
- •Iron triad physical properties
- •Iron triad trends
- •History Of Discovery
- •Iron (Ferrum). Cobalt (Cebafttim). Nickel (Niccolum)
- •Production
- •Chemical Properties Free elements
- •Iron(II) Compounds
- •Cobalt (II) compounds
- •Iron (III) compounds
- •Complexes of cobalt
- •Coordination compounds of nickel
- •Tests for iron triad elements
- •Themes for home preparation
- •Questions and tasks
- •Iron, cobalt, nickel
- •12. How is potassium ferrate obtained? How does it react with sulfuric acid? Give the equations of the relevant reactions Quiz problems
- •А. FeCl2
- •Make up the equations o f the reactions Make up the equations o f the reactions
- •Experimental section
- •2. Chemical properties of iron
- •3. Chemical properties of cobalt
- •4. Chemical properties of nickel
Iron (III) compounds
Iron. In this state, iron has five d electrons, but does not show any strong resemblance to manganese(II), except that most iron(III) compounds show high paramagnetism, i.e. its outermost shell electrons remain unpaired.
Iron(III) chloride is a black, essentially covalent solid, in which each iron atom is surrounded octahedrally by six chlorine atoms. It is prepared by direct combination of iron with chlorine or by dehydration of the hydrated chloride.
When the anhydrous solid is heated, it vaporises to form first Fe2Cl6 molecules, then the monomer FeCl3 and finally FeCl2 and chlorine. It fumes in air (with hydrolysis) and dissolves readily in water to give a yellow (dilute) or brown (concentrated) solution, which is strongly acidic. Crystallisation gives the yellow hydrate FeCl3.6H2O which has the structure [FeCl2(H2O)4]Cl2H2O, i.e. contains the octahedral complex ion [FeCl2(H2O)4]+; ions of this general type are responsible for the colours of the aqueous solution of iron(III) chloride. In the presence of excess chloride ion, both tetrahedral [FeCl4]- and octahedral [FeCl6]3- can be formed.
Iron(III) chloride forms numerous addition compounds, especially with organic molecules which contain donor atoms, for example ethers, alcohols, aldehydes, ketones and amines. Anhydrous iron(III) chloride is soluble in, for example, ether, and can be extracted into this solvent from water; the extraction is more effective in the presence of chloride ion.
Of other iron(III) halides, iron(III) bromide and iron(III) iodide decompose rather readily into the +2 halide and halogen.
Iron(III) sulfate Fe2(SO4)3 forms very hygroscopic white crystals. It forms the crystal hydrate Fe2(SO4)3·9H2O (yellow crystals). Iron(III) sulfate is greatly hydrolyzed In aqueous solutions, it forms double salts—alums— with alkali metal and ammonium sulfates. An example is ammonium iron alum (NH4)Fe(SO4)2·12H2O—light violet crystals well soluble in water. When roasted to above 500 °C, iron(III) sulfate decomposes as follows:
Fe2(SO4)3 = Fe2O3 + 3SO3↑.
Iron(III) sulfate is employed, like FeCl3, as a coagulant in water purification, and also for pickling metals. A solution of Fe2(SO4)3 can dissolve Cu2S and CuS to form copper(II) sulfate; this property is used in the hydrometallurgical preparation of copper.
When alkalis react with solutions of iron(III) salts, red-brown iron (III) hydroxide Fe(OH)3 precipitates. It is insoluble in an excess of the alkali.
Iron(III) oxides and hydroxide. If an aqueous solution of an iron(III) salt is treated with alkali, a red-brown precipitate of Iron(III) hydroxide' is obtained; this is probably best represented as FeO(OH). At strong heating it gives the red oxide Fe2O3. Iron(III) oxide occurs naturally as haematite, and can also be prepared by strong heating of iron(II) sulfate:
2FeSO4 = Fe2O3 + SO2 +SO3,
It shows some amphoteric behaviour, since it dissolves in alkali (concentrated aqueous or fused) to give a ferrate(III) ; the equation may be written as
Fe2O3 + 2OH- = 2FeO2- + H2O
Iron(II) oxide exists in two forms, the red a-form (paramagnetic) and the y-form (ferromagnetic) obtained by careful heating of FeO(OH). The -form is used as a red pigment, as a metal polish ("jeweller's rouge') and as a catalyst.
The mixed oxide Fe3O4 (triiron tetroxide) is a black solid, which occurs naturally as magnetite; it is formed when iron(III) oxide is strongly heated, and its structure is effectively made up of oxide (O2-) and iron(II) and iron(III) ions.
