
- •Iron triad physical properties
- •Iron triad trends
- •History Of Discovery
- •Iron (Ferrum). Cobalt (Cebafttim). Nickel (Niccolum)
- •Production
- •Chemical Properties Free elements
- •Iron(II) Compounds
- •Cobalt (II) compounds
- •Iron (III) compounds
- •Complexes of cobalt
- •Coordination compounds of nickel
- •Tests for iron triad elements
- •Themes for home preparation
- •Questions and tasks
- •Iron, cobalt, nickel
- •12. How is potassium ferrate obtained? How does it react with sulfuric acid? Give the equations of the relevant reactions Quiz problems
- •А. FeCl2
- •Make up the equations o f the reactions Make up the equations o f the reactions
- •Experimental section
- •2. Chemical properties of iron
- •3. Chemical properties of cobalt
- •4. Chemical properties of nickel
Chemical Properties Free elements
I ron.
Iron
combines with many non-metals at
when
heated forming oxides: Fe2O3
and Fe3O4
(when heated in air above 430 K). Steam produces with iron Fe3O4
and hydrogen. Iron is dissolved in dilute non-oxidant acids, giving
iron(II) solutions, e. g.:
ron.
Iron
combines with many non-metals at
when
heated forming oxides: Fe2O3
and Fe3O4
(when heated in air above 430 K). Steam produces with iron Fe3O4
and hydrogen. Iron is dissolved in dilute non-oxidant acids, giving
iron(II) solutions, e. g.:
Fe
+ 2H+(aq)![]() Fe2+(aq)
+ H2.
Fe2+(aq)
+ H2.
This follows from the value of E0 for the half-reaction:
Fe2+(aq) + 2e Fe(s), E0 = –0.44 V.
The impurities in ordinary iron provide fast dissolution of iron in acids. These impurities are responsible for the characteristic smell of hydrogen from this source.
In dilute nitric acid, ammonium nitrate is formed:
4Fe + 10H+ + NO3- 4Fe2+ + NH4+ + 3H2O.
Concentrated nitric acid renders the metal passive, i.e. chemically inactive, due to formation of a thin oxide surface film (which can be removed by scratching or heating in hydrogen). Iron is a good reducing agent (see the value of E° above): it reduces some cations to the metal (for example, copper) in aqueous solution, giving iron (II). Iron absorbs hydrogen readily and is a hydrogenation catalyst.
Hydrochloric acid of any concentration dissolves iron:
Fe + 2HC1 = FeCl2 + H2↑.
It is dissolved in dilute sulfuric acid similarly:
Fe + H2SO4 = FeSO4+H2.
In concentrated sulfuric acid solutions, iron is oxidized to iron(III):
2Fe + 6H2SO4 = Fe2(SO4)3 + 3SO2 ↑ + 6H2O.
Although in sulfuric acid, whose concentration is close to 100%, iron becomes passive and virtually no reaction occurs.
Iron dissolves in dilute and moderately concentrated solutions of nitric acid:
Fe + 4HNO3 = Fe(NO3)3 + NO ↑ + 2H2O.
At high concentrations of HNO3 dissolving retards and iron becomes passive.
Cobalt is similar to iron chemically; when heated in the air it gives oxides Co3O4 and CoO, but it is less actively attacked by dilute acids. Cobalt(II) halides are formed with halogens, excepting fluorine (CoF3 is obtained). Like iron and the next transition element, nickel, cobalt is not generally found in any oxidation state above +3, and this and +2 are the usual states.
Nickel is attacked by dilute aqueous acids but not by alkalis; it combines readily with many non-metals when heated.
Scheme of chemical properties of Fe triad elements
Low oxidation states of iron triad elements
Oxidation state +2
Oxidation state +3
Oxidation state +6
Electrode potentials
Low oxidation states of iron triad elements
If the coordination compound of Ni+2 K2[Ni(CN)4] is dissolved in liquid ammonia, the addition of potassium produces yellow K4[Ni(CN)4]. The [Ni(CN)4]4- ion contains nickel in oxidation state 0. It is believed to be tetrahedral.
