
- •Se “lugansk state medical university”
- •Classification of Gastritis
- •Clinicopathological approach to gastritis
- •Intestinal Metaplasia
- •Invasive Tests
- •Infectious gastritis (excluding helicobacter)
- •Viruses
- •Autoimmune gastritis
- •Lymphocytic gastritis
- •Granulomatous gastritis
- •Granulomatous Gastritis in Sarcoidosis and Crohn’s Disease
- •Isolated Granulomatous Gastritis
- •Chemical (reactive) gastropathy
- •Hemorrhagic gastropathy
- •Vascular gastropathies
- •Hypertrophic gastropathies
- •Gastric cardia
Se “lugansk state medical university”
CHAIR OF INTERNAL MEDICINE WITH BASIS OF PULMONOLOGY
SUBJECT №12: «GASTRIC DYSPEPSIA AND CHRONIC GASTRITIS»
Amount of education hours: 2
CONSPECT OF THE LECTURE
The term gastritis should be reserved for histologically documented inflammation of the gastric mucosa. Gastritis is not the mucosal erythema seen during endoscopy and is not interchangeable with “dyspepsia.” The etiologic factors leading to gastritis are broad and heterogeneous. Gastritis has been classified based on time course (acute vs. chronic), histologic features, and anatomical distribution or proposed pathogenic mechanism.
Classification of Gastritis
I. Acute gastritis |
II. Chronic atrophic gastritis |
A. Acute H. pylori infection |
A. Type A: Autoimmune, body-predominant |
B. Other acute infectious gastritides |
B. Type B: H. pylori–related, antral-predominant |
1. Bacterial (other than H. pylori) |
C. Indeterminant |
2. Helicobacter helmanni |
III. Uncommon Forms of Gastritis |
3. Phlegmonous |
A. Lymphocytic |
4. Mycobacterial |
B. Eosinophilic |
5. Syphilitic |
C. Crohn's disease |
6. Viral |
D. Sarcoidosis |
7. Parasitic |
E. Isolated granulomatous gastritis |
8. Fungal |
|
![]() The
correlation between the histologic findings of gastritis, the
clinical picture of abdominal pain or dyspepsia, and endoscopic
findings noted on gross inspection of the gastric mucosa is poor.
Therefore, there is no typical clinical manifestation of gastritis.
The
correlation between the histologic findings of gastritis, the
clinical picture of abdominal pain or dyspepsia, and endoscopic
findings noted on gross inspection of the gastric mucosa is poor.
Therefore, there is no typical clinical manifestation of gastritis.
Clinicopathological approach to gastritis
Gastritis, simply defined as the inflammation of the gastric mucosa, is a condition, not a disease. With rare exceptions (e.g., lymphocytic gastritis and the extremely rare phlegmonous gastritis), inflammation of the gastric mucosa per se does not produce signs or symptoms; its complications do. Thus, clinicians rarely search for gastritis. In dyspeptic patients with indications for endoscopy, biopsies from the stomach are often obtained to determine the patient’s Helicobacter pylori status. If an appropriate set of gastric mucosal specimens is collected and properly examined, valuable information in addition to the often implicit question “is there H.pylori?” may be obtained: the type, severity, and distribution of gastritis and perhaps other causes of the gastric inflammation. This chapter is a discussion of useful strategies gastroenterologists and pathologists can use to optimize the diagnosis of gastric diseases.
Sydney System
The discovery of H.pylori in 1982 coincided with a new trend in medicine: the birth of the expert group, whose task is to sift through the best available evidence (hence the term evidence-based medicine) and attempt to come to a consensus with regard to treatment strategies (clinical guidelines) or classifications of disease. Before the 1990 World Congress of Gastroenterology in Sydney, Australia, such a group of European gastroenterologists and pathologists set out to create a flexible matrix for the classification of gastritis. The resulting Sydney System had both endoscopic and histological divisions. The former was met with general indifference and faded into oblivion. The latter unleashed passions that were distributed largely along geocultural lines: enthusiasm in Europe, where the system was conceived; indignation in the Americas and Asia, where investigators felt excluded both politically (none were asked to participate in the expert group) and nosologically (the types of gastritis commonly seen in Asia and South America received limited attention). In spite of these operational shortcomings, the Sydney System described a framework useful to generate diagnoses and flexible enough to incorporate new ideas as they emerged. Four years after its introduction, the Sydney System was reapprised by a group of pathologists including a wider geographic and disease representation. This group established a terminology of gastritis and identified, defined, and attempted to resolve some of the problems associated with the original Sydney System. The Houston workshop resulted in what is known as the Updated Sydney System, which is currently the most widely used and cited method for the classification of gastritis.
