |
|
|
|
D |
|
|
|
|
|
|
|
|
|
Interstitial cell gate |
|
|
|
|
|
|
|
|
|
|
||
countCell |
Fibroblasts |
Fibroblasts |
|
|
|
|
|
|
|
|
||
(GFP+) |
(GFP |
+ |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
E |
|
|
|
|
|
|
|
|
100 |
102 |
104 |
|
|
|
|
|
|
|
|
|
|
|
GFP (FSP1) |
Mac-1+/GFP− |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
C
Non-fibroblasts fraction 4 10
Mac-1+/GFP-
-1 |
2 |
|
|
Mac |
10 |
|
|
|
0 |
Mac-1−/GFP- |
|
|
10 |
102 |
104 |
|
100 |
||
|
|
F4/80 |
|
F
Mac-1−/GFP−
G
Whole cell suspension RT (−)

2490 |
Inoue et al: Fibroblast and macrophage identification |
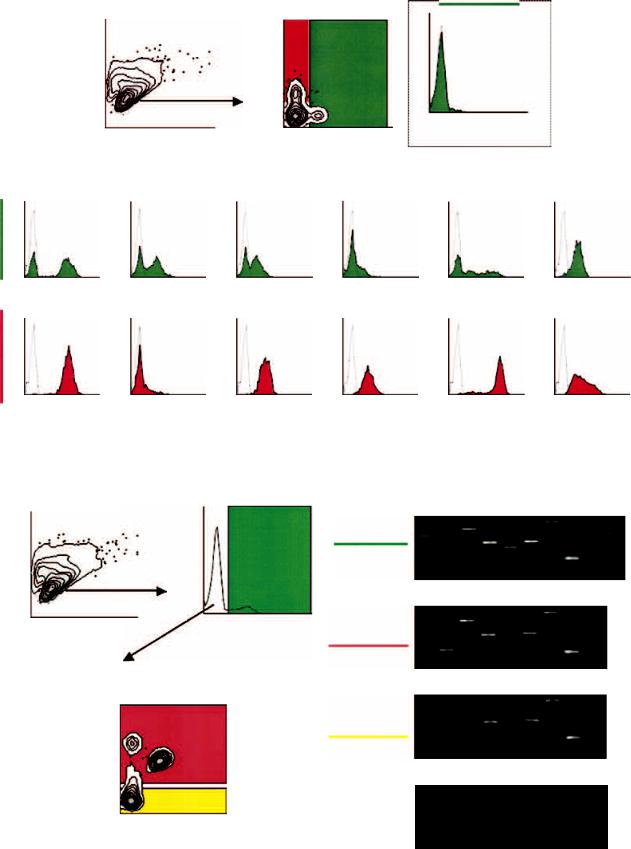
Fig. 1. Flow cytometric analysis of single cell suspensions from fibrotic kidneys. After gating on live cells by forward and side scatter (A), both macrophage and fibroblast populations were separately gated based on F4/80 staining and green fluorescent protein (GFP) expression under the control of the fibroblast-specific protein 1 (FSP1) promoter (B), F4/80+ staining represented 3.6% of the fibroblast population. Mac is macrophagered, Fib is fibroblast-green (C). Each macrophage marker staining was then compared between FSP1+ fibroblasts (D to I) and F4/80-defined macrophages (J to O). Representative histograms are shown with an average and standard deviation from five individual experiments. Broken lines indicate negative controls.
at room temperature with gentle stirring in Dulbecco’s modified Eagle’s medium (DMEM), including 0.1% collagenase (Sigma Chemical Co., St. Louis, MO, USA), 0.001% hyaluronidase (Sigma Chemical Co.), and 0.002% DNase (Sigma Chemical Co.) [3]. Cells were then fixed in PBS containing 4% paraformaldehyde, then incubated with 0.1% Tween-20 for 15 minutes at 37◦C [3, 8]. An Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA, USA) plus antimouse CD16/CD32 (Fc blocker) (BD Bioscience, San Jose, CA, USA) were employed before incubation with all primary antibodies. Cells were stained using optimum concentrations of primary antibody for 20 minutes at 4◦C in PBS containing 1% fetal bovine serum (FBS) and 0.002% saponin. At least, 1 × 104 cells per sample were analyzed in the Vanderbilt Flow Cytometry Core (FACSCalibur) (BD Bioscience). Mac-1 (M1/70), Mac-3 (M3/84), major histocompatibility complex (MHC) class II (M5/114.15.2), and CD45 (30-F11) were obtained from BD Bioscience; F4/80 (Cl:A3-1) and CD68 (FA-11) were obtained from Serotec Inc. (Raleigh, NC, USA); and Mac-2 (M3/38) was from Cedarlane Laboratories (Ontario, Canada). For biotinylated antibodies, streptavidin-dye (BD Bioscience) was employed. Isotype-specific antibodies were used as negative controls.
