- •In this chapter we will:
- •Structure of dna 195
- •Discovering the Structure of dna
- •Information, is a double helical molecule.
- •Polymerase Chain Reaction (pcr)
- •Replication of dna 201
- •Ames Test
- •One Gene—One Polypeptide
- •Expression of genetic information 207
- •212 Chapter 7 microbial genetics: replication and expression of genetic information
Understanding
the mechanism of DNA replication has enabled scientists to develop a
method for replicating segments of DNA in the laboratory from
slight traces that otherwise might be too small to analyze. The
method, called polymerase chain reaction (PCR), has enormous
practical importance because it allows rapid amplification of trace
DNA by making many additional copies through replication of specific
DNA sequences that can then be detected with great sensitivity (see
Figure). This permits the detection of even rare genes.
Already, PCR has permitted extremely sensitive detection of the
AIDS-causing virus in blood, which is essential for the protection
of the blood supply. The impact on microbiology and molecular
biology is enormous and PCR has become one of the most widely used
methods in science. In recognition of the importance of PCR, the
1993 Nobel Prize in chemistry was awarded to Kerry Mullis, who
discovered this method.
PCR
is based on the following facts about DNA replication: DNA serves as
a template for its own replication; the DNA double helix
separates into two chains for replication; a pool of free
nucleotides provides the nucleotides for the synthesis of new
chains; DNA polymerase catalyzes the formation of the new
chains; DNA polymerase adds only to the 3'-free OH end of a
nucleotide chain; and DNA polymerase requires a short chain of
nucleotides (oligonucleotide) to serve as a primer to initiate DNA
replication.
To
accomplish the replication of DNA outside of living cells by
using PCR, a source of template DNA is added along with a pool of
free nucleotides and a DNA polymerase. Also added are short
oligonucleotide primers that are complementary to the nucleotide
sequences flanking the region of the DNA that is to be
replicated. These primers define the region of DNA that is
replicated by providing the 3'-OH free ends onto which the DNA
polymerase can add nucleotides.
PCR
procedure uses heat to provide energy for breaking the hydrogen
bonds to separate the chains of the DNA double helix. Heating to 95°
C will break the hydrogen bonds without breaking the covalent bonds
that link the nucleotides in the chains. Once the chains are
separated the reaction is cooled, for example, to 40° C, which
allows hydrogen bonds to form between the oligonucleotide primers
and their complementary regions of the template DNA. The
temperature is then raised to approximately 72° C to allow the DNA
polymerase to quickly add nucleotides.
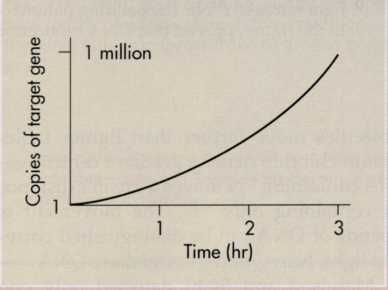
The
polymerase chain reaction (PCR) is an in
vitro
method for replicating DNA. A target nucleotide sequence is copied
repeatedly so that a million copies can be made in less than an
hour.
The
DNA polymerase used in PCR, called Taq
polymerase, comes from a bacterium—Thermus
aquaticus—
that lives in hot springs; it is not denatured at high
temperatures. Thus this DNA polymerase can withstand repeated
exposure to 95° C. This is critical because in PCR the temperature
is repeatedly cycled to separate the chains of the DNA double helix,
to bind the primers to the template DNA, and to allow the DNA
polymerase to synthesize new strands. Each cycle lasts only a few
minutes. The effect of repeated cycling is to exponentially
increase the number of copies of a defined segment of the DNA.
Within an hour a single copy of a gene can be amplified to a million
copies. PCR technology has applications for research and
diagnosis and is fast becoming a standard procedure in biotechnology
and medical diagnostic laboratories.