To create a report as suggested by the Sydney System, appropriate biopsy specimens should be methodically evaluated and the findings synthesized. Whereas gastroenterologists in some institutions have made a habit of obtaining mapped specimens from each patient who undergoes gastroscopy, others continue to take few and often topographically unidentified specimens. These diagnostic guidelines can be best followed by those gastroenterologist-pathologist teams who work together and communicate effectively.
Biopsy Protocol
To obtain adequately representative samples for the classification of gastritis, the biopsy protocol is recommended. Biopsy specimens from the three compartments (antrum, incisuraangularis, and corpus) should be separately identifiable when they are submitted to the laboratory. Proper orientation is indispensable for optimal histological evaluation; it may be accomplished either in the endoscopy suite when biopsy specimens are collected, or in the histopathology laboratory at the time of embedding. This latter option is generally preferable, unless endoscopy personnel are experienced and motivated to perform the precise and tedious work required to orientate minuscule fragments of fresh tissue properly.
To translate histopathological observations into well-defined topographic patterns, each feature is then graded using the standardized Visual Analogue Scale. The final diagnosis issued should synthesize all individual evaluations, for example, “H.pyloriantrum-predominant gastritis” or “corpus-restricted atrophic gastritis without H.pylori infection, suggestive of autoimmune gastritis.”
The Updated Sydney System is also suitable for evaluating and diagnosing the special types of gastritis. A sample diagnosis might read “lymphocytic gastritis, corpus predominant, with H.pylori infection.” In the case of gastropathies, the Updated Sydney System is mainly useful for helping in the orderly assessment of the histopathological features of the mucosa. This applies even in the unlikely situation that the system’s recommended set of five biopsy specimens is obtained from a patient with portal hypertension or watermelon stomach. However, in most such cases, attempting to grade each specimen individually is neither recommended nor necessary.
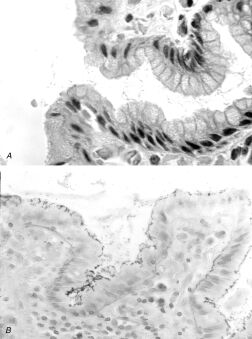
Chronic gastritis and Helicobacter pylori organisms.A. H&E stain of gastric mucosa showing surface foveolar cells, adherent mucus, and scattered bacillary forms within the mucus. B. Steiner silver stain of superficial gastric mucosa, showing abundant darkly staining microorganisms layered over the apical portion of the surface epithelium. Note that there is no tissue invasion
TOOLS TO DIAGNOSE AND CLASSIFY GASTRIC CONDITIONS
The different types of gastritides and gastropathies are characterized by various combinations of histological changes, many of which are expressions of immune, inflammatory, and adaptive responses common to several conditions. However, the presence, absence, and relative intensity of these responses provide important etiologic clues and are crucial in the categorization of the process. These histological changes can be viewed as the foundations for the terminology of gastritis, and some familiarity with them is indispensable to understand both the classification and the related manifestations of nonneoplastic gastric conditions.
Epithelial Degeneration
Surface epithelial degeneration is a nonspecific response to injury seen in all forms of gastritis. It is most conspicuous in chemical gastritis and H.pylori gastritis. In H.pylori gastritis, the intimate contact of bacteria with the surface cell membrane makes epithelial degeneration particularly prominent. Cell injury and necrosis can lead to erosions, which are seen endoscopically either as flat superficial lesions or as elevated lesions whose chronic nature is suggested by polypoid regenerative mucosa at the margins. The former are often the result of acute damage caused by drugs, bile reflux, or ischemia, whereas the latter are almost always associated with H.pylori gastritis.
Foveolar Hyperplasia
Elongation and increased tortuosity of gastric pits result from hyperplasia of the foveolar cells, a presumed adaptive response to increased cellular exfoliation from the surface epithelium. It can be viewed as a visual surrogate for increased epithelial cell turnover. Hyperplasia is accompanied by hyperchromatic nuclei and mitotic activity reaching an increased height of the pit and by other signs of cellular immaturity, such as mucin depletion and a high nucleocytoplasmic ratio. Marked foveolar hyperplasia is a prominent feature of chemical injury, but lesser degrees are commonly seen in H.pylori gastritis.
