
- •Introduction
- •87% Of strokes are ischemic, the rest are hemorrhagic.
- •Figure 4 Molecular process of neuronal ischaemia. (Colledge, Walker and Ralston., 2010, p. 1182)
- •Available treatments for stroke
- •Figure 5 Mechanism of t-pa action, (Genentech, 2014)
- •Induced pluripotent stem cells
- •Mesenchymal stem cells
- •MsCs for treatment of stroke
- •Migratory mechanisms of msCs
- •Infused msCs frequently transmigrate towards damaged regions in significant numbers and have the ability to decrease inflammation and facilitate tissue regeneration.
Migratory mechanisms of msCs
Homing refers to the ability of MSCs to migrate towards the damaged area, yet the mechanisms of homing are not well understood. It is unclear if the MSCs actively home via leukocyte–like endothelial cell adhesion (mediated by intravascular cellular adhesion, rolling and finally vascular extravasation, movement through stromal tissue and MSC engraftment) or become passively trapped in capillaries or microvessels (localization), instead of being arrested on endothelium by selectins and integrins(Figure 10) (Karp and Teo., 2009).
Ruster et al., (2006) provide evidence for active MSC homing. Their study shows that MSCs attach to endothelial cells relying on P-selectin-dependent manner, using VLA-4 /VCAM-1 interaction, inducing strong adhesion to endothelial cells and rolling.
Biochemical messages from the damaged area, MSCs reception of them and chemotactic ability, all influence MSC migration. It is challenging to determine the exact location of MSCs following infusion, as localization may occur, or MSCs may actually undergo transendothelial migration, leading to successful homing (Karp and Teo., 2009).
The conditions used to culture MSCs are very important, as they can have a significant impact on MSC function. The confluence of MSCs in culture, for example, affects migration potential, as an increased culture confluence inhibits MSC homing by increasing the production of a natural matrix metalloproteinase (MMP) inhibitor, TIMP-3 (De Becker et al., 2007).
After tissue damage, surviving local astrocytes, microglia and endothelium up-regulate the inflammatory chemoattractants, cytokines and adhesion ligands, directing the migration of MSCs towards the inflicted penubmra. Examples of cytokines and chemokines produced by damaged tissues include: TGF-α, IL-1, TNF-α and stromal cell-derived factor 1 (SDF-1 α). The cognate receptor for SDF-1α is CXCR4, which increases MSC tropism towards the source of chemokines, in order to home to the ischaemically damaged region and produce anti-inflammatory and anti-scarring molecules, participating in regeneration of damaged tissues (Figure 11). Wynn et al., (2004) looked into CXCR4 expression on human bone-marrow derived MSCs. The results showed low expression, with 1% of the cells expressing CXCR4 on the cell surface membrane; however 83% -98% of MSCs showed high level expression of intracellular CXCR4 receptor. Shi et al., (2007) suggested that the intracellular CXCR4 receptor can be rapidly induced to the cell surface within a few hours by stimulating with a cocktail of cytokines, therefore increasing MSC migration capacity and improving engraftment.
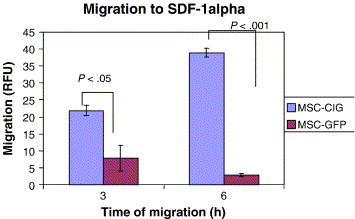
Bhakta et al., (2006) state that to optimize MSC migration and survival, CXCR4 gene can be expressed on cell surface using retroviral transduction. In this study, MSCs were isolated from healthy volunteers, cultured and transduced with either a CXCR4 containing retroviral vector and green fluorescent protein (GFP) or only GFP - control vector. As expected, MSCs transduced with CXCR4 migrated significantly more toward SDF-1α. (Figure 12) Findings by Cheng et al., (2008) also support this statement.
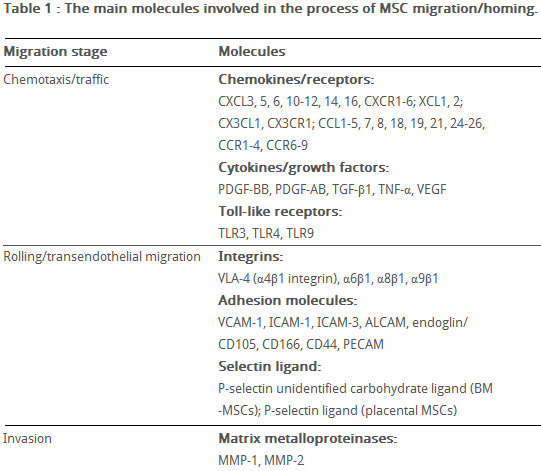
Hepatocyte growth/scatter factor (HGF) is also produced by inflamed and damaged tissues, both human and mouse MSCs express a cognate receptor for HGF, c-Met. HGF induces MSC chemotaxis, yet inhibits proliferation. Table 2 shows the main known molecules involved in MSC migration.
