
- •Structure of atom Development of the atomic structure conception
- •Rutherford's model
- •The Bohr Model of the Atom
- •The quantum revolution The nature and characteristics of radiant energy
- •Emission Spectrum of Hydrogen
- •The quantun theory of light
- •The quantitative bases for the vawe-mechanical model Wave Function
- •Quantum numbers
- •Table 2. The Quantum Numbers
- •Table 3. Summary of allowed combinations of quantum numbers for values n from 1 to 4.
- •Energies of orbitals and building up principles by electrons for many electron atoms
- •Valence Electrons
Structure of atom Development of the atomic structure conception
For a long time, the opinion that atoms are indivisible, i.e. contain no simpler constituent parts, dominated in science. Atoms were also considered to be unchangeable: an atom of a given element could never transform into an atom of another one. The end of the 19th century saw the establishment of a number of facts pointing to the complex composition of atoms and to the possibility of their mutual transformations.
Michael Faraday (1791-1867) greatly extended the new science of electrochemistry. His works with solutions and melts established that atoms are electrical in nature. Further detail in the structure of atoms had to wait for the development of gas discharge tubes and of still more powerful sources of electrical voltage. In 1879, Sir William Crookes devised an apparatus consisting of a evacuated glass tube containing two metal plates, called electrodes, which are connected to a source of electricity. When a high voltage is applied to evacuated tube, “cathode rays” pass between them. These rays could be detected by their ability to cause certain materials to give off greenish light, or fluoresce.
T
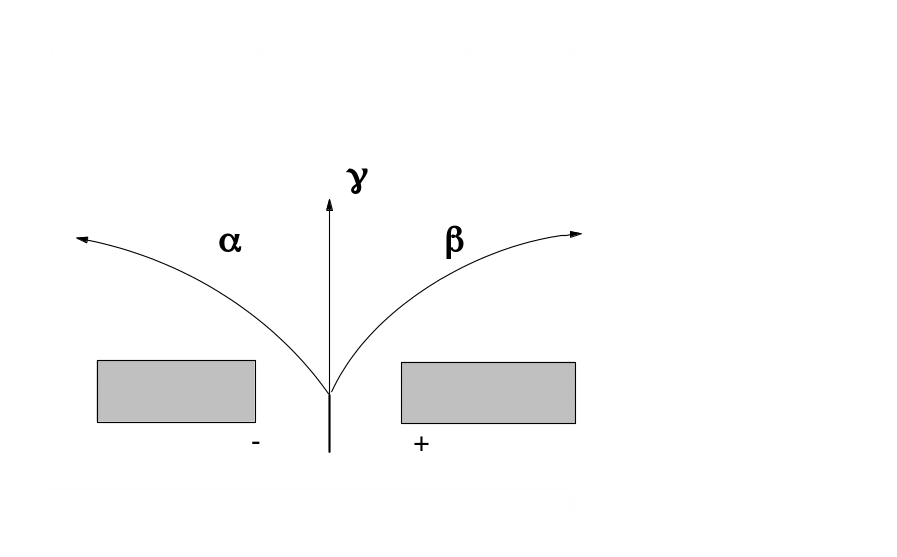 Fig.
2. Behavior of radioactive rays in an electric field.
Fig.
2. Behavior of radioactive rays in an electric field.
The demonstration by Thompson that all atoms contain units of negative electric charge led him in 1903 to the first model of the atom which envisaged the electrons being spread out uniformly throughout the spherical volume of the atom.
Since atom electrically neutral particles, the identification of electrons as negative components of all atoms naturally led to the search of positive particle. A beam consisting of the least massive positive particles was discovered in 1885 by E.Goldstein when hydrogen was used in the tube. This particles are called protons.
A major role in establishing the intricate nature of the atom and decoding its structure was played by the discovery and study of radioactivity.
The name radioactivity was given to the phenomenon of the emission by certain elements of radiation capable of penetrating through a substance, ionizing air, and of causing photographic plates to darken.
Investigations of the Curies and of the British physicist Ernest Rutherford showed that radioactive radiation is not homogeneous: a magnetic field causes it to divide into three beams, one of which does not change its original direction, while the other two deviate in opposite directions.
The rays that do not deviate in a magnetic field and, consequently, carry no electric charge, were called gamma rays. They are electromagnetic radiation similar to X-rays and having a very high penetrability. The deflection of the other two beams under the action of a magnetic field indicates that these beams consist of electrically charged particles. The opposite directions of the observed deflections witness that one beam contains negatively charged particles (this kind of radiation was previously named cathode rays or b rays ), while the other (alpha rays) contains positively charged particles. The beta rays were found to be a stream of rapidly moving electrons. This was another confirmation of the fact that electrons are among the particles which atoms consist of. The study of radioactivity confirmed the complexity of the composition of atoms. The problem appeared of revealing the structure of an atom.