Carbonyls. Carbonyls Fe(CO)5, Co2(CO)8, and Ni(CO)4 are during in the action of СО on fine powders of metals. СО reaction with Ni proceeds under the mildest conditions: at atmospheric pressure, t=50—80С. Fe and Co react with СО at 150—200 С and pressure 10 and 25 МPа, respectively.
All carbonyls are diamagnetics, since СО molecules are strong field ligands whereupon valence d-electrons of a central ion of metal are paired. These unshared electron pairs of the central ion form -bonds with CO molecules; -bonds are formed due to unshared electron pairs of CO molecules and free orbital’s of the central ion. If an element has a single electron when all other electrons are paired, it means that the formation of binuclear cluster coordination compound is expected.
π-bond σ-bond
dsp3-hybridisation |
|
|
π-bond σ-bond
sp3-hybridisation |
|
|
|
|
Fe(CO)5 is a yellow liquid, tm. = –20 С, tb. = +103 С;
Со2(CO)8 is a оrange crystalline solid, tm.= +51 С;
Ni(CO)4 is a colorless liquid, tm. = –19 С, tb. = 43 С.
Carbonyls are inflammable substances that ignite easily in the air forming metal oxide (for instance, Fe2O3) and СО2. Heating carbonyls at atmospheric pressure without air leads to their decomposition. Products of the reaction in this case are pure metal and CO and thereby this is an industrial process of fine powders of Fe, Co, and Ni of high purity production.
Fe(CO)5 reacts with alkalis in alcoholic solutions:
Fe(CO)5 + 4KOH = K2[Fe(CO)4] + K2CO3 +2H2O.
Сompounds М2[Fe(CO)4] are close to salts. Even the acid that corresponds to this salt can be obtained:
Fe(CO)5 + Ba(OH)2 BaCO3 + H2[Fe(CO)4].
Carbonyl Со2(CO)8 can be also reduced: carbonyl-hydride is formed under the action of hydrogen:
Со2(CO)8 + Н2 2Н[Со(CO)4].
This acid reacts with alkalis forming salts where oxidation state of cobalt is -1, forming K[Со(CO)4].
Iron. Iron forms the carbonyls Fe(CO)5, Fe2(CO)9 and Fe3(CO)12. In iron pentacarbonyl the iron(0) is 5-coordinated, as shown in the Figure below to give a trigonal bipyramid; Donation of an electron pair by each CO ligand gives the configuration of the nearest noble gas to iron of the coordination compound. Therefore, the [Fe(CO)4]2- ion and some octahedral carbonyl halides Fe(CO)4X2 (X – Cl, Br, I) are known.