Sackstein et al., (2008) state that MSCs do not express E-selectin ligands, but do express a CD44 cell surface receptor. In this study glycosylation of native CD44 receptor with 2,3-sialyl modifications occurred, using 1,3-fucosyltransferase to enzymatically engineer CD44 to induce E-selectin binding. CD44 was converted into hematopoietic cell E-selectin/L-selectin ligand (HCELL)6, able to induce strong E-selectin binding, which stimulates VLA-4 integrin activation, mediated by Rac1/Rap1 GTPase signaling pathway. Triggered VLA-4 adheres to vascular cell adhesion molecule 1 (VCAM-1), which mediates adhesion and signal transduction, leading to MSC transendothelial migration. (Thankamony and Sackstein., 2011)
MSC migration through type I collagen-rich stromal tissues, is dependent on MMP-2, MMP-9 and membrane-type-1 matrix metalloproteinase (MTI-MMP), which degrade components of the extracellular matrix, allowing for stem cell motility and later differentiation.
In addition, a study by Tsai et al., (2011) has found that priming MSCs with VPA or lithium increased homing to the cerebral infarcted regions via CXCR4 and MMP-9 upregulation, and copriming with VPA and lithium further enhanced this effect.
Therefore, both SDF-1α and MMP-9 expression appear to be essential for the homing ability of stem cells.
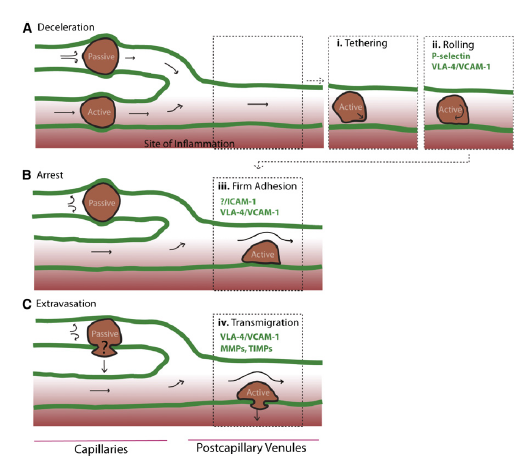
Figure 11 Inflammatory signals directing MSCs to the inflicted sites. (Vinagolu and Rajasekhar., 2009)
Figure 10, Model of passive vs active homing. (Karp and Teo., 2009) (A)MSCs can lose speed during the homing process, due to their large size and/or narrow capillaries leading to passive entrapment. Alternatively, MSCs that deform are likely to pass through capillaries to postcapillary venules, similar to leukocyte homing, and (1) tether, (2) roll on activated vasculature at sites of inflammation, where a chemokine gradient (red) is established.
(B) During passive arrest, an altered blood flow (arrows) may be observed. In contrast, during active arrest, cells quickly flatten and spread on the underlying endothelium, and blood flow is virtually unchanged, but the fate of passively arrested MSCs is unclear. (C) After active arrest, MSCs may transmigrate.

Table 2 The main molecules involved in MSC migration/homing. (Kholodenko et al., 2013)
Figure 12 MSCs transduced with the CXCR4 vector, compared to MSCs transduced with the control vector (GFP) demonstrated significantly more migration to SDF-1 α after both 3 and 6 hours. (Bhakta et al., 2006)
Discussion
Despite the data obtained by many researchers in their studies, it is apparent that MSCs from different sources show heterogeneity, particularly when expanded in vitro and express a great variety of chemokines and their receptors, which can lead to preferred transendothelial migration and homing of various MSCs into damaged tissues. Also, available knowledge from leukocytes or hematopoietic progenitor cells cannot be extrapolated to fully explain homing and migration of MSCs. Therefore, it is important to take into consideration the conditions of MSC isolation, cultivation, possibly priming and the number of passages undergone (number of times MSCs were removed from the culture and undergone a subculture process) before analysis.
