
теория 5 занят
.docTopic №5: The nature of chemical bond. Biogeneous s, p, d, f – elements and its biological role.Coordination compounds and its properties.Medical-biological role of coordination compounds.
Basic questions:
1. Energy of bond. Types of chemical bond.
2. The mechanism of the formation and properties of covalent bond: polarity, saturation, directivity, energy of bond.
3. Biogenic elements, contents in organism, position in P.S.E. Properties of biogenic elements.
4. Coordination theory of Werner. Structure of coordination compounds: complex-former, ligands, external and internal sphere of CC, coordination number.
5. Metal-ferment and other biocomplex, its biological role.
Coordination compounds of iron, cobalt, nickel, chromium, magnesium, its biological role.
Energy of bond. Types of chemical bond.
Chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are "strong bonds" such as covalent or ionic bonds and "weak bonds".
In chemistry, bond energy (E) is the measure of bond strength in a chemical bond. It is the heat required to break one Mole (unit) of molecules into their individual atoms.[1] For example, the carbon-hydrogen bond energy in methane E(C–H) is the enthalpy change involved with breaking up one molecule of methane into a carbon atom and 4 hydrogen radicals divided by 4.
Bond energy (E) should not be confused with bond-dissociation energy. It is a roughly transferable property, and enthalpy of formation can typically be roughly approximated by simply adding tabulated values for bond energies for all bonds in a molecule, with an error of sometimes just a few percent. However, to get a better approximation is much more difficult.
Types:
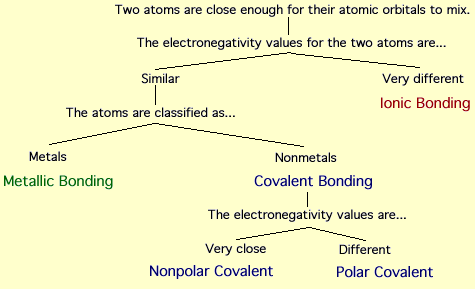
1)Ionic Bonds
An ionic bond is formed by the attraction of oppositely charged atoms or groups of atoms. When an atom (or group of atoms) gains or loses one or more electrons, it forms an ion. Ions have either a net positive or net negative charge. Positively charged ions are attracted to the negatively charged 'cathode' in an electric field and are called cations. Anions are negatively charged ions named as a result of their attraction to the positive 'anode' in an electric field.
Every ionic chemical bond is made up of at least one cation and one anion.Ionic bonding is typically described to students as being the outcome of the transfer of electron(s) between two dissimilar atoms. The Lewis structure below illustrates this concept.
2)Covalent
A covalent chemical bond results from the sharing of electrons between two atoms with similar electronegativities A single covalent bond represent the sharing of two valence electrons (usually from two different atoms). The Lewis structure below represents the covalent bond between two hydrogen atoms in a H2 molecule.
Types of covalent bonds
There are 3 types of this bond are possible depending on the number of electron pairs being shared between the bonded atoms:
Single covalent bonds: It is formed when one electron pair is shared between the two bonded atoms H:H or H-H
Double covalent bond: When two electron pairs are shared between the two bonded atoms, a double covalent bond is said to be formed. The two electron pairs are indicated by two lines between the two atoms :o: :o¨:
When three electron pairs are shared between two bonded atoms, a triple covalent bond comes into existence. :N⁞⁞N: or :N≡N:
3)Metallic
The valence electrons of pure metals are not strongly associated with particular atoms. This is a function of their low ionization energy. Electrons in metals are said to be delocalized (not found in one specific region, such as between two particular atoms).
Since they are not confined to a specific area, electrons act like a flowing “sea”, moving about the positively charged cores of the metal atoms. Delocalization can be used to explain conductivity, malleability, and ductility. Because no one atom in a metal sample has a strong hold on its electrons and shares them with its neighbors, we say that they are bonded.
In general, the greater the number of electrons per atom that participate in metallic bonding, the stronger the metallic bond.
2. The mechanism of the formation and properties of covalent bond: polarity, saturation, directivity, energy of bond.
1) Polarity- In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multiple moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in electronegativity between atoms in a compound and the asymmetry of the compound's structure. For example, a molecule of water.
