
- •Introduction
- •87% Of strokes are ischemic, the rest are hemorrhagic.
- •Figure 4 Molecular process of neuronal ischaemia. (Colledge, Walker and Ralston., 2010, p. 1182)
- •Available treatments for stroke
- •Figure 5 Mechanism of t-pa action, (Genentech, 2014)
- •Induced pluripotent stem cells
- •Mesenchymal stem cells
- •MsCs for treatment of stroke
- •Migratory mechanisms of msCs
- •Infused msCs frequently transmigrate towards damaged regions in significant numbers and have the ability to decrease inflammation and facilitate tissue regeneration.
Embryonic stem cells
Adult stem cells
Induced pluripotent stem cells
Embryonic stem cells
Embryonic stem cells (ESCs) are derived from the undifferentiated embryoblas of a blastocyst. Human embryos reach the blastocyst stage 4–5 days post fertilization. Removal of the embyoblast results in the destruction of a blastocyst, which raises ethical concerns. ESCs are pluripotent, meaning they are able to differentiate into more than 220 cell types of the three primary germ layers: ectoderm, endoderm and mesoderm and have the ability to replicate indefinitely under defined conditions. This and ESC plasticity makes them potentially useful for research and regenerative medicine.
Adult stem cells
Also known as somatic stem cells (SSC) are multipotent, undifferentiated cells found at different sites throughout the body, which can multiply by mitosis to regenerate damaged tissues. Multipotency refers to the adult stem cell's ability to generate into distinct cell types; however, research suggests that the transdifferentiation capacity of some SSC can be improved by modification of the growth medium used when cultured in vitro producing induced pluripotent stem cells. (Yamanaka and Blau, 2010) Many different types of SSCs from different origins have been identified, including: umbilical cord stem cells, mesenchymal stem cells, neural stem cells, hematopoietic stem cells and limbal stem cells. As these cells can be harvested from the patient, without ethical concerns, many researchers have focused on the SSC therapeutic potential for a variety of diseases. Yet, culturing SSCs up to the necessary numbers is still considered a challenge.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are specialized somatic cells, which have been genetically modified by injection of DNA or transcription factors to behave like ESC. IPSCs raise high hopes for future therapy, as they eliminate the risk of immune rejection by the patient, however, due to the novel nature of the technology and the not well understood process of reprogramming, unfortunately iPSC therapy is only theoretical at the moment. A way to improve iPSC safety needs to be found, as sometimes after genetic modification iPSCs have been shown to form tumours. (Liang et al., 2013)
F

Figure 7 Pluripotent ESC types and multipotent adult stem cell and pathological disorders that might benefit from ESC and SSC therapies.
Abbreviations: BASCs, bronchioalveolar stem cells; bESCs, bulge epithelial stem cells; CESCs, corneal epithelial stem cells; CSCs, cardiac stem cells; eNCSCs, epidermal neural crest stem cells; ESCs, embryonic stem cells; EPC, endothelial progenitor cell; HOCs, hepatic oval cells; HSCs, hematopoetic stem cells; KSCs, keratinocyte stem cells; MSCs, mesenchymal stem cells; NSCs, neuronal stem cells; PSCs, pancreatic stem cells; RSCs, retinal stem cells; SKPs, skin-derived precursors. (Mimeault et al., 2007)
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multi-potent cells with a strong capacity for self-renewal. They are capable of multilineage differentiation into mesoderm-type cells such as osteoblast, adipocyte, chondrocyte and possibly, but still controversial, other non-mesoderm type cells, for example, neuronal cells or hepatocytes. (Abdallah et al., 2008) (Figure 8) They can be isolated from a variety of tissues, such as bone marrow, adipose tissue, umbilical cord, and umbilical cord blood. MSCs have the ability to differentiate into a variety of cell types, depending on cues from their microenvironment. MSCs are identified by the expression of many molecular markers, such as: CD105, CD73 and are negative for the hematopoietic markers CD34, CD45, CD14. (Daminici et al., 2006) An important property of MSCs is their capacity for migration and homing in or around the zones damaged by ischaemia, inflammation, and trauma or tumour sites. Understandably, the efficacy and time course of cell invasion into the damaged tissues depends upon the route of transplantation. (Kholodenko et al., 2013) MSCs have great potential as therapeutic agents, since they can be obtained fairly effortlessly and can be rapidly expanded ex vivo for autologous transplantation, according to Dharmasaroja et al, (2009).
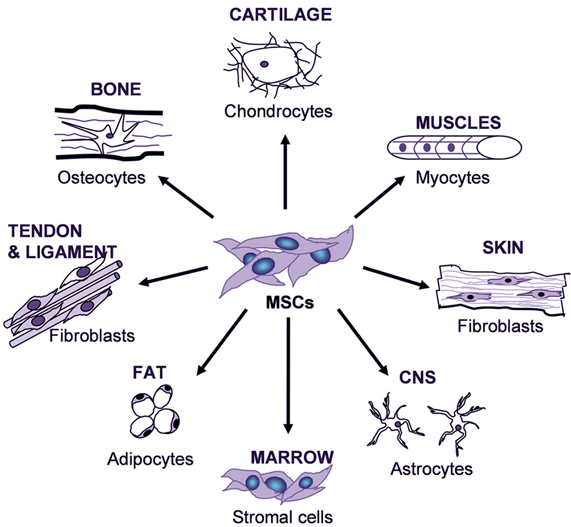
Figure 8 Variety of tissue types MSCs have the potential to differentiate into, depending on signals produced by the local physiological environment. (Kishk et al., 2010)