Honczarenko et al., (2009) conducted a study on in vitro-cultured, second passage MSCs, which were positive for CCR1, CCR7, CCR9, CXCR4, CXCR5, and CXCR6 cell surface receptors and secreted following chemokine ligands: CCL2, CCL4, CCL5, CCL20, CXCL12, CXCL8, CX3CL1. Some of these ligands can activate the MAP kinase and focal adhesion kinase (FAK) signaling pathways, by phosphorylation. These pathways are important for transcription of integrin signalling molecules and therefore, cell migration. For example, CCL5 activates STAT-1 and CXCL12 selectively triggers STAT-5 activation, inducing F-actin polymerization in the cytoskeleton. Studies that used culture-expanded MSCs showed loss of cell surface chemokine receptors at passage 12-16, leading to reduced chemotactic response, decreased presence of ICAM-1, ICAM-2, and VCAM-1adhesion molecules. This phenotypic change is related to slowing of cell growth during long-term cultivation and spontaneous apoptosis, dependent on cell confluency and the environmental conditions (e.g. hypoxic or normoxic) during incubation. MSCs in culture can gain or lose some surface receptors, which influences their transendothelial migratory ability. In the Ruster et al., (2006) study, MSCs were found expressing high rolling velocities, which could be due to lack of in vitro activation with TNF-α ,required for the expression of cell adhesion receptors that mediate rolling in inflamed or injured epithelia. Freshly isolated MSCs show improved homing capability, than culture-expanded MSCs. (Rombouts and Ploemacher, 2003). This data suggests that several chemokine axes exist in MSC biology and it is important to validate MSCs intended for regenerative therapy, prior to their use.
FAK regulates extracellular migration, which is integral for angiogenesis, therefore, increased expression of FAK can be correlated with increased metastasis, possible tumour formation and reduced safety of MSC treatment. Similarly to ichaemically damaged regions, different tumours also produce inflammatory mediators (cytokines, chemokines, chemoattractant molecules), which could direct MSCs to migrate towards the tumour environment. Zhu et al., (2006) show that both fetal bone marrow MSCs and adult MSCs could contribute to tumour growth in vivo, when transplanted subcutaneously into mice. Tumour tissues showed rich vascularisation and general invasion and necrosis of the surrounding normal tissues and formation of tumour-associated stroma. This progression of carcinogenesis could be due to spontaneous transformation of MSCs, that have reached primary tumour sites. These findings illustrate the necessity for further research to assure long-term safety of MSC therapy.
Lee et al., (2010) conducted a long-term, follow-up study in patients who received intravenous autologous MSC therapy after ischaemic stroke, to assess the long-term safety and efficacy of the treatment. Based on 5 years of follow-up, the results of this study showed that IV application of in vitro cultured MSCs is safe. No differences in comorbidities between the control and the MSC groups were found with little acute toxicity, the mortality in the MSC group was lower - 4 out of 16 patients (25%) died in the MSC group and 21 out of 36 (58.3%) died in the control group during the follow-up period. Serious adverse effects associated with MSC therapy include: arrhythmia, supraventricular tachyarrhythmia, seizures, caused by abnormal excitation from freshly formed neural circuits and vascular occlusion, caused by MSCs during IV infusion. The occurrence of these adverse effects did not differ between the groups. Researchers reported, that the level of SDF-1α in blood plasma during the infusion of MSCs, directly correlated with clinical improvements. In patients with ischaemic stroke circulating SDF-1α may be isolated within the vascular endothelium or infarcted area, increasing the local SDF-1α level. This emphasizes the importance of SDF-1α in post-stroke functional recovery following MSC treatment. It has also been suggested, that using serum SDF-1α levels could be useful for selecting patients who are likely to have favourable outcomes after MSC therapy.
Explaining MSC migration and engraftment is challenging, because to date, no clear, universally accepted criteria for classification of MSC phenotype and functional properties are available. More detailed research examining specific molecular signalling pathways mediating MSC migration, would aid novel therapeutic agent production and increase efficacy in clinical practice and regenerative medicine. Also, the method of MSC culturing has proven to affect MSC performance. The opportunity of modulating cultured MSCs with cytokines or using retroviral transduction opens broad possibilities in creating successful treatment, capable of directed tropism of greater number of transfused MSCs towards the site of the injury.
Conclusion
Based on the data from existing studies, the following can be concluded:
