
- •Introduction
- •Figure 1 Post stroke disabilities (Genetech 2014)
- •87% Of strokes are ischemic, the rest are hemorrhagic.
- •Figure 4 Molecular process of neuronal ischaemia. (Colledge, Walker and Ralston., 2010, p. 1182)
- •Available treatments for stroke
- •Induced pluripotent stem cells
- •Mesenchymal stem cells
- •Figure 8 Variety of tissue types msCs have the potential to differentiate into, depending on signals produced by the local physiological environment. (Kishk et al., 2010)
- •MsCs for treatment of stroke
- •Migratory mechanisms of msCs
Migratory mechanisms of msCs
Homing refers to the ability of MSCs to migrate towards the damaged area, yet the mechanisms of homing are not well understood. It is unclear if the MSCs actively home via leukocyte–like endothelial cell adhesion (mediated by intravascular cellular adhesion, rolling and finally vascular extravasation, movement through stromal tissue and MSC engraftment) or become passively trapped in capillaries or microvessels (localization), instead of being arrested on endothelium by selectins and integrins(Figure 10) (Karp and Teo., 2009).
(Ruster et al., 2006) provide evidence for active MSC homing. Their study shows that MSCs attach to endothelial cells relying on P-selectin-dependent manner, using VLA-4 /VCAM-1 interaction, inducing strong adhesion to endothelial cells and rolling.
Biochemical messages from the damaged area, MSCs reception of them and chemotactic ability, all influence MSC migration. It is challenging to determine the exact location of MSCs following infusion, as localization may occur, or MSCs may actually undergo transendothelial migration, leading to successful homing (Karp and Teo., 2009).
The conditions used to culture MSCs are very important, as they can have a significant impact on MSC function. The confluence of MSCs in culture, for example, affects migration potential, as increased culture confluence inhibits MSC homing by increasing the production of a natural matrix metalloproteinase (MMP) inhibitor, TIMP-3 (De Becker et al., 2007).
Tissue damage recruits MSCs into the inflicted sites, by cytokine and chemokine release and activation of adhesion ligands, so that MSCs can participate in tissue regeneration and modulation of the immune response. Examples of cytokines and chemokines produced by damaged tissues include: transforming growth factor (TGF-β), interleukin-1 (IL-1), tumour necrosis factor (TNF-α) and stromal derived factor (SDF-1). Hepatocyte growth/scatter factor (HGF) is also produced by inflamed and damaged tissues, both human and mouse MSCs express a cognate receptor for HGF, c-Met. HGF induces MSC chemotaxis, yet inhibits proliferation. Table 2 shows the main known molecules involved in MSC migration.
MSC migration through type I collagen-rich stromal tissues, is dependent on MMP-2, MMP-9 and membrane-type-1 matrix metalloproteinase (MTI-MMP), which degrade components of the extracellular matrix, allowing for stem cell motility and later differentiation.
Imitola et al., (2004) show that neural stem cells (NSCs) migrate in vivo toward an infarcted area, where surviving local astrocytes, microglia and endothelium up-regulate the inflammatory chemoattractants, including SDF-1α, which direct the migration of NSCs towards the penubmra. Both exogenous and endogenous NSCs express the cognate receptor for SDF-1α, CXCR4, which allows them to migrate towards the source of chemokines, home to the ischaemically damaged region and produce anti-inflammatory and anti-scarring molecules. (Figure 12)
Wynn et al., (2004) looked into CXCR4 expression on human bone-marrow derived MSCs. The results showed that 1% of the cells CXCR4 is present on the cell surface membrane, however 83% -98% of MSCs showed high level expression of intracellular CXCR4 receptor.
Bhakta et al., (2006) state that to optimize MSC migration and survival, CXCR4 gene can be expressed using retroviral transduction. In this study, MSCs were isolated from healthy volunteers, cultured and transduced with either a CXCR4 containing retroviral vector and green fluorescent protein (GFP) or only GFP - control vector. As expected, MSCs transduced with CXCR4 migrated significantly more toward SDF-1α. (Figure 13) Findings by Cheng et al., (2008) also support this statement.
