
- •Iron triad physical properties
- •Iron triad trends
- •History Of Discovery
- •Iron (Ferrum). Cobalt (Cebafttim). Nickel (Niccolum)
- •Production
- •Chemical Properties Free elements
- •Iron(II) Compounds
- •Cobalt (II) compounds
- •Iron (III) compounds
- •Complexes of cobalt
- •Coordination compounds of nickel
- •Tests for iron triad elements
- •Themes for home preparation
- •Questions and tasks
- •Iron, cobalt, nickel
- •12. How is potassium ferrate obtained? How does it react with sulfuric acid? Give the equations of the relevant reactions Quiz problems
- •А. FeCl2
- •Make up the equations o f the reactions Make up the equations o f the reactions
- •Experimental section
- •2. Chemical properties of iron
- •3. Chemical properties of cobalt
- •4. Chemical properties of nickel
Iron triad physical properties
Table 1. Some properties of iron triad elements
|
Fe |
Co |
Ni |
density, g/сm3 |
7.87 |
8.90 |
8.91 |
tm, oС |
1539 |
1492 |
1455 |
Rа, nm |
0.125 |
0.125 |
0.124 |
Ionisation energy Е ® Е+, kJ∙mol-1 |
762.5 |
760.4 |
737.1 |
Е+ ® Е2+, kJ∙mol-1 |
1561.9 |
1648 |
1753.0 |
Е2+ ® Е3+, kJ∙mol-1 |
2957 |
3232 |
3395 |
Еo(Е2+ + 2е = Е), V |
-0.44 |
-0.29 |
-0.25 |
Iron is a fairly soft metal, existing in different forms according to temperature (Fig. 1):
-Fe
![]() -Fe
-Fe![]() -Fe
-Fe
![]() -Fe.
-Fe.
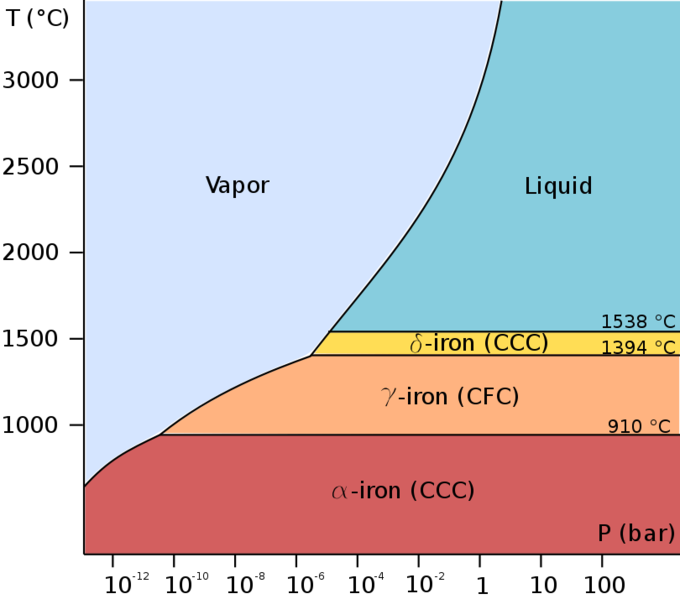
Figure 1. Phase diagram of pure iron
Alpha iron, also known as ferrite, is the most stable form of iron at normal temperatures. It is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).
Above 912 °C and up to 1400 °C α-iron undergoes a phase transition from bcc to the fcc configuration of γ-iron, also called austenite (Fig. 2). This one is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.
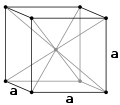
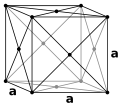
Figure 2. The body centered cubic unit cell (bcc or ccc) of -Fe and face-centered cubic unit cell (fcc) of γ-Fe
Magnetic properties of iron1. Below t = 770С it’s ferromagnetic substance (this temperature of ferro(ferri-)magnetic-paramagnetic transition is called the Curie point), in the range 770-910 оС it’s paramagnetic substance (-Fe). At 910-1401С it’s a paramagnetic (-Fe) with a face-centred cubic lattice. At 1401-1539С (b.p.) it’s -Fe with body-centred cubic lattice.
Table 2. Structure and magnetic properties of iron allotropes
-Fe*
|
-Fe |
-Fe |
-Fe |
“ferrite” ferromagnetic body-centred cubic lattice |
non-magnetic no change of structure |
face-centred cubic |
body-centred cubic |
Some Ra decrease in the series Fe-Co-Ni is the result of d-contraction, i.e. the growth of attraction energy of ns-electrons to the nucleus, whose charge is increased. This phenomenon also leads to the decrease of chemical activity of metals. Boiling point depression in the series is explained by the decrease of the number of electrons that participate in metallic bonding. Fe has lower density compared to Co and Ni because Fe forms body-centred cubic lattice at STP but Co and Ni have a close-packed hexagonal lattice.
Cobalt is a bluish silvery metal that exhibits ferromagnetism, and forms allotropes like iron.
Iron triad trends
The elements of iron triad are found in nature mostly as sulfides form strong complexes.
All elements also.
These elements belong to the d block. They have electron configurations:4s23dx with x=6,7, and 8 for Fe, Co and Ni, respectively. Therefore, oxidation state +2 is the most common.
In the chemistry of the triad, we observe the continuing tendency for the higher oxidation states to decrease in stability along the first transition series; unlike cobalt and iron, the +3 state of nickel is rare and relatively unimportant and the +2 state is the only important one.
Iron is characterized by two series of compounds: iron(II) and iron(III). The former corresponds to iron(II) oxide FeO, and the latter to iron(III) oxide Fe2O3. In addition, salts of ferric acid H3FeO4 are known in which the oxidation state of iron is +6.
The simple compounds of cobalt(III) are strongly oxidizing whereas crystalline field of ligands in complexes of Co(III) makes them relatively stable. Cobalt (II) compounds are the most stable.