Hyperemia and Edema of the Lamina Propria
Mucosal hyperemia—often visible endoscopically—is considered to be an indicator of bile reflux gastritis, and a significant correlation has been found with the concentration of bilirubin in gastric juice. Histologically, marked edema of the lamina propria with minimal inflammation is a characteristic finding in bile gastritis.
Neutrophilic Infiltration
The presence of neutrophils characterizes the “activity” in chronic gastritis. The cause is H.pylori in most cases, but other infectious and inflammatory conditions (e.g., syphilis and Crohn’s disease) may be responsible for the persistence of neutrophils, classically associated with “acute” inflammation. Neutrophils are found in virtually every patient with H.pylori infection. The intensity of neutrophil infiltration may help to distinguish among the acute phase of infectious gastritis, Helicobacter gastritis with a particularly active component, and acute hemorrhagic gastritis resulting from chemical injuries (e.g., nonsteroidalantiinflammatory drugs [NSAIDs] or alcohol), in which inflammation is a minor component. Neutrophils disappear rapidly after successful eradication therapy; their persistence is a highly sensitive indicator of therapeutic failure.
Eosinophilic Infiltration
Rare, scattered eosinophils may be present in the gastric lamina propria of healthy persons, particularly in underprivileged populations. Prominent eosinophilic infiltration of the gastric wall either may be part of the rare eosinophilic gastroenteritis or may represent a process confined to the stomach. In either case, the cause, suspected to have an allergic basis, is not known. Eosinophils are a major component in the responses to anisakiasis and may be a constituent of the granulomata that sometimes form around fragments of the helminths remaining in the gastric wall. In adults with H.pylori gastritis, there are usually small numbers of eosinophils. In contrast, children have been reported to have a greater eosinophilic component in the H.pylori–infected gastric mucosa. After eradication of the pathogen, eosinophils may increase for some time and then decline in parallel with mononuclear cells.
Mononuclear Cell Inflammation
Infiltration of the lamina propria by lymphocytes, plasma cells, and small numbers of eosinophils and mast cells is a major feature of H.pylori gastritis, except in areas of severe atrophy and metaplasia, in which the infiltrate tends to be sparse. When lymphocytes are seen infiltrating the surface or glandular epithelium, the possibility of lymphocytic gastritis should be considered. In autoimmune gastritis, there is a diffuse infiltrate of mucosal plasma cells and lymphocytes. The latter are also present around and within oxyntic glands.
Lymphoid Follicles
Lymphoid follicles are rare in the stomach of healthy, H.pylori–free adults. When an extensive biopsy protocol is used, lymphoid follicles or aggregates are found in virtually all patients with H.pylori gastritis. In infected children and young adults, these entities may produce a distinctive nodularity in the gastric antrum, known endoscopically as follicular gastritis. H.pylori infection is the major determinant of gastric acquired mucosa-associated lymphoid tissue (MALT) and, therefore, a crucial factor in the origin of primary gastric B-cell lymphomas (MALT lymphoma).
Atrophy
Gastric atrophy is defined as the loss of appropriate glands in a given gastric compartment; that is, glands that are expected to be present in the portion of gastric mucosa under examination (e.g., oxyntic glands in the mucosa of the corpus) have been replaced by tissues not normally found there. More recently, the narrower definition of loss of specialized cells has been proposed. Whenever the gastric mucosa is damaged, irrespective of the mechanism or cause, it may either regenerate or return to normal (restitutio ad integrum), or it may undergo adaptive reparative processes leading to the replacement of the native mucosa with other structures. Destroyed native glands may be replaced by fibroblasts and extracellular matrix, by glands of “pyloric” appearance ( pseudopyloric metaplasia), or by an intestinal-type epithelium ( intestinal metaplasia). During chronic H.pylori infection, all these types of repair occur, the respective proportion of each probably modulated by environmental, genetic, and bacterial factors. Widespread atrophy also occurs in autoimmune gastritis, as a consequence of the immune-mediated glandular destruction of the oxyntic mucosa. Atrophic foci found in stomachs with evidence of chemical gastropathy are probably the result of ulcer repair.