Tissue immunostaining
Paraffin embedded kidney sections were deparafinized, rehydrated, and then incubated with 0.05% trypsin and 10 lg/mL proteinase K in Hepes buffer saline for 15 minutes at room temperature [3]. Sections were next exposed to Fc blocker (see above) and washed in blocking buffer [PBS with 1.0% bovine serum albumin (BSA)]. Primary antibodies were diluted in blocking buffer and then applied for 1 hour. A tyramide signal amplification kit (Molecular Probes, Eugene, OR, USA) was used to heighten the reaction product. A laser scanning confocal microscope (LSM510-Meta) (Carl Zeiss, Thornwood, NY, USA) in the Vanderbilt Cell Imaging Core was used to capture digital images
for assembly and editing in Photoshop (Adobe Systems, Inc., San Jose, CA, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Nonfixed, nonpermeabilized cells were incubated with antimouse CD16/CD32 Fc blocker, and stained by Mac-1 antibody in PBS containing 1% FBS for 20 minutes at 4◦C. The cells were then sorted into PBS with 1% FBS based on GFP and Mac-1 using the Vanderbilt Flow Cytometry Core (FACSAria) (BD Bioscience). Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA concentrations were adjusted based on cell number. RQ1 RNase-Free DNase (Promega, Madison, WI, USA) and iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) were used for DNA digestion and reverse transcription [3]. All reactions ran on the following cycler schedule: 95◦C 5 minutes, then 95◦C 1 minute, 58◦C 1 minute, 72◦C 1 minute for 25 to 35 cycles (midlinear range) with a finish at 72◦C for 7minutes. Amplicons were separated in 2% agarose gel and stained by ethidium bromide. The following primers were used: FSP1 [3] forward 5 -GTGATTTGGGTCATGCT CAG-3 , reverse 5 -CATTGCACATCATGGCAATG- 3 ; Mac-1 [9] forward 5 -CAGATCAACAATGTGACC GTATGG-3 , reverse 5 -CATCATGTCCTTGTACTG CCGC-3 ; Mac-2 [10] forward 5 -GCCCCGCATGCT GATCACAATC-3 , reverse 5 -GTTGTACTGCAGTA GGTGAGCATCGT-3 ; CD68 [11] forward 5 -CTTCC CACAGGCAGCACAG-3 , reverse 5 -AATGATGAG AGGCAGCAAGAGG-3 ; F4/80 [11] forward 5 -CTT TGGCTATGGGCTTCCAGTC-3 , reverse 5 -GCAAG GAGGACAGAGTTTATCGTG-3 ; MHC class II [12] forward 5 -GGCTCCTCAAGCGACTGTGT-3 , reverse 5 -GGGGCTGGAATCTCAGGTTC-3 ; CD45 [13] forward 5 -GAGACCAGGAAGTCTGTGCTCAGTA-3 , reverse 5 -CGCAGAACCATTGGCAGCATGTTCT- 3 ; procollagen, type I [14] forward 5 -GCATGGCCA AGAAGACATCC-3 , reverse 5 -CCTCGGGTTTCC ACGTCTC-3 ; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [14] forward 5 -GCACAGTCA
Fig. 3. Cell sorting for analysis of mRNA encoding fibroblast-specific protein 1 (FSP1) and macrophage markers. Using the same gates for the flow cytometric analysis shown in Figure 1 (A), cells were sorted into several pools: FSP1+ fibroblasts [green fluorescent protein (GFP+ )] (B), and the remaining GFP− cells into Mac-1+ and Mac-1− groups (C). Data shown in (C) is representative of the previous staining pattern with macrophage antibodies. Three pools of cells were sorted for reverse transcription-polymerase chain reaction (RT-PCR) analysis: FSP1+ fibroblasts (GFP+ ) (D), macrophage-enriched (Mac-1+ /GFP− ) (E), and macrophage/fibroblast-depleted (Mac-1− /GFP− ) (F). mRNA from unsorted cells was used for negative control without reverse transcriptase (RT) (G).
