
- •Chemistry 5b072600, 5b073300 (300 tests)
- •1. The elementary object is …
- •Number of equivalents of substance a per 1 liter of solution
- •Mass of substance a to the total mass
- •There is no unit
- •There is no unit
- •There is no unit
- •167. Using standard warmth of combustion for substances to calculate thermal effect of reaction
- •Values are resulted in the table:
- •If values s are given in the table.
- •172. Using standard warmth of combustion of substances to calculate thermal effect of reaction at 298к
- •Values are resulted in the table:
167. Using standard warmth of combustion for substances to calculate thermal effect of reaction
C2H5OH(ж)+СH3СООН(ж)
→ CH3COOС2H5(ж)+H2О(ж)
при
250С,
if warmth of combustion of substances are following
 -1366,9
кJ/mol,
-1366,9
кJ/mol,
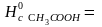 -873,8
кJ/mol,
-873,8
кJ/mol,
 -2254,2
кJ/mol
:
-2254,2
кJ/mol
:
13,5 kJ;
15,4 kJ;
17,8 kJ;
7,9 kJ;
11,5 kJ
168. Calculate thermal effect of reaction at 292K and 1, 0133⋅105 Па for reaction: Fe2O3(тв)+3CO(г)=2Fe(тв)+3CO2(г),
 -821,32
кJ/mol,
-821,32
кJ/mol,
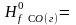 -110,5
кJ/mol,
-110,5
кJ/mol,
 0,
0,
 -393,51
кJ/mol
:
-393,51
кJ/mol
:
-27,71 кJ;
13,5 кJ;
141,82 кJ;
-354,8 кJ;
0;
169. To define thermal effect of reaction: СН СН + СО + Н2О (ж) → СН2 = СНСООН (ж)
at standard pressure, if warmth of formation are known
-
Substance
 [kJ/mol
]
[kJ/mol
]СН СН
226,75
СО
–110,50
Н2О (ж)
–285,84
СН2 = СНСООН (ж)
–384,37
-214,78 кJ
107,36 кJ
1007,36 кJ
–623,09 кJ
–154,2 кJ
170. Calculate thermal effect of reaction (kJ) at Т = 298К: CuCl2 + Zn = ZnCl2 + Cu
Values are resulted in the table:
-
Substance
ZnCl2
–478,2
CuCl2
–262,3
-215,9
740,5
–903,6
904,2
370,2
171. Calculate entropy change under standard conditions for reaction: 2H2S + SO2 = 2 Н2О (ж) + 3S,
If values s are given in the table.
-
Substance
 [J/mol
К]
[J/mol
К]Н2О
69,95
S
32,55
SO2
248,1
H2S
205,64
–421,81
–102,50
513,24
350,72
231,11
172. Using standard warmth of combustion of substances to calculate thermal effect of reaction at 298к
С2Н5ОН(ж) + СН3СООН(ж) → СН3СООС2Н5(ж) + Н2О (ж)
Substance |
Нсг. [kJ/mol ] |
С2Н5ОН(ж) |
–1366,9 |
СН3СООН(ж) |
–873,8 |
СН3СООС2Н5(ж) |
–2254,2 |
Н2О (ж) |
0 |
13,5 кJ
-2254,2 кJ
–240,2 kJ
–3,21 kJ
–1,34 kJ
173. Calculate standard change of entropy at 298К for reaction: Cd (тв) + 2AgCl (тв) = 2Ag (тв) + CdCl2 (тв)
on absolute values entropy of chemical compounds
Substance |
S [J/mol К] |
Cd |
51,76 |
AgCl |
96,07 |
Ag |
42,69 |
CdCl2 |
115,3 |
–43,22 J/К
10,16 J/К
0
325,82 J/К
–10,16 J/К
174. Calculate thermal effect of chemical reaction (kJ) at Т=298К, Р=1,013105 Па
Cd (тв) + 2AgCl (тв) = 2Ag (тв) + CdCl2 (тв), if warmth of formation of substances are known
Substance |
298 [КJ/mol ] |
Сd |
0 |
Ag |
0 |
AgCl |
–126,8 |
CdCl2 |
–389,0 |
–135,4
–263,2
–515,8
443,2
344,8
175. Calculate standard change of energy of Gibbs (kJ) for reaction: Cd (тв) + 2AgCl (тв) = 2Ag (тв) + CdCl2 (тв),
if are known Н298 = –135,4 kJ; S298 = –76,84 J/К.
–112,5
1393,55
–141,7
24,61
41,42
176. Calculate thermal effect of reaction (kJ) at Т =298К: CuCl2 + Zn = ZnCl2 + Cu