2) Saturation is the point at which a solution of a substance can dissolve no more of that substance, and additional amounts of it will appear as a solid precipitate.
This point of maximum concentration, the saturation point, depends on the temperature of the liquid as well as the chemical nature of the substances involved. If a change in conditions (e.g. cooling) means that the concentration is actually higher than the saturation point, the solution has become supersaturated.
3.Biogenic elements, contents in organism, position in P.S.E. Properties of biogenic elements.
The Biogenic Elements: The Chemical Elements. Essential To Earth's Living Systems
Almost every one of the chemical elements plays some role in Earth's living systems, however, ~20 elements account for the vast majority of material in living systems. These biogenic elements are divided into:
-six major biogenic elements (elements found in almost all of Earth's living systems, often in relatively large quantities)
-five minor biogenic elements (elements found in many of Earth's living systems, and/or in relatively small quantities)
-trace elements (essential elements necessary only in very small quantities to maintain the chemical reactions on which life.
Major Biogenic Elements in organism
Carbon
Hydrogen
Oxygen
Nitrogen
Sulfur
Phosphorous
Macroelements (12 elements in total) form up to 99 % of any organism, and can be further subdivided into:
a) a group of stable primary elements (1-60 % of total organism weight). These are: O,C, H,N
b) a group of stable secondary elements (0.05/1 % of total organism weight). These are Ca, S, Mg, Cl, Na, K, Fe
Microelements can be divided into three categories:
a) a subgroup of 8 stable elements (less than 0.05%). These are the elements: Cu, Zn, Mn, Co, B, Si, F, I
b) a subgroup of approximately 20 elements that are present at conc. of 0.001% and lower.
c) a subgroup of contaminating elements: Their constant excess in the organism leads to disease: Mn, He, Ar, Hg, Tl, Bi, Al, Cr, Cd.
4. Coordination theory of Werner. Structure of coordination compounds: complex-former, ligands, external and internal sphere of CC, coordination number.
It was only in 1893, that Werner presented a theory known as Werner's coordination theory which could explain all the observed properties of complex compounds. Important postulates of this theory are:
Most elements exhibit two types of valencies:
(a) Primary valency
This corresponds to the oxidation state of the metal ion. This is also called principal, ionisable or ionic valency. It is satisfied by negativeous and its attachment with the central metal ion is shown by lines.
(b) Secondary valency
It is also termed as coordination number of the central metal ion. It is non-ionic or non-ionasable. This is satisfied by either negative ions or neutral molecules. The ligands, which satisfy the coordination number are directly to the metal atom or ion and are shown by thick lines.
Every element tends to satisfy both its primary and secondary valences. In order to meet this requirement a negative ion may often show a dual behavior.
Coordination complex
Although our primary focus in this unit is on bonding, the topic of coordination complexes is so important in chemistry and biochemistry that some of their basic features are worth knowing about, even if their detailed chemistry is beyond the scope of this course. These complexes play an especially crucial role in physiology and biochemistry. Thus heme, the oxygen-carrying component of red blood cells (and the source of the red color) is basically a complex of iron, and the part of chlorophyll that converts sunlight into chemical energy within green plants is a magnesium complex.
Internal sphere
 Ligand
Ligand
K



 [Ag(CN2)]
[Ag(CN2)]
Coordination
Number or ligand
external
sphere amins neutral molecule
O H2Oᴼ-aqua
CN-cyanO NH3ᴼ-ammine
complex former Cl-chlorO COᴼ-carbonyl
Br-bromO
SO42-sulphatO
NO3-nitratO
NO2-nitritO
CO32-carbonatO
5. Metal-ferment and other biocomplex, its biological role.
Werner was able to show, in spite of considerable opposition, that transition metal complexes consist of a central ion surrounded by ligands in a square-planar, tetrahedral, or octahedral arrangement. This was an especially impressive accomplishment at a time long before X-ray diffraction and other methods had become available to observe structures directly. His basic method was to make inferences of the structures from a careful examination of the chemistry of these complexes and particularly the existence of structural isomers. For example, the existence of two different compounds AX4 having the same composition shows that its structure must be square-planar rather than tetrahedral.