Ferrites. When iron(III) oxide is fused with sodium or potassium carbonates, ferrites are formed—salts of ferrous acid HFeO2 that has not been obtained in the free state, for instance sodium ferrite NaFeO2:
Fe2O3 + Na2CO3 = 2NaFeO2+ C02↑.
In engineering, the name ferrites or ferrite materials is applied to the products obtained in sintering powders of iron(III) oxide and oxides of some divalent metals, for instance, nickel, zinc, and manganese. Sintering is conducted at 1000 to 1400 °C. Ferrites have valuable magnetic properties and high electrical resistance, which underlies the small value of the electrical losses in them. Ferrites find broad application in communication equipment, computers, and in automatic and remote, control equipment.
Cobalt (III) compounds
The simple compounds of cobalt(III) are strongly oxidising:
[Co(H2O)6]3+ +e- = [Co(H2O)6]2 +, E0 = +1.92 V.
and hence the simple cobalt(III) cation cannot exist in aqueous solution (which it would oxidise to oxygen). However, the chemistry of cobalt is known for the ease of forming complexes, and for the big effect which complex formation has on relative stabilities of the + 2 and + 3 states.
As already noted, the simple salts in this oxidation state are powerful oxidizing agents and oxidise water. Since, Co(III) would also oxidise any halide except fluoride to halogen, the only simple halide salt is CoF3, Cobalt(III) fluoride, obtained by reaction of fluorine with cobalt(II) fluoride can be used in some fluorination reactions and reacts vigorously with water.
Cobalt(III) oxide is obtained as a brown precipitate Co2O3 when cobalt(II) hydroxide is oxidised in alkaline conditions (or when a cobalt(III) is decomposed by aqueous alkali). On heating it gives the black mixed oxide Co3O4.
Hydrated cobalt(III) sulfate, Co2(SO4)3.18H2O is obtained when cobalt(II) sulfate is oxidised electrolytically in moderately concentrated sulfuric acid solution: it is stable when dry but liberates oxygen from water. Some alums, for example KCo(SO4)2.12H,O can be obtained by crystallisation from sulfuric acid solutions. In these and the sulfate, the cation [Co(H2O)6]3+ may exist; it is both acidic and strongly oxidizing.
Cobalt(III) nitrate Co(NO3)3 has been prepared by the reaction of dinitrogen pentoxide with cobalt(III) fluoride.
Nickel (III) compounds
The number of these compounds is limited. Nickel(III) oxide (Ni2O3) has been referred to in the literature but is not a well defined compound. The substance black nickel oxide is sometimes described as being Ni2O3 however the composition has a nickel content of around 77% by weight whereas Ni2O3 would have 70.98% Ni by weight. So it may be non-stoichiometric NiO.
Nickel oxide hydroxide. The related nickel oxide hydroxide (NiOOH) can be prepared by reaction of nickel(II) chloride with sodium hypochlorite. NiOOH is used as the cathode in many rechargeable batteries, including nickel-cadmium, nickel-iron, nickel hydrogen, and nickel-metal hydride.
COMPLEXES
COMPLEXES OF IRON
Iron(III) readily forms octahedral complexes. The hexaaquo-ion [Fe(H2O)6]3+ is found only in a few solid hydrated salts (or in their acidified solutions), for example Fe2(SO4)3.9H2O, Fe(ClO4)3.10H2O. In many other salts, the anion may form a complex with the iron(III) and produce a consequent colour change. For example, iron(III) chloride hydrate or solution. Stable anionic complexes are formed with a number of ions, for example with oxalate, C2O42–, and cyanide. The redox potential of the iron(II) – iron (III) system is altered by complex formation with each of these ligands; indeed, the hexacyanoferrate(III) ion, [Fe(CN)6]3–, is most readily obtained by oxidation of the corresponding iron(II) complex, because
[Fe(H2O)6]3+ + e– [Fe(H2O)6]2+, E0 = +0.77 V,
[Fe(CN)6]3– + e– [Fe(CN)6]4–, E0 = +0.36 V.
The thiocyanate ion SCN- forms an intensely red-coloured complex (most simply represented as [Fe(SCN)(H2O)5]2+) which is a test for iron(III). However, unlike cobalt(III), iron(III) does not form stable hexammines in aqueous solution, although salts containing the ion [Fe(NH3)6]3+ can be obtained by dissolving anhydrous iron(III) salts in liquid ammonia.