Inoue et al: Fibroblast and macrophage identification |
2491 |
Merge |
Immunostain |
GFP (FSP1) |
F4/80
Mac-2
MHCII
CD45
M
Immunohistochemistry
|
Fibroblast: FSP1+ |
Tubular epithelial cells |
F4/80 |
− |
− |
Mac-2 |
+ |
+ |
Mac-3 |
+/− |
+ |
MHCII |
+ |
+/− |
CD45 |
+ |
− |
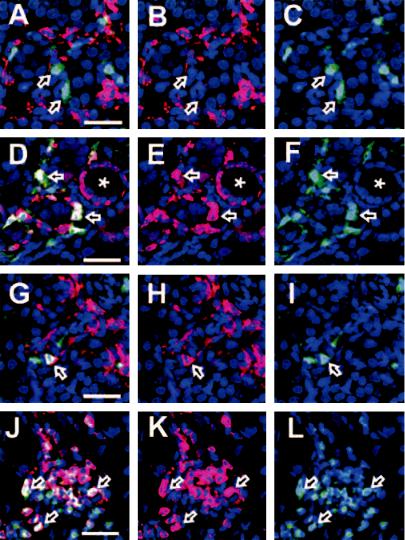
Fig. 2. Immunostaining of fibrotic tissues.
Stainings revealed that F4/80 localized to green fluorescent protein (GFP− ) [fibroblastspecific protein 1 (FSP1− )] cells (A, B, and C, arrows). Mac-2–stained cytoplasm of both GFP+ (FSP1+ ) fibroblasts (D, E, and F, arrows) and GFP− (FSP1− ) cells; also, many tubular epithelial cells were positive (D, E, and F, ). Major histocompatibility complex (MHC) class II staining was in a cell membrane pattern in both GFP+ fibroblasts (G, H, and I, arrows) and GFP− cells. Tubular epithelial staining of MHC class II was similar to interstitial cell staining in our experiments. Many inflammatory cells were stained by CD45, which also costained FSP1+ (GFP+ ) fibroblasts (J, K, and L, arrows). Bar = 25 lm. Results are summarized on the table (M). Results are indicated by + (positive), +/− (weak positive), or − (negative).
AGGCCGAGAAT-3 , reverse 5 -GCCTTCTCCATGG TGGTGAA-3 .
RESULTS AND DISCUSSION
Various studies using antibodies of overlapping specificity have given mixed messages regarding the expression of FSP1 in monocytes, macrophages, lymphocytes, and in some cell lines [1, 15–19]. No systematic com-
parison of antibodies with specificity for macrophages has been made with marked fibroblasts harvested from fibrotic tissues. Here, we used transgenic mice carrying the GFP gene under the control of the FSP1 promoter to properly mark fibroblasts; the high selectivity of FSP1 in fibroblasts has been previously established [1–3, 7]. UUO following surgical ligation produces an acute nephropathy associated with significant infiltration by macrophages and lymphocytes leading to fibrosis
2492 |
Inoue et al: Fibroblast and macrophage identification |
and eventual loss of the kidney [3, 7]. Cells suspensions were prepared from fibrotic kidney tissues or from contralateral control kidneys. Macrophage gates were established using highly specific antisera against F4/80; F4/80 does not stain established fibroblast cell lines in culture [20].
In Figure 1, all antibodies against monocytes/ macrophages, except F4/80, stained both populations of cells. The staining intensities for Mac-1, Mac-3, CD68, CD45, and MHC class II were often higher in monocytes/macrophages than in fibroblasts. The staining with Mac-2 was also cross-reactive but with less intensity in fibroblasts. CD68 also stained only one third of fibroblasts, and those reactions were relatively faint. F4/80 was largely nonoverlapping (<3%) with FSP1+ fibroblasts, which is within the error of normal experimental variation [20].
