
- •Навчальний посібник для студентів-технологів
- •Костянтинівка
- •Introduction то chemistry
- •Vocabulary
- •Exercises
- •1. Answer the questions.
- •2. Match the English word combinations with their Ukrainian equivalents;
- •3. Match the Ukrainian word combinations with their English equivalents
- •From the history of chemistry
- •Vocabulary
- •Exercises
- •Answer the questions
- •6. Translate the words in the brackets into English:
- •7. Translate the text using a dictionary. Some facts about chemistry
- •D. I. Mendeleyev
- •Exercises
- •1. Answer the questions
- •2. Translate the sentences paying attention to the passive constructions:
- •3. Open the brackets choosing the suitable word. Translate them.
- •Chemistry: key to progress and abundance
- •Vocabulary
- •Fields of chemistry
- •Vocabulary
- •Exercises
- •2.Answer the questions.
- •3.Fill in the gaps with suitable words given below.
- •4.Make up sentences out of these words.
- •5. Translate into English.
- •Symbols, formulas and equations
- •Vocabulary
- •Inorganic molecules and compounds
- •Vocabulary
- •Periodic law
- •Vocabulary
- •Exercises
- •Answer the questions.
- •True or false?
- •Найважливіші хімічні елементи
- •Rules of reading formulas and equations Правила читання хімічних формул
- •Приклади:
- •The periodic table of d.I. Mendeleyev
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Read and translate the text with vocabulary Joseph Priestley
- •Laboratory equipment
- •2.Learn the words and special term from the list.
- •Describe the functions of each piece of equipment. An experiment in the laboratory
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Give Ukrainian equivalents:
- •3. Translate the sentences:
- •4. Make the questions to the sentences:
- •The molecular theory of matter and the states of matter
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Give English equivalents:
- •3. Give Ukrainian equivalents:
- •4. Translate the sentences:
- •Atomic structure
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Give Ukrainian equivalents:
- •3. Give English equivalents:
- •8. Read and translate the text Molecules
- •Chemical and physical changes
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •Find the pairs of synonyms:
- •Find the pairs of antonyms:
- •4. Translate the following sentences, mind the Participles:
- •5. Open the brackets translating the Ukrainian words:
- •Nuclear fission
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Translate the sentences into Ukrainian:
- •Open the brackets choosing the suitable word and translate them into
- •4. Translate the text in writing
- •Vocabulary
- •Exercises
- •5. Read and translate the text The Temperature Scales
- •Exercises
- •1. Give Ukrainian equivalents:
- •2. Give English equivalents:
- •Liquids
- •Vocabulary
- •Exercises
- •Exercises
- •1. Find Ukrainian equivalents:
- •2. Find English equivalents:
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Give synonyms:
- •3. Translate the following sentences:
- •Acids and bases
- •1. Extremely useful – надзвичайно корисні
- •2. Are common to all – загальні для всіх
- •3. Acetic acid - оцтова кислота
- •Vocabulary
- •Exercises
- •1. Answer the following questions.
- •2. Complete the sentences (use the text).
- •3. Characterize acids and bases using the following plan.
- •Vocabulary
- •Exercises
- •Chlorine
- •Vocabulary
- •Exercises
- •1. Answer the questions.
- •Make up a description of any element you like. Hydrochloric acid
- •Vocabulary
- •Exercises
- •Match English word combinations with their Ukrainian equivalents.
- •Answer the questions.
- •Solutions
- •Vocabulary
- •Exercises
- •Answer the questions
- •2. Translate the following sentences:
- •Nitrogen
- •Vocabulary
- •Exercises
- •Match English word combinations with their Ukrainian equivalents.
- •Answer the questions.
- •Silicon
- •Vocabulary
- •Exercises
- •Match English word combinations with their Ukrainian equivalents.
- •Answer the questions
- •Cellulose
- •Vocabulary
- •Exercises
- •Answer the questions.
- •Analytical chemistry methods of analysis
- •Methods of separation
- •Ion exchange methods in analytical chemistry
- •Ionization
- •Vocabulary
- •Exercises
- •Chromatography and ion exchange technique
- •Chromatography techniques
- •Gas analysis
- •Some physical methods used in gas analysis
- •Extraction
- •Precipitation
- •Electrolysis
- •Polymers
- •Notes and commentary
- •Vocabulary
- •Exercises
- •1. Answer the questions.
- •2. Match English word combinations with their Ukrainian equivalents.
- •3. Match Russian word combinations with their English equivalents.
- •Retell text using questions from Ex. 1 as a plan.
- •5. Read, translate and do the tasks.
- •Some applications of polymers
- •Vocabulary
- •Exercises
- •1. Read and translate the sentences. Correct the false statements.
- •2. Read the text, translate it in written form using dictionary.
- •The nature of polymeric materials
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •Find the pairs of synonyms:
- •Find the pairs of antonyms:
- •Choose the Ukrainian equivalents from the right column:
- •5. Translate the sentences
- •6. Open the brackets choosing the suitable verb:
- •7. Open the brackets choosing the correct form of the verb:
- •7. Translate the text in writing
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2.Translate the following word-combinations:
- •Translate into English:
- •4. Open the brackets translating the Ukrainian words into English:
- •5. Translate the sentences into Ukrainian:
- •6. Translate the text using a dictionary
- •Microbiological production of industrial chemicals
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •Translate the following sentences into Ukrainian, mind the sentences of the predicate:
- •3. Translate the following sentences into English, mind the use of the tenses:
- •4. Translate the following sentences into Ukrainian
- •5. Translate from Ukrainian into English
- •The chemical elements essential to life
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Find the pairs of synonyms:
- •Find the pairs of antonyms:
- •4. Translate paying attention to the meanings of the word “provide”
- •5. Open the brackets translating the Ukrainian words into English
- •6. Translate the text with a dictionary Hydrogen in industry
- •Plastics
- •Vocabulary
- •Exercises
- •Answer the questions.