As for the +3 state, iron(II) forms a variety of complexes which are usually 6-coordinate and octahedral. Replacement of the water ligands in green [Fe(H2O)6]2+ by ammonia molecules is incomplete in aqueous ammonia, but reaction of the anhydrous chloride with gaseous or liquid ammonia gives the complex [Fe(NH3)6]Cl2. The water ligands are more easily replaced by cyanide ions to give the hexacyanoferrate(II) ion, [Fe(CN)6]4– Many salts of this ion are known, for example, the soluble yellow hydrate K4[Fe(CN)6].3H2O, and the insoluble brown copper(II) salt Cu2[Fe(CN)6].
The reaction between aqueous Fe3+ ions and [Fe(CN)6]4– yields an intense blue precipitate, prussian blue, which is iron(III) hexacyanoferrate( II), Fe4[Fe(CN)6]3; the same material, called Turnbull’s blue is obtained by addition of Fe2+ (aq.) ions to [Fe(CN)6]3+ ions. The intense colour of this compound is due to charge-transfer. The formation of [Fe(CN)6]4– ions causes the iron(II) to change its properties (for example it is not precipitated as the hydroxide with alkali or as the sulfide with S2- ions); it is more readily oxidised to the +3 state, since
[Fe(CN)6]3–(aq) + e– [Fe(CN)6]4–(aq); E0 = +0.36 V,
When concentrated sulfuric acid is added to a nitrate in the presence of aqueous iron(II) sulfate, the liberated nitrogen oxide forms a brown complex [Fe(H2O)5NO]2+ which appears as a "brown ring' at the acid-aqueous interface.
Perhaps the most important complex of iron(II) is heme (or haeme). Haemoglobin, the iron-containing constituent of the blood, consists essentially of a protein, globin, attached through a nitrogen atom at one coordination position of an octahedral complex of iron(II). Of the other five coordination positions, four (in a plane) are occupied by nitrogen atoms, each of which is part of an organic ring system—the whole system is a porphin. The sixth position (see Figure below), is occupied either by an oxygen molecule or a water molecule, and here reversible oxygen uptake can occur, as shown, thereby enabling oxygen to be transported from one part of the body to another. Coordination of a ligand CN or CO instead of water prevents this process, and the toxicity of cyanide or carbon monoxide is, in part due to this fact.
Fig.
Schematic representantiom of haem (porphin rings not shown)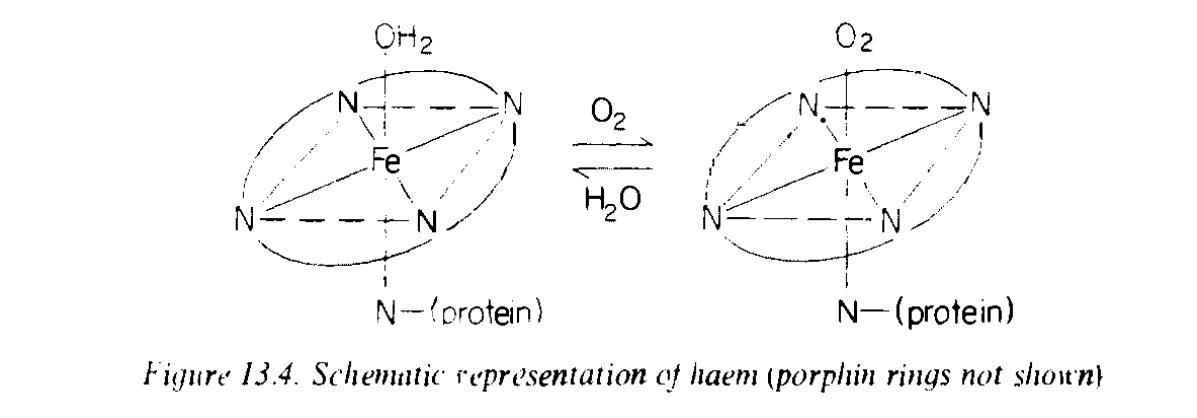

Fe(ІІ), Co(ІІ), and Ni(ІІ) form very interesting coordination compounds with cyclopentadienyl, С5Н5. The first compound prepared was the compound of Fe(ІІ) by the reaction:
FeCl2 + 2NaC5H5 Fe(C5H5)2 + 2NaCl,
that was performed in diethylether medium. This compound is called ferrocene.

The general name metallocene is derived from ferrocene, (C5H5)2Fe or Cp2Fe, systematically named bis(η5-cyclopentadienyl)iron(II). Thus, metallocenes are sometimes referred to as sandwich compounds.