In addition, a study by Tsai et al., (2011) has found that priming MSCs with VPA or lithium increased homing to the cerebral infarcted regions via CXCR4 and MMP-9 upregulation, and copriming with VPA and lithium further enhanced this effect. MSC were labelled with BrdU before transplantation. Homing MSCs were detected using immunohistochemistry. (Figure 14) Therefore, both SDF-1α and MMP-9 expression appear to be essential for the homing ability of stem cells.
Sackstein et al., (2008) state that MSCs do not express E-selectin ligands, but do express a CD44 cell surface receptor. In this study glycosylation of native CD44 receptor with 2,3-sialyl modifications occurred, using 1,3-fucosyltransferase to enzymatically engineer CD44 to induce E-selectin binding. CD44 was converted into hematopoietic cell E-selectin/L-selectin ligand (HCELL)6, able to induce strong E-selectin binding, which stimulates VLA-4 integrin activation, mediated by Rac1/Rap1 GTPase signaling pathway. Triggered VLA-4 adheres to vascular cell adhesion molecule 1 (VCAM-1), which mediates adhesion and signal transduction, leading to MSC transendothelial migration. (Thankamony and Sackstein., 2011)
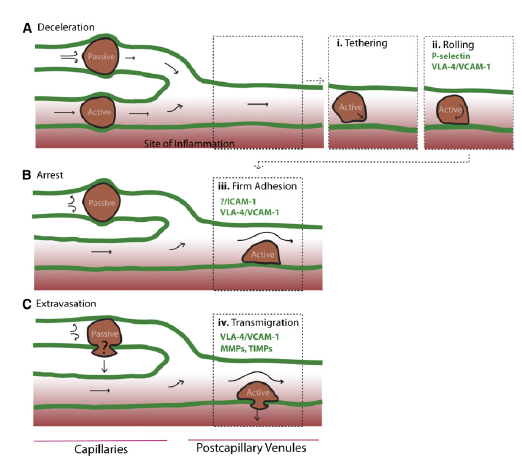
Figure 11 Inflammatory signals directing MSCs to the inflicted sites. (Vinagolu and Rajasekhar., 2009)
Figure 10, Model of passive vs active homing. (Karp and Teo., 2009) (A)MSCs can lose speed during the homing process, due to their large size and/or narrow capillaries leading to passive entrapment. Alternatively, MSCs that deform are likely to pass through capillaries to postcapillary venules, similar to leukocyte homing, and (1) tether, (2) roll on activated vasculature at sites of inflammation, where a chemokine gradient (red) is established.
(B) During passive arrest, an altered blood flow (arrows) may be observed. In contrast, during active arrest, cells quickly flatten and spread on the underlying endothelium, and blood flow is virtually unchanged, but the fate of passively arrested MSCs is unclear.
(C) After active arrest, MSCs may transmigrate.
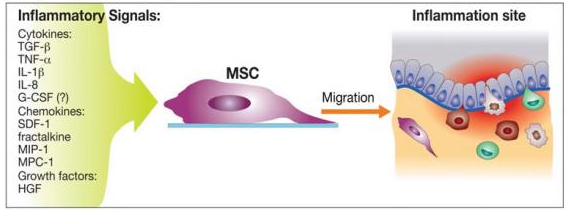
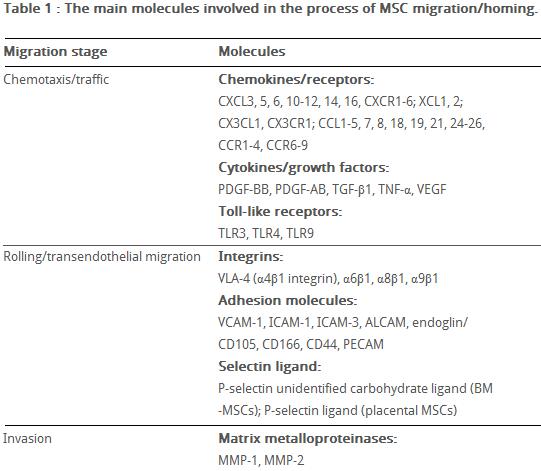
Figure 13 MSCs transduced with the CXCR4 vector, compared to MSCs transduced with the control vector (GFP) demonstrated significantly more migration to SDF-1 α after both 3 and 6 hours. (Bhakta et al., 2006)
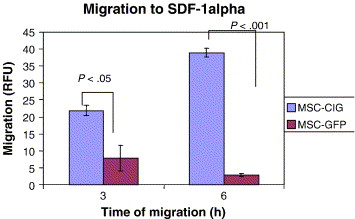
Table 2 The main molecules involved in MSC migration/homing. (Kholodenko et al., 2013)
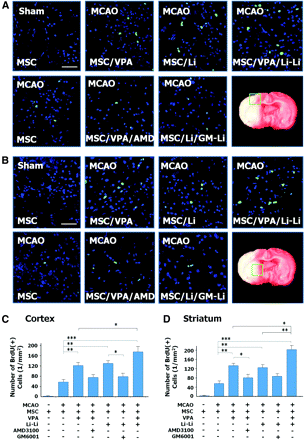
Figure 14 Priming with VPA and/or lithium promotes MSC (green) homing into infarcted brain regions. In the ischemic frontal cortex (A) and striatum (B), the number of BrdU-positive cells increased when MSC were primed with sodium VPA and/or lithium chloride before transplantation. (Tsai et al., 2011)
Discussion
Despite the data obtained by many researchers in their studies, it is apparent that MSCs from different sources show heterogeneity, particularly when expanded in vitro and express a great variety of chemokines and their receptors, which can lead to preferred transendothelial migration and homing of various MSCs into damaged tissues. Also, available knowledge from leukocytes of hematopoietic progenitor cells cannot be extrapolated to fully explain homing and migration of MSCs. Therefore, it is important to take into consideration the conditions of MSC isolation, cultivation, possibly priming and the number of passages undergone (number of times MSCs were removed from the culture and undergone a subculture process) before analysis.
