
2) CrCl3
3) AlCl3
4) NaNO3
5) NH4NO2
Задание #47
Вопрос: Which one of the following orders correctly represents the increasing acid strengths of the given acids?
1) HOClO2 < HOClO3 < HOClO < HOCl
2) HOCl < HOClO < HOClO2 < HOClO3
3) HOClO < HOCl < HOClO3 < HOClO2
4) HOClO3 < HOClO2 < HOClO < HOCl
5) HOClO3 < HOClO < HOCl < HOClO2
Задание #48
Вопрос: What is the equivalent weight of KMnO4 when it is converted in К2MnO4?
1) M/4
2) M/5
3) M/2
4) M/3
5) M/1
Задание #49
Вопрос: In an electrochemical galvanic cell:
1) potential energy decreases
2) potential energy changes into electrical energy
3) chemical energy changes into electrical energy
4) kinetic energy decreases
5) electrical energy changes into chemical energy
Задание #50
Вопрос: What salt is not hydrolyzed?
1) Na2CO3
2) Pb(NO3)2
3) BaCl2
4) K3PO4
5) K2S
Задание #51
Вопрос: Which of the following metals is often found in pure state?
1) Copper
2) Magnesium
3) Iron
4) Gold
5) Aluminum
Задание #52
Вопрос: What is the sign of EMF for a galvanic cell?
1) Zero
2) Positive
3) 1
4) Either positive or negative
5) Negative
Задание #53
Вопрос: Elements found in D-block of periodic table are known as ...
1) alkali metals
2) transition elements
3) earth alkali metals
4) noble elements
5) metaloids
Задание #54
Вопрос: Which metal is common between brass, bronze and German silver?
1) Mg
2) Ag
3) Zn
4) Cu
5) Al
Задание #55
Вопрос: Where does the acid base indicator phenolphthalein gain of crimson (purple) colour?
1) Na2СO3
2) SnCl2
3) MgSO4
4) KI
5) AgNO3
Задание #56
Вопрос: A potassium permanganate KMnO4 is a strong oxidizing agent in ................ medium
1) acidic
2) in water
3) neutral
4) in ammonium
5) basic
Задание #57
Вопрос: The Nernst equation is described by:
1)

2)
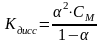
3)

4)

5)

Задание #58
Вопрос: Corrosion of iron is essentially an electrochemical phenomenon where the cell reaction are ...
1) Fe is oxidised to Fe2+ and H2O is reduced to O2
2) Fe is oxidised to Fe3+ and H2O is reduced to O2-
3) Fe is oxidised to Fe2+ and dissolved oxygen in water is reduced to OH-
4) Fe is oxidised to Fe2+ and H2O is reduced to O2-
5) Fe2+ is oxidised to Fe3+ and H2O is reduced to H2
Задание #59
Вопрос: On heating lead nitrate form oxides of nitrogen and lead. The oxides formed are ...
1) NO and PbO
2) NO2 and PbO
3) NO2 and PbO2
4) N2O and PbO2
5) NO and PbO2
Задание #60
Вопрос: An intramolecular reaction in which the atoms of one element is reduced and simultaneously increase the degree of oxidation state is called:
1) Displacement
2) Disproportionation
3) Combustion
4) Decomposition
5) Intermolecular
Задание #61
Вопрос: This type of corrosion occurs when the metal comes in contact with a conducting liquid or when two dissimilar metals are immersed or dipped partly in a solution. There is the formation of a galvanic cell on the surface of metals. Some parts of the metal surface act as anode and rest act as cathode. Water must be present to serve as a medium for the transport of ions. What is the corrosion type?
1) electrochemical corrosion
2) liquid corrosion
3) gas corrosion
4) atmospheric corrosion
5) chemical corrosion
Задание #62
Вопрос: Select the reduction process:
1)

2)
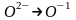
3)

4)

5)

