
- •1) Concentration of h2s is equal 50 ppm. What will be concentration of h2s in mg/m3, if molecular mass 34 g/mol, temperature of air equal 10 ºC; pressure 680 mmHg.
- •2) Concentration of ch4 is equal c ppm. What will be concentration of ch4 in mg/m3, if molecular mass 16 g/mol, temperature of air equal 15 ºC; pressure 690 mmHg.
- •3) Concentration of co2 is equal c ppm. What will be concentration of co2 in mg/m3, if molecular mass 44 g/mol, temperature of air equal 20 ºC; pressure 700 mmHg.
- •4) Concentration of so2 is equal c ppm. What will be concentration of so2 in mg/m3, if molecular mass 64 g/mol, temperature of air equal 30 ºC; pressure 710 mmHg.
- •5) Concentration of h2 is equal c ppm. What will be concentration of h2 in mg/m3, if molecular mass 2 g/mol, temperature of air equal 40 ºC; pressure 720 mmHg.
- •6) Concentration of no2 is equal c ppm. What will be concentration of no2 in mg/m3, if molecular mass 46 g/mol, temperature of air equal 50 ºC; pressure 730 mmHg.
- •7) Concentration of n2 is equal c ppm. What will be concentration of n2 in mg/m3, if molecular mass 28 g/mol, temperature of air equal 60 ºC; pressure 740 mmHg.
1) Concentration of h2s is equal 50 ppm. What will be concentration of h2s in mg/m3, if molecular mass 34 g/mol, temperature of air equal 10 ºC; pressure 680 mmHg.
Standard sources: T = 273 K; P = 101325 Pa = 760 mmHg; R = 8.31 J/(mol*K)
C(H2S) = 50 ppm 50 ppm = (v/v) = 50 μL/ L = 50 ml/m3
Mr(H2S) = 34 g/mol Now we need to find the weight of 50 mL of
T = 10 ºC hydrogen sulfide. For that purpose, we can use ideal
Pa = 680 mmHg gas law:
T = 273 K pV = mRT/M
P = 101325
Pa = 760 mmHg
R = 8.31 J/(mol*K)
V = 50 mL; R=8.31 L· kPa / (moL K); M (H2S) = 34 g/moL
- Pressure and temperature are not given. But let’s imagine that we are in Almaty now. The pressure is 680 mmHg, temperature 10°C.
- We need to convert temperature to K: T = 273 + 10 = 283 K
- The pressure must be converted to kPa. We know that 760 mmHg = 101.325 kPa. P = 101.325 kPa x 680 mmHg / 760 mmHg = 90.66 kPa
m
= *
*

m=0,0655 g = 65,5 mg
c(mg/m3) = 65,5 mg/m3
2) Concentration of ch4 is equal c ppm. What will be concentration of ch4 in mg/m3, if molecular mass 16 g/mol, temperature of air equal 15 ºC; pressure 690 mmHg.
Standard sources: T = 273 K; P = 101325 Pa = 760 mmHg; R = 8.31 J/(mol*K)
C(CH4) = 50 ppm 50 ppm = (v/v) = 50 μL/ L = 50 ml/m3
Mr(CH4) = 16 g/mol Now we need to find the weight of 50 mL of
T = 15 ºC hydrogen sulfide. For that purpose, we can use ideal
Pa = 690 mmHg gas law:
T = 273 K pV = mRT/M
P = 101325
Pa = 760 mmHg
R = 8.31 J/(mol*K)
V = 50 mL; R=8.31 L· kPa / (moL K); M (CH4) = 16 g/moL
- Pressure and temperature are not given. But let’s imagine that we are in Almaty now. The pressure is 690 mmHg, temperature 15°C.
- We need to convert temperature to K: T = 273 + 15 = 288 K
- The pressure must be converted to kPa. We know that 760 mmHg = 101.325 kPa. P = 101.325 kPa x 690 mmHg / 760 mmHg = 91.99 kPa
m
=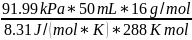 *
*
m=0,03075 g = 30,75 mg
c(mg/m3) = 30,75 mg/m3
3) Concentration of co2 is equal c ppm. What will be concentration of co2 in mg/m3, if molecular mass 44 g/mol, temperature of air equal 20 ºC; pressure 700 mmHg.
Standard sources: T = 273 K; P = 101325 Pa = 760 mmHg; R = 8.31 J/(mol*K)
C(CO2) = 50 ppm 50 ppm = (v/v) = 50 μL/ L = 50 ml/m3
Mr(CO2) = 44 g/mol Now we need to find the weight of 50 mL of
T = 20 ºC carbon dioxide. For that purpose, we can use ideal
Pa = 700 mmHg gas law:
T = 273 K pV = mRT/M
P = 101325
Pa = 760 mmHg
R = 8.31 J/(mol*K)
V = 50 mL; R=8.31 L· kPa / (moL K); M (CO2) = 44 g/moL
- Pressure and temperature are not given. But let’s imagine that we are in Almaty now. The pressure is 700 mmHg, temperature 20°C.
- We need to convert temperature to K: T = 273 + 20 = 293 K
- The pressure must be converted to kPa. We know that 760 mmHg = 101.325 kPa. P = 101.325 kPa x 700 mmHg / 760 mmHg = 93,33 kPa
m
=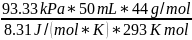 *
*
m=0,0843 g = 84,3 mg
c(mg/m3) = 84,3 mg/m3