Figure. a
trigonal bipyramid
of iron
pentacarbonyl, Fe(CO)5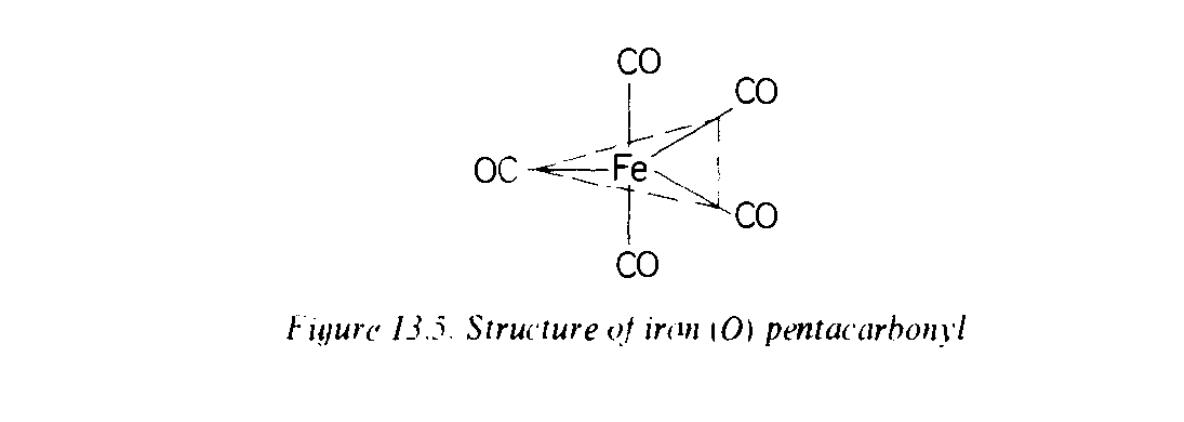
Cobalt. Cobalt(0) has an odd number of electrons, and forms no simple carbonyl in this oxidation state. However, carbonyls of formulae Co2(CO)8, Co4(CO)12 and Co6(CO)16 are known. Co2(CO)8 is important catalyst for organic syntheses. In the so-called oxo reaction, where an alkene reacts with carbon monoxide and hydrogen, under pressure, to give an aldehyde, dicobalt octacarbonyl is used as catalyst:
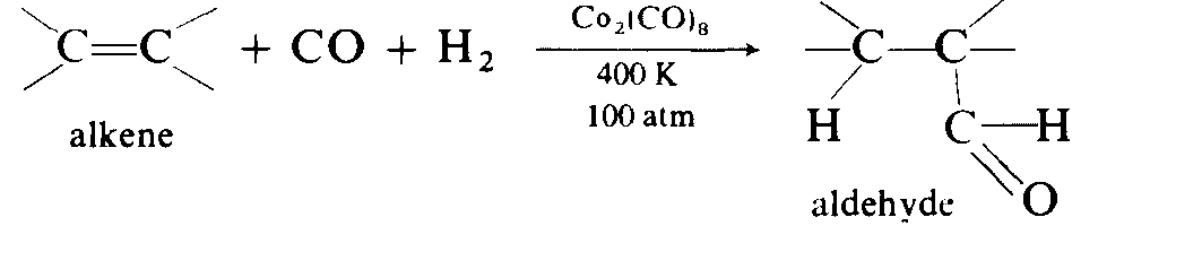
Nickel. Nickel tetracarbonyl Ni(CO)4 was the first metal carbonyl to be discovered, by Mond in 1890. It is obtained by passing of carbon monoxide over nickel metal heated to 320 K. It is a volatile, toxic liquid (b.p. 315 K), and has a tetrahedral structure. It has considerable stability, but inflames in the air; More systematically named butanedione dioxime. there is some double bonding between the nickel and carbon atoms, e.g.
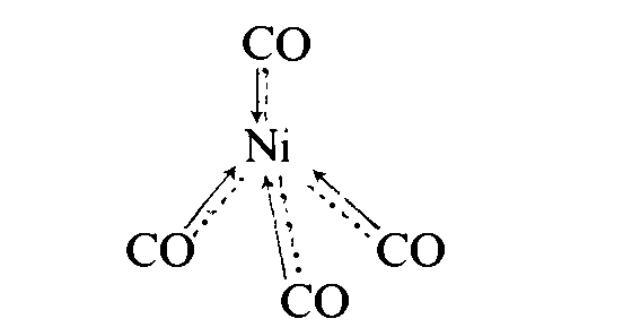 or
or

Compounds +2
Oxides FeO, СоО і NiO can be prepared as follows:
Fe2O3
+ Н2
![]() 2FeO + H2O,
2FeO + H2O,
FeC2O4 FeO + CO + CO2.
All oxides are unstable against oxygen attack:
4FeO + O2 = 2Fe2O3 (excess of O2)
6СоO + O2 = 2Со3O4 (400-900С)
NiO also absorbs O2 from the air, but this process gives no additional compounds of Ni.
ЕО oxides have basic properties. They are readily soluble in strong acids, don’t react with alkalis and water. СоО is a single exception because it reacts partially with concentrated alkalis.
.
Hydroxydes М(ОН)2 can be obtained by double exchange reactions of Me(II) with alkalis. White-greenish Fe(ОН)2 is formed in the absence of O2. Usually precipitate formed contains Fe(ІІ) and Fe(ІІІ). When solutions of alkalis act on solutions of Со(ІІ) salts the blue precipitate of basic salts forms at the beginning The basic salts are gradually transformed into rose Со(ОН)2 under the action of an alkali in excess:
Co(NO3)2
+ NaOH
![]() (CoOH)NO3
+ NaNO3,
(CoOH)NO3
+ NaNO3,
(CoOH)NO3+
NaOH
![]() Co(OH)2
+ NaNO3.
Co(OH)2
+ NaNO3.
Fe(OH)2, Co(OH)2 and Ni(OH)2 have basic properties:
Ni(OH)2 + 2HCl = NiCl2 + 2H2O.