- •Glass and glass products
- •Vocabulary
- •Exercises
- •Translate into Ukrainian the following international words.
- •Match English word combinations with their Ukrainian equivalents.
- •Answer the questions.
- •The nature of ceramics
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Translate the sentences:
- •7. Read and translate the texts
- •Ceramics
- •Vocabulary
- •Exercises
- •Translate the following international words into Ukrainian.
- •Answer the questions.
- •What is ecology?
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Translate the following sentences:
- •3. Translate the sentences:
- •The water problem
- •Pollution
- •Air pollution
- •Water pollution
- •Earth pollution
- •Vocabulary
- •Exercises
- •1. Answer the questions
- •2. Translate the following word-combinations:
- •3. Translate the following sentences into Ukrainian:
- •4. Translate the sentences into Ukrainian:
- •5. Write the translation of the following text Lead
- •The environmental protection
- •Vocabulary
- •Exercises
- •1. Match the words:
- •2. Translate the sentences into English:
- •3. Put 4 types of the questions to the sentences:
- •4. Translate the text
- •Radioactivity
- •Notes on the text
- •Vocabulary
- •Exercises
- •4. Read and translate the text The discovery of X-Rays and Radioactivity
- •5. Open the brackets and translate the sentence into Ukrainian:
- •Chernobyl nuclear power station
- •Vocabulary
- •Exercises
- •Protection of the environment
- •Industry of ukraine
- •Chemical industry
- •Texts for reading glass
- •Glass history natural glasses
- •Early glasses
- •Blowing, (b) cutting and (c) flattening. Modern glasses soda-lime-silica glasses
- •Cutting and drilling of glass
- •Glass cutting principle (scribing, flexuring).
- •Applications of glass
- •Glazing
- •Containers
- •Optical glass
- •Glass fibres for insulation and reinforcement
- •Borate and related glasses
- •Window glass
- •Sheet wire glass
- •Stemalite
- •Hardened glass for ship’s port holes
- •Safety glass for ground transport
- •Slag glass-ceramic
- •Mechanics of Glass Processes
- •Batching
- •Melting
- •Float Process
- •Fusion Draw
- •Pressing
- •Fibre Process
- •Tensile Drawing
- •Centrifugal Drawing
- •Wool fibre drawing process
- •Types of glass
- •Glass industry of ukraine
- •Glossary
- •Reference list
- •Contents
Borate and related glasses
Boron (B) is in group III of the Mendeleev table and is a network former with strong Dietzel field. It tends to bond to three different oxygen ions adopting a triangular coordination.
Tetrahedral
coordination is also found. As regards the three coordinated B
atoms, it has been suggested that these form boroxyl groups
B3O6.These groups are planar and linked to form a three-dimensional
network by boron–oxygen–boron bonds. Such a structure is in good
agreement with X-ray diffraction results. Such a structure is then
much different from that of silica. When adding alkali metals to
borates the induced changes are opposite to those generated in
silica; this is called in the literature the ‘boron oxide
anomaly’. In fact, the viscosity may increase and the thermal
expansion decrease when adding alkali metal, contrary to what is
generally observed in silica-based glasses. It is believed that
such a trend could be related to a change in the coordination of
boron atoms. Borosilicate glasses play an important role in glass
manufacturing since they are corrosion resistant to water and allow
for low thermal expansion. They are composed of Si and B atoms which
form the network, Si being four coordinated and B three coordinated.
The resulting network is softer than silica with lower viscosity and
lower melting temperature. 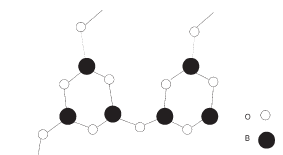 Contrary
to what happens when modifiers are introduced in silica, the bond
potential remains symmetrical in borosilicates inducing low
variation in the mean distances with increasing temperature, that
is, a low thermal expansion. In borosilicate glasses, alkali metal
ions like Na+ ions will either modify the network introducing NBOs
or change the boron coordination (from3 to 4). The latter phenomenon
is believed to be responsible for the ‘boron oxide anomaly’
where viscosity may increase with increasing alkali metal
concentration as stated for borate glasses.
Contrary
to what happens when modifiers are introduced in silica, the bond
potential remains symmetrical in borosilicates inducing low
variation in the mean distances with increasing temperature, that
is, a low thermal expansion. In borosilicate glasses, alkali metal
ions like Na+ ions will either modify the network introducing NBOs
or change the boron coordination (from3 to 4). The latter phenomenon
is believed to be responsible for the ‘boron oxide anomaly’
where viscosity may increase with increasing alkali metal
concentration as stated for borate glasses.
Window glass
The window glass is manufactured by upward drawing. It is designed for filling the fenestrations of residential and industrial buildings and structures, glazing door sashes, skylights and plant growing and cattle breeding buildings. The window glass is available of the first and second grades. The window glass sheets are supplied in sizes specified by the customer, and if not specified, in the manufacturer’s assortment with the length and width multiple of 25. Upon agreement with the customer, window glass sheets can be produced in length and width other than those specified above. The glass sheets are of a rectangular shape. The difference in the length of diagonals of sheets does not exceed 7 mm, while that for the high-grade glass is not in excess of 5 mm. the glass sheets are colourless with a greenish or bluish tint, which does not affect the external transmittance. No optical distortion of the lines of the “brick wall” screen are permitted when viewing in through the sheet of the second-grade glass at an angle of 90˚and when looking through the sheet of the first-grade glass at an angle of 60˚.