200
CHAPTER
7 MICROBIAL GENETICS: REPLICATION AND EXPRESSION OF GENETIC
INFORMATION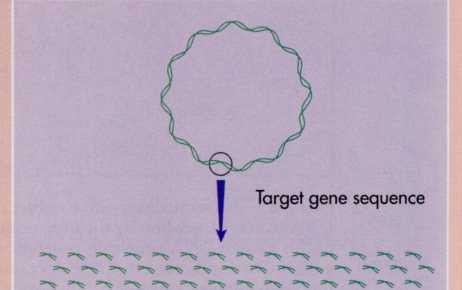

Polymerase Chain Reaction (pcr)
FIG.
7-9 During
DNA replication, enzymes separate the two strands of DNA in a
localized region called the replication fork. At this site, new
nucleotides align opposite base pairs and new strands of DNA are
synthesized.
one
site, with two replication forks moving from the initiation site in
opposite directions around the circular bacterial chromosome.
As the replication forks move around the bacterial chromosome, an
enzyme—DNA
gyrase—twists
the DNA. This enzyme is unique to bacteria and hence a potential
site for the action of an antimicrobial agent. In fact, a new class
of antibacterial agents, the quinolones,
have
been discovered that interfere with DNA gyrase. By preventing
the formation of replication forks in bacterial cells, quinolones
block bacterial reproduction and, hence, can be used to treat
bacterial infections. The quinolone ciprofloxacin, for example, is
useful in treating Pseudomonas
infections.
DNA
gyrase untwists the DNA
of
the bacterial chromosome.
Quinolones
are antibacterial agents that inhibit DNA
gyrase.
Formation
of a New Chain of Nucleotides—DNA Polymerase
Free
nucleotides within the cell in association with DNA polymerase are
positioned opposite their complementary nucleotides in the
template. This process of aligning [ə'laɪnɪŋ]
complementary nucleotides (A opposite T and C opposite G) is
called base pairing['pɛə(r)ɪŋ].
The order of
the
nucleotides is specified by the template DNA. After the
nucleotides are aligned by base pairing ['pɛə(r)ɪŋ],
an enzyme called DNA
polymerase
links
the nucleotides by forming phosphodiester bonds. The action of DNA
polymerase can be likened ['laɪk(ə)n]
to a zipper ['zɪpə]
where the teeth of the zipper are initially aligned and
progressively linked together in a continuous motion.
DNA
polymeras adds nucleotides to the free 3'-OH end of an existing
nucleotide ['n(y)o͞oklēəˌtīd]
chain of nucleotides (FIG. 7-10). Because DNA polymerase adds
nucleotides only to the 3'-OH free end, the direction of DNA
synthesis is 5'-P —3'-OH.
Since the two chains of the double helical DNA molecule are
antiparallel (one running from the 5'-P —> 3'-OH free end
and the other running from the 3'-OH —» 5'-P free end) this
indicates that the synthesis of the two complementary DNA chains
must proceed in opposite directions.
One
DNA chain can be continuously synthesized. It is the chain that runs
in the appropriate direction for the continuous addition of new free
nucleotides to the free 3'-OH end. This is the continuous
or
leading
strand of DNA.
Its
synthesis occurs simultaneously with the unwinding of the
double helical molecule and progresses toward the replication fork.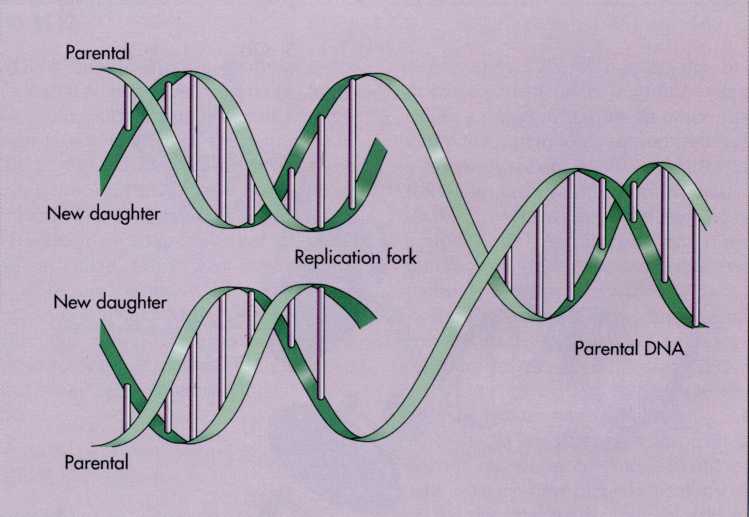
Replication of dna 201
FIG.
