
THE MINISTRY OF AGRICULTURE THE REPUBLIC OF KAZAKHSTAN
S. SEIFULLIN KAZAKH AGROTECHNICAL UNIVERSITY
DEPARTMENT OF PHYSICS AND CHEMISTRY
Author: Mukhanbetova N. A, senior teacher,
master of chemistry
Test for 2nd control on the discipline “general and inorganic chemistry” for students of technical specialties:
5B072700 – Technology of food products
and
5B080600 – Agrarian technique and technology
Test for 2nd control was discussed at the meeting of the chair chemistry and physics "_____" ___________ 2017, protocol № _______
Head of the Department ________________Alimkulova E.J., associate prof.
Astana 2017
Задание #1
Вопрос: Which metals will serve as the anode for lead?
1) Ag, Fe
2) Co, Pt
3) Pb, Cu
4) Cu, Al
5) Cr, Ni
Задание #2
Вопрос: Use the activity series given to you to determine which of the following reactions will NOT take place:
1) Cu + HCl -->
2) Mg + H2O -->
3) Ag + AuCl3 -->
4) Zn + HNO3 -->
5) Ca + Pb(NO3)2 -->
Задание #3
Вопрос: What metals will not react with diluted acids?
1) Pt, Au
2) Ni, Mg
3) Zn, Fe
4) Au, Cа
5) Cd, Cu
Задание #4
Вопрос: If iron filings are put in different test tubes containing ZnSO4, CuSO4, Al2(SO4)3, CaCl2, CrCl3 in which one changes will be observed?
1) CuSO4
2) CaCl2
3) Al2(SO4)3
4) ZnSO4
5) CrCl3
Задание #5
Вопрос: A current of x A flowing for 10 min deposits 3g of the metal which is monovalent. The atomic mass of the metal is 50. Find the value of x?
1) 0.10
2) 9.65
3) 6.72
4) 8.75
5) 10.5
Задание #6
Вопрос: The Earth's atmosphere or air is composed of several gases.By far, the most abundant gas in the Earth's atmosphere is ...
1) Nitrogen
2) Hydrogen
3) Carbon dioxide
4) Argon
5) Oxygen
Задание #7
Вопрос:
The
electrode potential at an copper electrode, dipped in a 0,01М
aqueous solution of copper sulfate at 25°C, is equal .......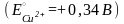 :
:
1) +0,34В
2) +0,369В
3) +0,281В
4) +0,399В
5) +0,311В
Задание #8
Вопрос: What is the pOH of the solution, with a content of [H+] = 10-4 mol /L?
1) 10
2) 8
3) 4
4) 6
5) 9
Задание #9
Вопрос: Galvanization of iron sheets is done by:
1) Pb plating
2) Tin plating
3) Ag plating
4) Zn plating
5) Cu plating
Задание #10
Вопрос: Select the definition of Faraday's 1st law:
1) A chemical compound always contains exactly the same proportion of elements by mass.
2) For non-electrolyte solutions, the partial vapor pressure of a solvent over a solution (P1) is equal to the vapor pressure of the pure solvent (P0) multiplied by the mole fraction of the solute (X2).
3) At constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of reacting species, with each concentration term raised to the power equal to the numerical coefficient of the species in the chemical equilibrium
4) The mass of a substance produced at an electrode during electrolysis is proportional to the number of moles of electrons (the quantity of electricity) transferred at that electrode
5) The mass of a substance deposited or liberated at any electrode on passing a certain amount of charge is directly proportional to its chemical equivalent weight
Задание #11
Вопрос: In a comparison with S-block elements, melting point of transition elements are ...
1) higher
2) does not matter because they are same
3) same
4) lower
5) constant
Задание #12
Вопрос: In a redox reaction if the EMF is positive then the reaction is:
1) Reversible
2) Non-spontaneous
3) None of these
4) Spontaneous
5) Irreversible
Задание #13
Вопрос: All alloys contain this element if they are amalgama. The element is:
1) platinum
2) gold
