
- •Iron triad physical properties
- •Iron triad trends
- •History Of Discovery
- •Iron (Ferrum). Cobalt (Cebafttim). Nickel (Niccolum)
- •Production
- •Chemical Properties Free elements
- •Iron(II) Compounds
- •Cobalt (II) compounds
- •Iron (III) compounds
- •Complexes of cobalt
- •Coordination compounds of nickel
- •Tests for iron triad elements
- •Themes for home preparation
- •Questions and tasks
- •Iron, cobalt, nickel
- •12. How is potassium ferrate obtained? How does it react with sulfuric acid? Give the equations of the relevant reactions Quiz problems
- •А. FeCl2
- •Make up the equations o f the reactions Make up the equations o f the reactions
- •Experimental section
- •2. Chemical properties of iron
- •3. Chemical properties of cobalt
- •4. Chemical properties of nickel
Coordination compounds of nickel
Nickel(II) forms a great variety of coordination compounds, in which there may be either six ligands (octahedral or distorted octahedral), five ligands (square pyramidal or trigonal biprism) or four (tetrahedral or square planar), and which may be cationic, neutral or anionic. The simple hydrated cation [Ni(H2O)6]2+ is octahedral; addition of concentrated aqueous ammonia in excess to an aqueous solution of a nickel(II) salt gives the purple octahedral complex [Ni(NH3)6]2+ by replacement of the water ligands ; this forms sparingly soluble salts with some anions, for example Br-. The scarlet-coloured coordination compounds formed when dimethylglyoxime is added to a nickel(II) solution is a neutral planar coordination compounds:
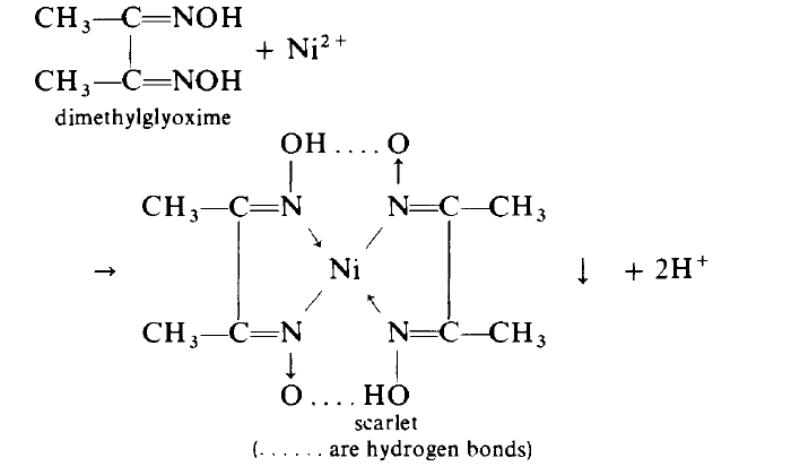
If nickel(II) cyanide, Ni(CN)2, is dissolved in excess potassium cyanide, the orange-red complex salt K2Ni(CN)4.H2O can be crystallized out; this contains the stable square-planar [Ni(CN)4]2– anion.
Compounds IV
Oxide and fluoride can stabilize Co4+ derivatives, e.g. caesium hexafluorocobaltate (Cs2CoF6)) and potassium percobaltate (K3CoO4).
Compounds VI
Iron. As might be expected, these higher oxidation states are found almost exclusively in the anionic form, and are produced only under strongly oxidising conditions. Alkali metal ferrates(VI), for example K2FeO4, are obtained by oxidation of a suspension of hydrous iron(III) oxide (assumed to be Fe(OH)3 in the equation below) by chlorate(I) in concentrated alkali:
2Fe(OH)3 + 3ClO– + 4OH = 2FeO42– + 3C1- + 5H2O
The deep red FeO42- is stable only in alkali; in acid, iron(III) is produced:
2 FeO42– + 10H+ =2Fe3 + (aq) + 5H2O + O2
Ferrate(VI) has powerful oxidizing properties, for example ammonia is oxidized to nitrogen. Potassium ferrate(VI) is isomorphous with potassium chromate(VI), and both anions are tetrahedral. Decomposition of potassium ferrate(VI) at 1000 K gives a ferrate(V), K3FeO4, and several types of ferrate(IV), for example FeO32–, FeO44– are known; these ferrates(IV) have no solution chemistry and are probably best regarded as mixed oxides, since the FeO44– ion has no identifiable structure.
Tests for iron triad elements
TESTS FOR IRON
Reagent
|
Fe2+ |
Fe3+
|
Ammonia or sodium hydroxide (hydroxyl ions)
|
Green precipitate. Turns brown on exposure to air |
Red-brown precipitate
|
Potassium hexacyanoferrate(II), K4[Fe(CN)6]
|
White precipitate, rapidly turning blue |
Prussian blue precipitate
|
Potassium hexacyanoferrate(III), K3[Fe(CN)6]
|
Dark blue precipitate (Turnbull's blue)
|
Reddish-brown coloration (no precipitate)
|
Potassium thiocyanate, KCNS
|
No coloration*
|
Blood red coloration
|
*This test is extremely sensitive and usually sufficient Fe3+ ions are present in an iron(II) salt to give some coloration. The blood red colour appears to be due to a complex.
TESTS FOR COBALT
For a cobalt(II) salt the precipitation of the blue-pink cobalt(II) hydroxide by alkali, or precipitation of black cobalt(II) sulfide by hydrogen sulfide provide useful tests; the hydroxide is soluble in excess alkali and is oxidised by air to the brown CoO(OH). Addition of excess potassium nitrite acidified with acetic acid gives a precipitate of the potassium hexanitrocobaltate(III), K3[Co(NO2)6].
Decomposition of most cobalt(III) complexes by boiling with alkali gives a brown precipitate of the hydrated oxide Co2O3.H2O. This will quantitatively oxidise iodide to iodine.
TESTS FOR NICKEL
The reactions of aqueous solutions of nickel(II) salts with hydroxide ions, with excess ammonia, with sulfide ion and with dimethylglyoxime all provide useful tests for nickel(II) ions.
