
- •Copper subgroup physical properties
- •Copper subgroup trends
- •Preparation
- •Electronic Configurations & Oxidation States
- •Chemical Properties Free elements
- •Compounds Cu(I).
- •Compounds of Ag
- •Mendeleev's predicted elements
- •The Pourbaix diagram for copper in pure water, acid or alkali conditions. Note that copper in neutral water is more noble than hydrogen.
- •Aqua regia or aqua regis
- •Dissolving gold
- •Similar equations can be written for platinum. As with gold, the oxidation reaction can be written with either nitric oxide or nitrogen dioxide as the nitrogen oxide product.
- •Decomposition of aqua regia
- •History
- •Cuprates. High Temperature Superconductors (hts)
- •History
- •Synthesis
- •Laboratory Preparation
- •Leaching
- •Gold smelting Mercury removal
Chemical Properties Free elements
All copper subgroup elements have position after hydrogen in the electromotive series of metals and, therefore, don’t displace hydrogen of non-oxidant acids. The single exception is the interaction of copper with concentrated hydrochloric acid since coordination of Cu+ with chloride-ions in acid medium significantly decreases electrode potential to negative values:
2Cu + 4HCl = 2H[CuCl2] + H2
Copper readily reacts with oxidant-acids:
3Cu + 8HNO3(dil) = 3Cu(NO3)2 + 2NO + 4H2O
Cu + 2H2SO4(conc) = + SO2 + 2H2O
Ag reacts slowly with diluted and to a great extent with concentrated HNO3:
Ag + 2HNO3(conc) = AgNO3 + NO2 + H2O
2Ag + 2H2SO4(conc) = Ag2SO4 + SO2 + 2H2O
Au does not react with these acids, although it is dissolved at the action of aqua regia (HCl : HNO3 = 3 : 1):
Au + 4HCl + HNO3 = H[AuCl4] + NO + 2H2O
The mixture of concentrated sulfuric acid with hydrogen sulfates or sulfates of alkali metals, selenic acid, melts that contain alkalis and nitrates of alkali metals also dissolve Au(0):
Au +7H2SeO4 = H[Au(SeO4)2] +3SeO2 + 6H2O
2Au + 2NaOH + 3NaNO3 = 2Na[AuO2]+3NaNO2 +H2O
Gold is stable against corrosion at atmospheric conditions but the surface of copper is gradually covered by green basic carbonate:
2Cu
+ O2
+ H2O
+ CO2
![]() (CuOH)2CO3;
(CuOH)2CO3;
Silver is coated by black film of silver sulfide, Ag2S:
4Ag + O2 + 2H2S 2Ag2S + H2O.
It is worth to note that Ag inactive to oxygen attack at the absence of H2S. Oxide film does not exist at the surface of silver and gold. Thus, the latter reaction takes place namely due to the formation of virtually insoluble compound, Ag2S (the solubility of silver sulfide, Ag2S, in water is 2.83×10-15 g/L, KSP (Ag2S) = 6×10–51).
It is only metal copper in the subgroup that reacts with oxygen:
2Cu + O2 = 2CuO
Copper monoxide decomposes at to > 800°С:
4CuO = 2Cu2O + O2
Oxides of Ag and Au can be prepared indirectly.
Free metals react with chlorine and fluorine:
Cu + Cl2 = CuCl2
2Ag + Cl2 = 2AgCl
2Au + 3Cl2 = 2AuCl3
Scheme of chemical properties of copper
Oxidation state +1
Oxidation state +2
Oxidation state +3
Compounds of copper subgroup elements
The most stable oxidation states of copper subgroup elements are Cu(II), Ag(I) and Au(III) and only compounds of Cu(I), Ag(I) and Au(I) have similarity of composition and properties. The significant distinction of properties of compounds of copper subgroup elements is the cause of their independent consideration below.
Compounds of copper
Oxidation state +2
Compounds Cu(II). Oxidants usually oxidize copper to Cu+2.