7-10 DNA polymerases add nucleotides only to the 3'-OH ends of the
newly synthesized DNA polynucleotide chains. One chain is
elongated continuously along the direction of formation of the
replication fork. The other strand is synthesized as discontinuous
segments (Okazaki fragments) that are then joined together by DNA
ligase.
The
other strand of DNA, however, cannot be synthesized
continuously. This is because it runs 3'-OH to 5'-P but DNA
polymerase only adds nucleotides in the 5'-P to 3'-OH direction. The
initiation of its synthesis can begin only after the double
helix has undergone some unwinding. Synthesis of this strand
involves formation of short DNA fragments (called Okazaki
fragments after the husband and wife team that discovered them) in
the direction opposite the direction in which the parent DNA
unwinds. Because it is synthesized discontinuously and only after
synthesis of the continuous strand has begun, it is called
the
discontinuous
or
lagging
strand of DNA. The
short
DNA fragments of the discontinuous strand are joined together by
enzymes called ligases.
The
combined action of DNA polymerase and DNA ligase, thus,
accomplishes the synthesis of both complementary strands of DNA
during replication.
To
make a complementary copy of DNA, the double helix is pulled [pul]
apart to form a replication fork, complementary nucleotides are
aligned by base pairing, and phosphodiester linkages are formed by
DNA polymerase.
202
CHAPTER
7 MICROBIAL GENETICS: REPLICATION AND EXPRESSION OF GENETIC
INFORMATION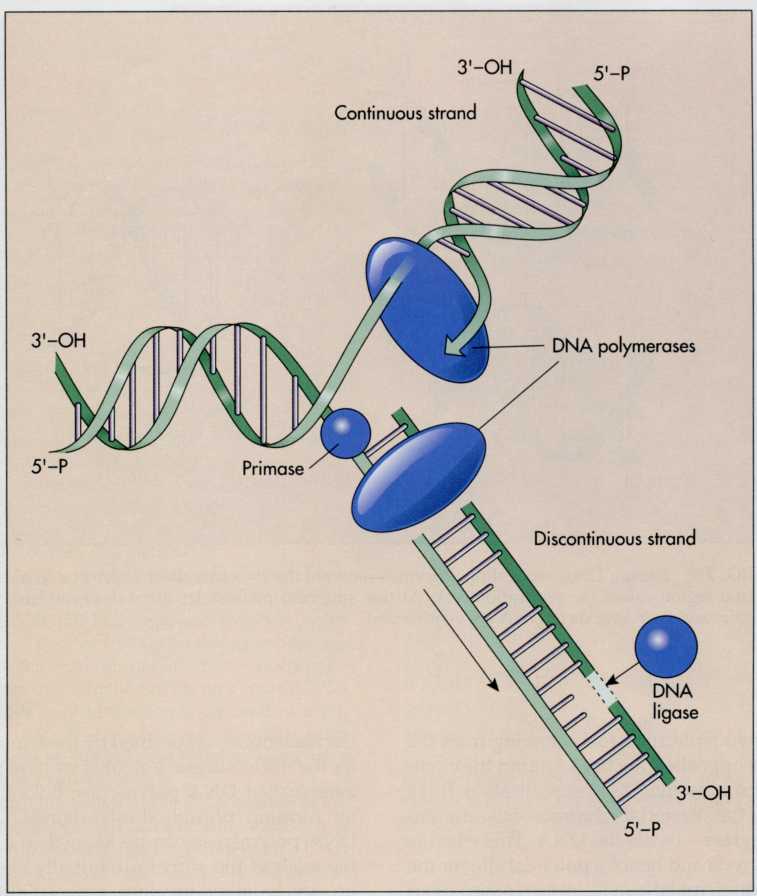
MUTATIONS
Replication
of DNA should always produce exact copies of the hereditary
information. Errors, however, sometimes occur. Such errors are
called mutations. A mutation
is any
change in the sequence of nucleotides within DNA. Mutations can
involve the addition, deletion, or substitution of nucleotides. Even
a simple change, such as the deletion or addition of a single
nucleotide, can greatly alter the characteristics of an
organism. Once they occur, these changes in the DNA are heritable
and are passed from one generation to the next. Mutations introduce
genetic variability that makes evolutionary change possible. They
also sometimes increase the virulence of pathogens and make some
microorganisms resistant to antibiotics.