Задание #63
Вопрос: Why is it necessary to add some salts to water for the electrolysis of water to proceed?
1) Water are electrolyzed spontaneously
2) Salts are necessary to prevent the explosion of the dangerous gasses hydrogen and oxygen
3) Salts are not needed for the reaction to proceed
4) Due to the production of acid during the electrolysis, a buffer is needed to maintain a constant pH
5) Water, as a poor electrical conductor needs some electrolyte solution to give the necessary conduction for the electrolysis to proceed
Задание #64
Вопрос: Aluminium displaces hydrogen from acids, but copper does not. A galvanic cell prepared by combining Cu/Cu2+ and Al/Al3+ has an emf of 2.0 V at 298 K. If the potential of copper electrode is +0.34 V, that of aluminium electrode is ...
1) - 1.66 V
2) - 0.34 V
3) - 2.34 V
4) + 1.66 V
5) + 2.34 V
Задание #65
Вопрос: Find the valency of the metal whose atomic weight is 96 and 0.3605g of a metal is deposited on the electrode by passing 1.2 ampere current for 15 minutes:
1) 1
2) 4
3) 2
4) 3
5) None
Задание #66
Вопрос: The coordination number of the complex compound Au(NH3)42(SO4)3 is equal ...
1) 4
2) 8
3) 3
4) 2
5) 6
Задание #67
Вопрос: A solution with a pH of 12 is:
1) strongly acidic
2) weakly basic
3) weakly acidic
4) water
5) strongly basic
Задание #68
Вопрос: What is the temporary hardness of water, if for titration of 100 ml of water was consumed 5.65 mL of 0.02 N hydrochloric acid solution (mmol / L)?
1) 8,85
2) 1,13
3) 2,8
4) 4,0
5) 11,3
Задание #69
Вопрос: NO2 is not obtained on heating ...
1) AgNO3
2) KNO3
3) Cu(NO3)2
4) Pb(NO3)2
5) none of these
Задание #70
Вопрос: The constitute of a Daniell cell is:
1) Zn - Cu cell
2) Cu - Ag cell
3) Mg - Cu cell
4) Zn - Ag cell
5) None of these
Задание #71
Вопрос: When the value of the electrode potential of the metal increases in the electrochemical series, we observe that .....
1) chemical activity of metals increases
2) reducing ability of the metal cation increases
3) oxidizing properties of the metal atom increases
4) metal solubility in acid increases
5) metal solubility in water increases
Задание #72
Вопрос: Magnesium metal can be attached to the steel pipeline to prevent rusting. What name is given to this type of protection provided by magnesium?
1) Galvanising
2) Anodising
3) Electroplating
4) Natural protection
5) Use a Sacrificial anode
Задание #73
Вопрос: When electrolysis of aqueous NaCl solution is carried out, the products are:
1) Na at cathode and Cl2 at anode
2) H2 at cathode and Cl2 at anode
3) NaOH at cathode and Cl2 at anode
4) H2 at cathode and O2 at anode
5) Na at cathode and O2 at anode
Задание #74
Вопрос: An equivalent weight of oxidizer KMnO4 in a water neutral medium is equal ... (g/mol):
1) 158
2) 31,6
3) 79
4) 25,02
5) 52,6
Задание #75
Вопрос: Redox reactions in which the both oxidizing and reducing agents are composed into one molecule is called:
1) Intramolecular
2) Diplacement
3) Combustion
4) Intermolecular
5) Disproportionation
Задание #76
Вопрос: Which of the following statements is true?
1) H3PO3 is a stronger acid than H2SO3
2) HClO4 is a weaker acid than HClO3
3) HNO3 is a stronger acid than HNO2
4) In aqueous medium HF is a stronger acid than HCl
5) HNO2 is a stronger acid than HCl
Задание #77
Вопрос: When Cl2 gas reacts with hot and concentrated sodium hydroxide (NaOH) solution, the oxidation number of chlorine changes from ...
1) Zero to - 1 and zero to + 5
2) Zero to + 1 and zero to -5
3) Zero to - 1 and zero to + 3
4) Zero to + 3 and zero to + 5
5) Zero to + 1 and zero to -3
Задание #78
Вопрос: When KMnO4 acts as an oxidizing agent and ultimately forms MnO4 2-, MnO2, Mn2O3 and Mn2+, then the number of electrons transferred in each case is ...
1) 3, 5, 7, 1
2) 6, 4, 3, 2
3) 1, 5, 3, 7
4) 1, 3, 4, 5
5) 4, 3, 1, 5
Задание #79
Вопрос: The chemical destruction of metal in fluids, electrolytes which are not is called:
1) Atmospheric corrosion
2) Gas corrosion
3) Liquid corrosion
4) Electrochemical corrosion
5) Biocorrosion
Задание #80
Вопрос: Which of the following reactions is NOT a redox reaction?
1) NaI + Cl2 ---> NaCl + I2
2) NaOH + HCl ---> NaCl + H2O
3) H2O2 ---> H2O + O2
4) Ni2+ + Fe ---> Fe2+ + Ni
5) Na + Cl2 ---> NaCl
Задание #81
Вопрос: For an aqueous solution with pOH=5, hydrogen (H+) ions concentration will be equal to.... (mol/L):
1) 10-1
2) 10-2
3) 10-9
4) 10-4
5) 10-5
Задание #82
Вопрос: What is the oxidation state of gold in complex ion [Au(CN)4]- ?
1) + 3
2) + 6
3) + 4
4) + 2
5) + 5
Задание #83
Вопрос: A certain current liberates 0.504 g of hydrogen in 2 hours. How many grams of oxygen can be liberated by the same current in same time?
1) 1.0 g
2) 4.0 g
3) 2.0 g
4) 0.252 L
5) 8.0 g
Задание #84
Вопрос: Choose substances that form aqueous solution with acidic medium (pH<7):
1) KBr 2) CuSO4 3) KI 4) KNO3 5) Na2SiO3
Задание #85
Вопрос: The method used for galvanisation of nuts, bolts, screws and washers is known as:
1) Hot dipping
2) Electro-galvanisation
3) Sheradizing
4) Cathodic protection
5) Metallizing
Задание #86
Вопрос: Which of the followinf p-block elements does not show allotropy?
1) phosphorus
2) chlorine
3) carbon
4) sulfur
5) arsenic
Задание #87
Вопрос: Which of the following terms refers to the number of molecules or ions attached to a central metallic atom?
1) none of these
2) connection number
3) no answer
4) coordination number
5) bonding number
Задание #88
Вопрос: Which of the following is NOT produced during the electrolysis of water?
1) Electricity