Honczarenko et al., (2009) conducted a study on in vitro-cultured, second passage MSCs, were positive for CCR1, CCR7, CCR9, CXCR4, CXCR5, and CXCR6 cell surface receptors and secreted following chemokine ligands: CCL2, CCL4, CCL5, CCL20, CXCL12, CXCL8, CX3CL1. Some of these ligands can activate the MAP kinase and focal adhesion kinase (FAK) signaling pathways, by phosphorylation. These pathways are important for transcription of integrin signalling molecules and cell migration. For example, CCL5 activates STAT-1 and CXCL12 selectively triggers STAT-5 activation, inducing F-actin polymerization in the cytoskeleton. Studies that used culture-expanded MSCs showed loss of cell surface chemokine receptors at passage 12-16, leading to reduced chemotactic response, decreased presence of ICAM-1, ICAM-2, and VCAM-1adhesion molecules. This phenotypic change is related to slowing of cell growth during long-term cultivation and spontaneous apoptosis, dependent on cell confluency and the environmental conditions (e.g. hypoxic or normoxic) during incubation. MSCs in culture can gain or lose some surface receptors, which influences their transendothelial migratory ability. In the Ruster et al., (2006) study, MSCs were found expressing high rolling velocities, which could be due to lack of in vitro activation with TNF-α ,required for the expression of cell adhesion receptors that mediate rolling in inflamed or injured epithelia. Freshly isolated MSCs show improved homing capability, than culture-expanded MSCs. (Rombouts and Ploemacher, 2003). This data suggests that several chemokine axes exist in MSC biology and it is important to validate MSCs intended for regenerative therapy, prior to their use.
It is challenging to explain MSC migration and homing, due to a lack of universally accepted criteria for defining MSC functional properties and phenotype, as well as which method out of many to use for MSC culturing.
FAK regulates extracellular migration, which is integral for angiogenesis, therefore, increased expression of FAK can be correlated with increased metastasis, possible tumour formation and reduced safety of MSC treatment. Similarly to ichaemically damaged regions, different tumours also produce inflammatory mediators (cytokines, chemokines, chemoattractant molecules), which could direct MSCs to migrate towards the tumour environment. Zhu et al., (2006) show that both fetal bone marrow MSCs and adult MSCs could contribute to tumour growth in vivo, when transplanted subcutaneously into mice. Tumour tissues showed rich vascularisation and general invasion and necrosis of the surrounding normal tissues and formation of tumour-associated stroma. This progression of carcinogenesis could be due to spontaneous transformation of MSCs, that have reached primary tumour sites. These findings illustrate the necessity for further research to assure long-term safety of MSC therapy.