Immunohistochemistry was employed to ascertain the distribution of cellular staining in fibrotic tissue because tubular epithelial cells are also reported to be Mac-2 and/or Mac-3 positive in normal adult kidney [21]. Moreover, CD45 and MHC class II are expressed by many leukocytes, including macrophages, in inflammatory or pathologic tissues [22]. Representative stainings for a few of the markers are shown in Figure 2. Overall, the results of our immunohistochemistry were consistent with findings from flow cytometry, and Mac-3 showed approximately the same distribution as Mac-2 (data not shown). F4/80 again was largely nonoverlapping (<3%) with FSP1+ fibroblasts. Of special interest was the apparent coexpression of the CD45 common leukocyte antigen in FSP1+ fibroblasts.
Finally, in Figure 3A and B, whole cell suspensions from fibrotic kidneys were sorted by flow cytometry into the following three pools: FSP1+ fibroblasts (GFP+), macrophage-enriched (Mac-1+ and GFP−), or macrophage/fibroblast-depleted (Mac-1− and GFP−). Mac-1 was used as the second marker for cell sorting because Mac-1 is a surface antigen while F4/80 is distributed in both cytoplasm and cell membrane. Each pool was then subjected to cDNA amplification to determine the selective presence of mRNA encoding the various macrophage markers used for flow cytometry in Figure 1 (Fig. 3D to F). Except for mRNA encoding F4/80 being absent in GFP+ (FSP1+) fibroblast pool and mRNA encoding FSP1being absent in the Mac-1+/GFP− and Mac- 1−/GFP− pools, the mRNA encoding our other test set of macrophage markers were present in all pools of cells except control. mRNA encoding CD45 was found in all cell pools, including fibroblasts (Fig. 3D). Although CD45 is expressed by all hematopoietic lineages [23], it is also found in fibrocytes from peripheral blood [24], and in our hands is also expressed by FSP1+ fibroblasts in tissue. Thus, CD45 does not discriminate between macrophages and fibroblasts, either.
This distribution of amplicons confirmed the selectivity of F4/80 as the clearest marker of macrophages. Approximately 70% of the cells in the Mac-1+/GFP− group were F4/80+ (Fig. 3C), confirming that Mac-1 is also present in other nonfibroblast cells besides macrophages [21]. We suspect the remaining 30% is made up of granulocytes and dendritic cells. Furthermore, mRNA transcripts encoding type I collagen were coexpressed with FSP1 in GFP+ fibroblasts. Mac-1− leucocytes, tubular epithelia, mesangial cells, podocytes, or cells from blood vessels in the Mac-1−/GFP− pool also express mRNA encoding nonselective macrophage markers as well as type I collagen, as expected.
F4/80 is well-known as a highly restricted macrophage molecule in mice [20]. Anti-CD68 antibody has been extensively used to characterize monocytes/macrophage populations in humans. CD68 is also known as macrosialin, a murine homologue [25]. Although these two antibodies distinguish macrophages from fibroblasts, F4/80 is a substantially better macrophage marker than CD68 for mice in terms of specificity and intensity of expression. Mac-2 antigen, galectin-3, was originally described as carbohydrate-binding protein 35 (CBP35) in 3T3 fibroblasts [26]. It is also highly expressed in thioglycollate-elicited peritoneal macrophages [27]. Finally, around 70% of FSP1+ fibroblasts are Mac-1+ and unlike the other markers, levels of Mac-1 expression are similar in both fibroblasts and macrophages.
CONCLUSION
We demonstrate the Mac-series of antibodies are overlapping while F4/80 best distinguishes monocytes/macrophages from FSP1+ fibroblasts, and is the preferred linage marker for macrophages.
ACKNOWLEDGMENT
This study was supported by NIH grants DK-46282, HL-68121, CA098131, and CA-68485.
Reprint requests to Eric G. Neilson, M.D., Hugh Jackson Morgan Professor of Medicine and Cell and Developmental Biology Chairman, Department of Medicine, D-3100 MCN, Vanderbilt University School of Medicine, Nashville, TN 37232–2358.