CuO can be obtained by the direct synthesis reaction between simple substances and also by thermal decomposition of many oxygencontaining compounds Cu(II):
(CuOH)2CO3 = 2CuO + H2O + CO2;
2Cu(NO3)2 = 2CuO + 4NO2 + O2.
CuO is a powder of black colour decomposed at tо > 800°С:
4CuO = 2Cu2O + O2
It is reduced readily at 250°С by hydrogen or СО:
CuO + Н2 = Cu + Н2O
Basic properties of CuO are most noticeable. This oxide reacts with strong acids:
CuO + 2HCl = CuCl2 + Н2O
while it interacts also with alkalis when melting:
CuO + 2NaOH = Н2O + Na2CuO2 (cuprates).
Solutions of Cu(ІІ) form blue precipitate of Cu(ОН)2 with alkalis. Cu(ОН)2 can be decomposed easily when heating:
Cu(ОН)2 = CuО + Н2О
Cu(ОН)2 can react with concentrated solutions of alkalis. Deep-blue hydroxocuprates are formed:
Cu(ОН)2 + 2NaOH = Na2[Cu(ОН)4]
Na2[Cu(ОН)4] loses water at 200°С and becomes transformed into oxocuprate Na2CuО2.
Aquaions Cu2+ exist as aquacomplexes [Cu(Н2О)6]2+ that are coloured blue.
The most practically important compound is copper sulfate that can be deposited with crystalline water CuSO4×5H2O (blue vitriol). It loses water after heating and nonaqueous CuSO4 becomes colourless.
Some salts of copper (ІІ) are insoluble in water. The least soluble among them is black CuS that is formed at the action of H2S or soluble sulfides on compounds of Cu(ІІ):
Сu2+ + H2S = CuS + 2H+
Copper carbonate CuСО3 has not been prepared up to the present. Insoluble basic salt appears at the action of carbonates on Cu(ІІ):
2СuSO4 + 2Na2СО3 + H2О = (CuОН)2СО3↓+ 2Na2SO4 + СО2
Crystalline basic carbonates are encountered in nature in the form of beautiful green-coloured mineral malachite (CuОН)2СО3 (or Cu(ОН)2CuCО3) and deep blue azurite 2CuCО3×Cu(ОН)2.
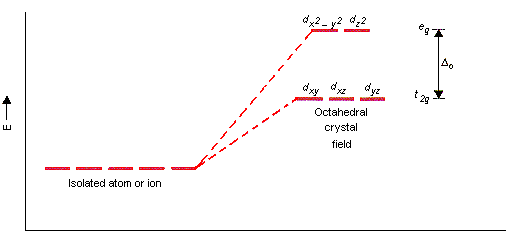
Complexes. Formation of complexes is a quite typical behavior of Сu(ІІ). All coordination compounds are coloured since d-sublevel of Сu2+ is not completed (3d9). Coordination number can be varied: 4 (square planar) or 6 (octahedral).
Nonuniformity of ligands of Cu(II) complexes with coordination number 6 can be shown by the Crystal Field Theory (see scheme below). The least repulsion occurs if unpaired 3d-electron is situated at dx2-y2-orbital that is closest to 4 ligands with negative charge. The appearance of electron pair of dz2-orbital initiates strong repulsion of ligands along z-axis. Therefore, complexes of Cu(II) frequently have space configuration of distorted octahedron. These complexes are shown sometimes in literature as follows: [Cu(H2O)4(H2O)2]2+, [Cu(OH)4(OH)2]4-.

Water molecules of the inner sphere are substituted at the action of NH3 on Cu(II). In this case [Cu(NH3)4(H2O)2]2+ forms of deep blue colour. Cu(NH3)4SO4·H2O compound can be prepared by adding concentrated ammonia solution, NH3, to a saturated aqueous solution of copper sulfate, CuSO4, until all the copper(II) hydroxide that is initially formed redissolves into a clear deep blue solution. The displacement of the fifth water molecule takes place in the concentrated ammonia only. It can be proved by stability constants of complexes (К1=1,4×104, К2=3,2×103, К3=7,8×102, К4=1,4×102 і К5=0,3).