Mutations
are stable heritable changes in the nucleotide sequences of
DNA.
Types
of Mutations
There
are several types of mutations (FIG. 7-11). One type of mutation,
base
substitution,
occurs
when one pair of nucleotide bases in the DNA is replaced by another
pair of nucleotides. A deletion
mutation
involves
removal of one or more nucleotide base pairs from the DNA. An
insertion
mutation
involves
the addition of one or more base pairs. Even though they may
represent minor changes in the sequence of nucleotides, mutations
can have major effects, sometimes proving lethal to the progeny
(offspring or descendants) of the organism.
Sometimes
a mutation results in the death of the microorganism or its
inability to reproduce. This is called a lethal
mutation.
In other
cases, the mutation alters the nutritional requirements for the
progeny of a microorganism. Such a mutation is called a nutritional
mutation.
Often,
nutritional mutants will be unable to synthesize essential
biochemicals, such as amino acids. Auxotrophs
are
nutritional mutants that require growth factors that are not needed
by the parent (prototroph)
strain.
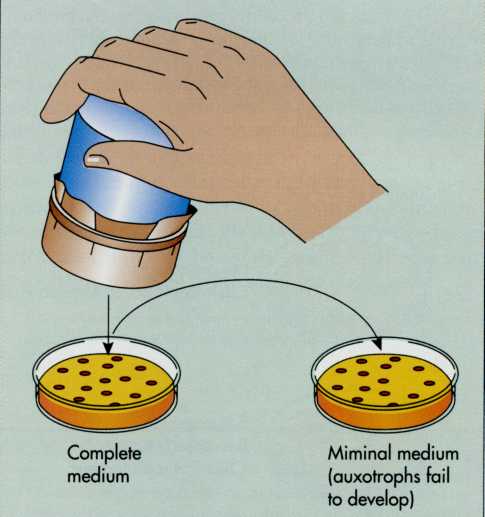
Replica
plating
is a
method frequently used to detect auxotrophs (FIG. 7-12). In
this method, bacterial cells are grown on a master plate and then
transferred to sterile plates by repeatedly stamping a pad over
the master plate and pressing the pad into plates with media of
differing composition. The distribution of microbial colonies
should be replicated exactly on each new plate. If a colony is
unable to grow on the minimal media, which lacks a specific growth
factor, but will grow on the complete medium, this indicates that
nutritional mutants, or auxotrophs, are occurring. This method
allows an investigator to screen a large number of bacteria for
mutations.
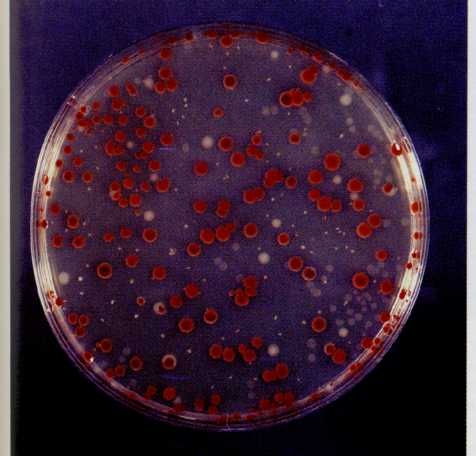
FIG,
7-11 Plate showing growth of Serratia
marcescens. The
wild type colonies are red and the mutant colonies are
gray.
FIG.
7-12 Replica
plating is used to identify mutants by transferring identical
colonies to different types of media and comparing the colonies that
develop on the respective plates. This method is critical in
identifying auxotrophic mutants. All colonies develop on a complete
medium that satisfies the nutritional needs of both the parental and
mutant strains. Colonies of the auxotrophic mutant fail to
develop on a minimal medium lacking the specific nutritional
growth factors required by the mutant.
203