Thus, in order to create the most complete picture of the mechanisms of MSC migration and homing require more detailed studies aimed at investigating the various molecular signaling pathways involved in these processes. A more complete understanding of the mechanisms of MSC homing and migration will help effectively apply these cells in clinical practice and regenerative medicine when administered systemically. The possibility to modulate the migration ability of these cells, for example, by inflammatory cytokines, opens broad prospects for the development of directed migration of greater number of iv injected cells to the injury site.
Conclusion
Based on the data from existing studies, the following can be concluded:
(1) There is evidence of active MSCs migration towards inflamed or injured tissues, following cytokine and chemokine gradients. However, more work is required to substantiate this model, and the origin and mechanisms of trafficking of the mobilized MSCs remain unresolved.
(2) Systemically infused MSCs are frequently observed in significant concentrations within the bone marrow compartment, or within an injury or inflammatory site, and these cells have potential to reduce inflammation and promote tissue regeneration. However, the exact location of the MSCs (within the vessel or tissue) and their phenotype remain elusive, and thus broad conclusions cannot be substantiated regarding their engraftment or mechanisms that mediate their functional properties.
(3) Direct methods of assessing native MSCs and their trafficking properties is a big unmet need required to conclusively elucidate mechanisms of MSC trafficking during physiological and pathological states. Detection of infused MSCs that remain in an undifferentiated state compared to their differentiated progeny also presents a significant challenge.
(4) Homing of culture-expanded MSCs is inefficient compared to leukocytes and HSCs, which apparently is due to a lack of relevant cell-adhesion and chemokine receptors; however, engineering strategies are available to enhance the homing response. The increased size of MSCs likely promotes passive cell entrapment and reduces the number of MSCs that reach the target site.
As our understanding of the mechanisms of MSC trafficking grows, the ability to enhance homing to specific tissues through engineered approaches should significantly reduce the number of cells required to achieve a therapeutic effect, and presumably provide better outcomes for patients. Accumulating evidence suggests that MSCs have a significantly larger role in regulating wound healing and inflammatory diseases than previously thought. Given the systemic nature of many diseases and the desire to have minimally invasive therapies, systemic infusion of MSCs that can promote tissue regeneration and immunosuppressive effects represents an attractive therapeutic approach. The number of potential therapeutic applications and their efficiency and efficacy will continue to grow as the fundamental biology that is responsible for the MSC regenerative properties and homing responses continues to be elucidated.
Figure 3. Problems Faced in Field of MSC
Trafficking
Given the complexities involved in identifying
MSCs and tracking their position and the lack of
standardized methods for culturing and characterizing
them, new studies in this area should
consider the common problems/challenges that
are experienced and the available methods to
address them.
References
National Institutes of Health, U.S. Department of Health and Human Services. (2002). Stem Cell basics. Available: http://stemcells.nih.gov/info/basics/pages/basics1.aspx. Last accessed: 15/04/2014.
Kishk N et al. (2010). Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 24 (8), p. 702-8.
Liang Y et al. (2013). The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chinese Journal of Cancer, 32 (4), p. 205-212.
Yamanaka S. and Blau H., (2010). Nuclear reprogramming to a pluripotent state by three approaches. Nature, 465, p. 704–712
Dominici M. et al., (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8 (4), p. 315-317.
Colledge N., Walker B., Ralston S. (2010). Davidson's Principles and Practice of Medicine. 21st ed. Elsevier. p. 1181-1182.
Bhakta S. Et al., (2005) The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovascular Revascularization Medicine. 7 (1), p. 17-24.
Chen L., et al. (2008). Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE. 3, (4)
Tsai L. et al.,. (2011). Mesenchymal Stem Cells Primed With Valproate and Lithium Robustly Migrate to Infarcted Regions and Facilitate Recovery in a Stroke Model. Stroke. 42, p. 2932-2939
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3185169/