E-mail: eric.neilson@vanderbilt.edu
REFERENCES
1.STRUTZ F, OKADA H, LO CW, et al: Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130:393–405, 1995
2.BHOWMICK NA, CHYTIL A, PLIETH D, et al: TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303:848–851, 2004
3.IWANO M, PLIETH D, DANOFF TM, et al: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110:341– 350, 2002
4.KALLURI R, NEILSON EG: Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784, 2003
5.XUE C, PLIETH D, VENKOV C, et al: The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res 63:3386–3394, 2003
Inoue et al: Fibroblast and macrophage identification |
2493 |
6.OKADA H, BAN S, NAGAO S, et al: Progressive renal fibrosis in murine polycystic kidney disease: An immunohistochemical observation. Kidney Int 58:587–597, 2000
7.IWANO M, FISCHER A, OKADA H, et al: Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther 3:149–159, 2001
8.YANG YH, HUTCHINSON P, LITTLEJOHN GO, BOYCE N: Flow cyto-
metric detection of anti-neutrophil cytoplasmic autoantibodies. J Immunol Methods 172:77–84, 1994
9.LIM LH, FLOWER RJ, PERRETTI M, DAS AM: Glucocorticoid receptor activation reduces CD11b and CD49d levels on murine eosinophils: Characterization and functional relevance. Am J Respir Cell Mol Biol 22:693–701, 2000
10.RONG JX, SHAPIRO M, TROGAN E, FISHER EA: Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA 100:13531–13536, 2003
11.WEISBERG SP, MCCANN D, DESAI M, et al: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796– 1808, 2003
12.TSUYUKI Y, FUJIMAKI H, HIKAWA N, et al: IFN-gamma induces coordinate expression of MHC class I-mediated antigen presentation machinery molecules in adult mouse Schwann cells. Neuroreport 9:2071–2075, 1998
13.HOWELL JC, LEE WH, MORRISON P, et al: Pluripotent stem cells identified in multiple murine tissues. Ann N Y Acad Sci 996:158– 173, 2003
14.ZUR NIEDEN NI, KEMPKA G, AHR HJ: In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 71:18–27, 2003
15.GRIGORIAN M, TULCHINSKY E, BURRONE O, et al: Modulation of
mts1 expression in mouse and human normal and tumor cells. Electrophoresis 15:463–468, 1994
16.KLINGELHOFER J, AMBARTSUMIAN NS, LUKANIDIN EM: Expression
of the metastasis-associated mts1 gene during mouse development. Dev Dyn 210:87–95, 1997
17.TAKENAGA K, NAKAMURA Y, SAKIYAMA S: Cellular localization of
pEL98 protein, an S100-related calcium binding protein, in fibroblasts and its tissue distribution analyzed by monoclonal antibodies.
Cell Struct Funct 19:133–141, 1994
18.TAKENAGA K, NAKAMURA Y, SAKIYAMA S: Expression of a calcium binding protein pEL98 (mts1) during differentiation of human promyelocytic leukemia HL-60 cells. Biochem Biophys Res Commun 202:94–101, 1994
19.TAYLOR S, HERRINGTON S, PRIME W, et al: S100A4 (p9Ka) protein in colon carcinoma and liver metastases: Association with carcinoma cells and T-lymphocytes. Br J Cancer 86:409–416, 2002
20.AUSTYN JM, GORDON S: F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11:805– 815, 1981
21.FLOTTE TJ, SPRINGER TA, THORBECKE GJ: Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol 111:112–124, 1983
22.PENNINGER JM, IRIE-SASAKI J, SASAKI T, OLIVEIRA-DOS-SANTOS AJ:
CD45: New jobs for an old acquaintance. Nat Immunol 2:389–396, 2001
23.TROWBRIDGE IS, THOMAS ML: CD45: An emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol 12:85–116, 1994
24.ABE R, DONNELLY SC, PENG T, et al: Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol 166:7556–7562, 2001
25.RAMPRASAD MP, TERPSTRA V, KONDRATENKO N, et al: Cell surface
expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci USA 93:14833–14838, 1996
26.ROFF CF, WANG JL: Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J Biol Chem 258:10657–10663, 1983
27.SPRINGER TA: Monoclonal antibody analysis of complex biological systems. Combination of cell hybridization and immunoadsorbents in a novel cascade procedure and its application to the macrophage cell surface. J Biol Chem 256:3833–3839, 1981
